-
Name
(R)-2-PROPYLOCTANOIC ACID
- EINECS
- CAS No. 185517-21-9
- Article Data19
- CAS DataBase
- Density 0.908 g/cm3
- Solubility
- Melting Point
- Formula C11H22O2
- Boiling Point 289.3 °C at 760mmHg
- Molecular Weight 186.294
- Flash Point 154.2 °C
- Transport Information
- Appearance
- Safety
- Risk Codes
-
Molecular Structure
- Hazard Symbols
- Synonyms Acide arundique [INN-French];Acidum arundicum [INN-Latin];Arundic acid [INN];Acido ar?ndico [INN-Spanish];(2R)-2-Propyloctanoic acid;
- PSA 37.30000
- LogP 3.45770
Synthetic route
-
-
213914-70-6
(2S)-2-(2-propenyl)octanoic acid

-
-
185517-21-9
(R)-(-)-arundic acid

| Conditions | Yield |
|---|---|
| With hydrogen; platinum on activated charcoal In isopropyl alcohol under 7600 Torr; for 1.5h; Catalytic hydrogenation; | 99% |
| With hydrogenchloride; sodium chloride; hydrogen; platinum In hexane; ethyl acetate; isopropyl alcohol | |
| With hydrogen; palladium 10% on activated carbon In methanol; ethyl acetate at 20℃; for 1h; | n/a |
| With platinum on carbon; hydrogen In isopropyl alcohol at 30℃; under 3750.38 Torr; for 3.5h; Autoclave; Large scale; | 1045 g |
-
-
1333204-12-8
(R)-2-propyloctan-1-ol

-
-
185517-21-9
(R)-(-)-arundic acid

| Conditions | Yield |
|---|---|
| Stage #1: (R)-2-propyloctan-1-ol With sodium hypochlorite; sodium chlorite; 2,2,6,6-Tetramethyl-1-piperidinyloxy free radical In water; acetonitrile at 35℃; for 7h; pH=6.7; Inert atmosphere; aq. phosphate buffer; Stage #2: With sodium hydrogencarbonate; sodium sulfite In water; acetonitrile at 0℃; pH=8; Inert atmosphere; Stage #3: With hydrogenchloride In water pH=2; Inert atmosphere; | 98% |
| With sodium hypochlorite solution; 2,2,6,6-Tetramethyl-1-piperidinyloxy free radical; sodium chlorite In aq. phosphate buffer; acetonitrile at 35℃; for 7h; Inert atmosphere; | 98% |
| With sodium hypochlorite; sodium chlorite; 2,2,6,6-Tetramethyl-1-piperidinyloxy free radical In aq. phosphate buffer; acetonitrile at 35℃; for 7h; pH=6.7; | 95% |
| With sodium hypochlorite; sodium chlorite; 2,2,6,6-Tetramethyl-1-piperidinyloxy free radical In aq. phosphate buffer; water; acetonitrile at 35℃; for 7h; pH=6.7; | 95% |

-
-
185517-21-9
(R)-(-)-arundic acid

| Conditions | Yield |
|---|---|
| With potassium hydroxide In water at 20℃; for 0.166667h; | 97.1% |
-
-
185463-37-0
(2S)-2-(2-propynyl)octanoic acid

-
-
185517-21-9
(R)-(-)-arundic acid

| Conditions | Yield |
|---|---|
| With hydrogen; palladium on activated charcoal In ethyl acetate at 20℃; for 7305h; | 97% |
| With hydrogen; platinum on activated charcoal In isopropyl alcohol under 3800 Torr; for 2.5h; Catalytic hydrogenation; | 94.6% |
| With hydrogen; 5%-palladium/activated carbon In monoethylene glycol diethyl ether; water under 3800.26 Torr; for 5h; | 89% |
| palladium In methanol; ethyl acetate | |
| With hydrogenchloride; sodium chloride; hydrogen; palladium In 1,2-dimethoxyethane; hexane; ethyl acetate |

-
-
185517-21-9
(R)-(-)-arundic acid

| Conditions | Yield |
|---|---|
| With palladium on activated charcoal; hydrogen In methanol under 3040.2 Torr; for 2h; | 96% |
-
-
1192553-22-2
(S)-2-[(E)-1-propenyl]-(Z)-oct-4-enoic acid

-
-
185517-21-9
(R)-(-)-arundic acid

| Conditions | Yield |
|---|---|
| With palladium 10% on activated carbon; hydrogen In methanol at 20℃; under 3040.2 Torr; for 2h; | 96% |
-
-
1087312-90-0
(1R)-2-endo-[(2R)-propyloctanoyl]-1,7,7-trimethylbicyclo[2.2.1]heptan-2-ol

-
A
-
464-49-3
(1R,4R)-1,7,7-trimethylbicyclo[2.2.1]heptan-2-one

-
B
-
185517-21-9
(R)-(-)-arundic acid

| Conditions | Yield |
|---|---|
| With ammonium cerium(IV) nitrate In water; acetonitrile at 0℃; for 1h; | A n/a B 95% |

-
-
185517-21-9
(R)-(-)-arundic acid

| Conditions | Yield |
|---|---|
| With 1‐methyl‐2‐azaadamantane‐N‐oxyl; sodium hypochlorite; sodium chlorite In aq. phosphate buffer; acetone at 20℃; for 3h; pH=6.8; | 92% |

-
-
185517-21-9
(R)-(-)-arundic acid

| Conditions | Yield |
|---|---|
| With ferric nitrate In 1,4-dioxane for 20h; Heating; | 60% |
-
-
213914-72-8
N-[(2S)-2-propyloctanoyl]-(1S)-(-)-10,2-camphorsultam

-
-
185517-21-9
(R)-(-)-arundic acid

| Conditions | Yield |
|---|---|
| With tetra(n-butyl)ammonium hydroxide; dihydrogen peroxide In 1,2-dimethoxyethane; water at -20℃; for 0.833333h; | 59% |
| With tetra(n-butyl)ammonium hydroxide; dihydrogen peroxide In tetrahydrofuran at -20℃; for 0.833333h; Hydrolysis; | 59.3% |

-
-
185517-21-9
(R)-(-)-arundic acid

| Conditions | Yield |
|---|---|
| Stage #1: N-(2R-(2-propyl)octanoyl)-(1S)-(-)-2,10-camphorsultam With tetra(n-butyl)ammonium hydroxide; dihydrogen peroxide In tetrahydrofuran; water at -20℃; for 0.833333h; Stage #2: With sodium sulfite In tetrahydrofuran; water at 20℃; for 0.5h; | 59.3% |

-
A
-
807363-10-6
(+)-(S)-2-propyloctanoic acid

-
B
-
185517-21-9
(R)-(-)-arundic acid

| Conditions | Yield |
|---|---|
| Stage #1: (1,2:5,6-di-O-isopropylidene-α-D-glucofuranos-3-O-yl) 2-hexylpent-3-enoate With hydrogen; platinum(IV) oxide In diethyl ether at 20℃; under 760 Torr; for 12h; Stage #2: With lithium hydroxide; dihydrogen peroxide In methanol; water for 6h; Title compound not separated from byproducts; | |
| Stage #1: (1,2:5,6-di-O-isopropylidene-α-D-glucofuranos-3-O-yl) 2-hexylpent-3-enoate With hydrogen; platinum(IV) oxide In diethyl ether at 20℃; under 760 Torr; for 12h; Stage #2: With titanium(IV) isopropylate; benzyl alcohol In toluene for 7h; Heating; Stage #3: With hydrogen; palladium on activated charcoal In methanol at 20℃; under 760 Torr; for 6h; Title compound not separated from byproducts; |

-
-
185517-21-9
(R)-(-)-arundic acid

| Conditions | Yield |
|---|---|
| Multi-step reaction with 2 steps 1: H2 / 10 percent Pd/C / methanol / 1 h 2: 60 percent / aq. Fe(NO3)3 / dioxane / 20 h / Heating View Scheme |
-
-
141341-55-1
N-octanoyl-(1S)-(-)-10,2-camphorsultam

-
-
185517-21-9
(R)-(-)-arundic acid

| Conditions | Yield |
|---|---|
| Multi-step reaction with 3 steps 1.1: LDA / tetrahydrofuran; hexane / 0.5 h / -78 °C 1.2: 85.3 percent / LiI / tetrahydrofuran; hexane; various solvent(s) / 3.5 h / -78 - -30 °C 2.1: 89.6 percent / 2-methyl-2-butene; H2O2; tetrabutylammonium hydroxide / 1,2-dimethoxy-ethane; H2O / 0.17 h / -10 °C 3.1: 94.6 percent / H2 / Pt/C / propan-2-ol / 2.5 h / 3800 Torr View Scheme | |
| Multi-step reaction with 3 steps 1.1: LDA / tetrahydrofuran; hexane / 0.5 h / -78 °C 1.2: 71.7 percent / LiI / tetrahydrofuran; hexane; various solvent(s) / 6 h / -78 - 0 °C 2.1: 100 percent / H2 / Pd/C / ethyl acetate; methanol / 1 h 3.1: 59.3 percent / aq. H2O2; tetrabutylammonium hydroxide / tetrahydrofuran / 0.83 h / -20 °C View Scheme | |
| Multi-step reaction with 3 steps 1.1: LDA / tetrahydrofuran; hexane / 0.5 h / -78 °C 1.2: 71.7 percent / LiI / tetrahydrofuran; hexane; various solvent(s) / 6 h / -78 - 0 °C 2.1: 82.2 percent / 2-methyl-2-butene; H2O2; tetrabutylammonium hydroxide / 1,2-dimethoxy-ethane; H2O / 2 h / -10 °C 3.1: 99 percent / H2 / Pt/C / propan-2-ol / 1.5 h / 7600 Torr View Scheme | |
| Multi-step reaction with 3 steps 1.1: lithium diisopropyl amide / tetrahydrofuran; n-heptane; ethylbenzene / 0.5 h / -60 °C / Large scale 1.2: 4 h / -65 - 5 °C / Large scale 2.1: 2-methyl-but-2-ene; dihydrogen peroxide; tetra(n-butyl)ammonium hydroxide / water; 1,2-dimethoxyethane / -10 - 5 °C 3.1: platinum on carbon; hydrogen / isopropyl alcohol / 3.5 h / 30 °C / 3750.38 Torr / Autoclave; Large scale View Scheme | |
| Multi-step reaction with 3 steps 1.1: lithium cyclohexylisopropylamide / tetrahydrofuran 1.2: -5 - 5 °C 2.1: 2-methyl-but-2-ene; dihydrogen peroxide; tetra(n-butyl)ammonium hydroxide / water; 1,2-dimethoxyethane / -10 - 5 °C 3.1: platinum on carbon; hydrogen / isopropyl alcohol / 3.5 h / 30 °C / 3750.38 Torr / Autoclave; Large scale View Scheme |
-
-
111-64-8
n-octanoic acid chloride

-
-
185517-21-9
(R)-(-)-arundic acid

| Conditions | Yield |
|---|---|
| Multi-step reaction with 4 steps 1.1: 98.6 percent / Et3N; 4-dimethylaminopyridine / tetrahydrofuran / 1 h / 0 °C 2.1: LDA / tetrahydrofuran; hexane / 0.5 h / -78 °C 2.2: 85.3 percent / LiI / tetrahydrofuran; hexane; various solvent(s) / 3.5 h / -78 - -30 °C 3.1: 89.6 percent / 2-methyl-2-butene; H2O2; tetrabutylammonium hydroxide / 1,2-dimethoxy-ethane; H2O / 0.17 h / -10 °C 4.1: 94.6 percent / H2 / Pt/C / propan-2-ol / 2.5 h / 3800 Torr View Scheme | |
| Multi-step reaction with 4 steps 1.1: 98.6 percent / Et3N; 4-dimethylaminopyridine / tetrahydrofuran / 1 h / 0 °C 2.1: LDA / tetrahydrofuran; hexane / 0.5 h / -78 °C 2.2: 71.7 percent / LiI / tetrahydrofuran; hexane; various solvent(s) / 6 h / -78 - 0 °C 3.1: 100 percent / H2 / Pd/C / ethyl acetate; methanol / 1 h 4.1: 59.3 percent / aq. H2O2; tetrabutylammonium hydroxide / tetrahydrofuran / 0.83 h / -20 °C View Scheme | |
| Multi-step reaction with 4 steps 1.1: 98.6 percent / Et3N; 4-dimethylaminopyridine / tetrahydrofuran / 1 h / 0 °C 2.1: LDA / tetrahydrofuran; hexane / 0.5 h / -78 °C 2.2: 71.7 percent / LiI / tetrahydrofuran; hexane; various solvent(s) / 6 h / -78 - 0 °C 3.1: 82.2 percent / 2-methyl-2-butene; H2O2; tetrabutylammonium hydroxide / 1,2-dimethoxy-ethane; H2O / 2 h / -10 °C 4.1: 99 percent / H2 / Pt/C / propan-2-ol / 1.5 h / 7600 Torr View Scheme |
-
-
213914-74-0
N-[(2S)-2-hexyl-4-pentynoyl]-(1S)-(-)-10,2-camphorsultam

-
-
185517-21-9
(R)-(-)-arundic acid

| Conditions | Yield |
|---|---|
| Multi-step reaction with 2 steps 1: 89.6 percent / 2-methyl-2-butene; H2O2; tetrabutylammonium hydroxide / 1,2-dimethoxy-ethane; H2O / 0.17 h / -10 °C 2: 94.6 percent / H2 / Pt/C / propan-2-ol / 2.5 h / 3800 Torr View Scheme |
-
-
213914-68-2
N-(2S)-(2-hexyl-4-pentenoyl)-(1S)-(-)-10,2-camphorsultam

-
-
185517-21-9
(R)-(-)-arundic acid

| Conditions | Yield |
|---|---|
| Multi-step reaction with 2 steps 1: 100 percent / H2 / Pd/C / ethyl acetate; methanol / 1 h 2: 59.3 percent / aq. H2O2; tetrabutylammonium hydroxide / tetrahydrofuran / 0.83 h / -20 °C View Scheme | |
| Multi-step reaction with 2 steps 1: 82.2 percent / 2-methyl-2-butene; H2O2; tetrabutylammonium hydroxide / 1,2-dimethoxy-ethane; H2O / 2 h / -10 °C 2: 99 percent / H2 / Pt/C / propan-2-ol / 1.5 h / 7600 Torr View Scheme | |
| Multi-step reaction with 2 steps 1: 2-methyl-but-2-ene; dihydrogen peroxide; tetra(n-butyl)ammonium hydroxide / water; 1,2-dimethoxyethane / -10 - 5 °C 2: platinum on carbon; hydrogen / isopropyl alcohol / 3.5 h / 30 °C / 3750.38 Torr / Autoclave; Large scale View Scheme |
-
-
807362-94-3
(2R)-2-propyloctanamide

-
-
185517-21-9
(R)-(-)-arundic acid

| Conditions | Yield |
|---|---|
| With hydrogenchloride; water; acetic acid at 130℃; for 10h; | n/a |
| With methanesulfonic acid In acetic acid at 105 - 112℃; for 13h; Product distribution / selectivity; | n/a |
| With methanesulfonic acid; acetic acid at 40 - 105℃; for 13h; |

-
-
185517-21-9
(R)-(-)-arundic acid

| Conditions | Yield |
|---|---|
| Stage #1: With potassium hydroxide In water at 20℃; for 0.166667h; Stage #2: With hydrogenchloride In n-heptane; Isopropyl acetate; water Product distribution / selectivity; | n/a |
-
-
1333204-09-3
(R)-2-(benzo-1,3-dithiol-2-yl)octan-1-ol

-
-
185517-21-9
(R)-(-)-arundic acid

| Conditions | Yield |
|---|---|
| Multi-step reaction with 4 steps 1.1: sodium hydride / tetrahydrofuran; mineral oil / 0 °C / Inert atmosphere 1.2: 18 h / 20 °C / Inert atmosphere 2.1: n-butyllithium / tetrahydrofuran; hexane / 0 °C / Inert atmosphere 2.2: 0.08 h / Inert atmosphere 3.1: hydrogen / ethanol / 3 h / 760.05 Torr / Inert atmosphere 3.2: Inert atmosphere 4.1: sodium chlorite; sodium hypochlorite; 2,2,6,6-Tetramethyl-1-piperidinyloxy free radical / water; acetonitrile / 7 h / 35 °C / pH 6.7 / Inert atmosphere; aq. phosphate buffer 4.2: 0 °C / pH 8 / Inert atmosphere 4.3: pH 2 / Inert atmosphere View Scheme |
-
-
1333204-10-6
2-((R)-1-(benzyloxy)octan-2-yl)benzo-1,3-dithiole

-
-
185517-21-9
(R)-(-)-arundic acid

| Conditions | Yield |
|---|---|
| Multi-step reaction with 3 steps 1.1: n-butyllithium / tetrahydrofuran; hexane / 0 °C / Inert atmosphere 1.2: 0.08 h / Inert atmosphere 2.1: hydrogen / ethanol / 3 h / 760.05 Torr / Inert atmosphere 2.2: Inert atmosphere 3.1: sodium chlorite; sodium hypochlorite; 2,2,6,6-Tetramethyl-1-piperidinyloxy free radical / water; acetonitrile / 7 h / 35 °C / pH 6.7 / Inert atmosphere; aq. phosphate buffer 3.2: 0 °C / pH 8 / Inert atmosphere 3.3: pH 2 / Inert atmosphere View Scheme |
-
-
1333204-11-7
2-((R)-1-(benzyloxy)octan-2-yl)-2-ethylbenzo-1,3-dithiole

-
-
185517-21-9
(R)-(-)-arundic acid

| Conditions | Yield |
|---|---|
| Multi-step reaction with 2 steps 1.1: hydrogen / ethanol / 3 h / 760.05 Torr / Inert atmosphere 1.2: Inert atmosphere 2.1: sodium chlorite; sodium hypochlorite; 2,2,6,6-Tetramethyl-1-piperidinyloxy free radical / water; acetonitrile / 7 h / 35 °C / pH 6.7 / Inert atmosphere; aq. phosphate buffer 2.2: 0 °C / pH 8 / Inert atmosphere 2.3: pH 2 / Inert atmosphere View Scheme |

-
-
185517-21-9
(R)-(-)-arundic acid

| Conditions | Yield |
|---|---|
| Multi-step reaction with 5 steps 1.1: sodium tetrahydroborate / methanol / 0.5 h / 0 °C / Inert atmosphere 2.1: sodium hydride / tetrahydrofuran; mineral oil / 0 °C / Inert atmosphere 2.2: 18 h / 20 °C / Inert atmosphere 3.1: n-butyllithium / tetrahydrofuran; hexane / 0 °C / Inert atmosphere 3.2: 0.08 h / Inert atmosphere 4.1: hydrogen / ethanol / 3 h / 760.05 Torr / Inert atmosphere 4.2: Inert atmosphere 5.1: sodium chlorite; sodium hypochlorite; 2,2,6,6-Tetramethyl-1-piperidinyloxy free radical / water; acetonitrile / 7 h / 35 °C / pH 6.7 / Inert atmosphere; aq. phosphate buffer 5.2: 0 °C / pH 8 / Inert atmosphere 5.3: pH 2 / Inert atmosphere View Scheme |
-
-
124-13-0
Octanal

-
-
185517-21-9
(R)-(-)-arundic acid

| Conditions | Yield |
|---|---|
| Multi-step reaction with 6 steps 1.1: (R)-5-benzyl-2,2,3-trimethylimidazolidin-4-one; sodium dihydrogenphosphate; benzoic acid / water; acetonitrile / 24 h / 0 °C / Inert atmosphere 2.1: sodium tetrahydroborate / methanol / 0.5 h / 0 °C / Inert atmosphere 3.1: sodium hydride / tetrahydrofuran; mineral oil / 0 °C / Inert atmosphere 3.2: 18 h / 20 °C / Inert atmosphere 4.1: n-butyllithium / tetrahydrofuran; hexane / 0 °C / Inert atmosphere 4.2: 0.08 h / Inert atmosphere 5.1: hydrogen / ethanol / 3 h / 760.05 Torr / Inert atmosphere 5.2: Inert atmosphere 6.1: sodium chlorite; sodium hypochlorite; 2,2,6,6-Tetramethyl-1-piperidinyloxy free radical / water; acetonitrile / 7 h / 35 °C / pH 6.7 / Inert atmosphere; aq. phosphate buffer 6.2: 0 °C / pH 8 / Inert atmosphere 6.3: pH 2 / Inert atmosphere View Scheme |
-
-
1192553-20-0
(2S,3S,4S)-1-benzyloxy-4-[(E)-1-propenyl]-(Z)-dec-6-ene-2,3-diol

-
-
185517-21-9
(R)-(-)-arundic acid

| Conditions | Yield |
|---|---|
| Multi-step reaction with 3 steps 1: sodium periodate; sodium hydrogencarbonate / dichloromethane; water / 6 h / 20 °C 2: sodium chlorite; sodium dihydrogen phosphate monohydrate; cyclohexene / water; tert-butyl alcohol / 12 h / 20 °C 3: palladium 10% on activated carbon; hydrogen / methanol / 2 h / 20 °C / 3040.2 Torr View Scheme |

-
-
185517-21-9
(R)-(-)-arundic acid

| Conditions | Yield |
|---|---|
| Multi-step reaction with 2 steps 1: sodium chlorite; sodium dihydrogen phosphate monohydrate; cyclohexene / water; tert-butyl alcohol / 12 h / 20 °C 2: palladium 10% on activated carbon; hydrogen / methanol / 2 h / 20 °C / 3040.2 Torr View Scheme |

-
-
185517-21-9
(R)-(-)-arundic acid

| Conditions | Yield |
|---|---|
| With dihydrogen peroxide; lithium hydroxide In tetrahydrofuran; water at 0 - 20℃; | 1.65 g |

| Conditions | Yield |
|---|---|
| In 1,2-dimethoxyethane; water at 60℃; for 0.166667 - 0.416667h; Product distribution / selectivity; | 100% |
| In isopropyl alcohol; acetonitrile at 60℃; for 0.166667 - 0.416667h; Product distribution / selectivity; | 85% |
| In water; acetonitrile at 60℃; for 0.166667 - 0.416667h; Product distribution / selectivity; | 79% |
-
-
3886-69-9
(R)-1-phenyl-ethyl-amine

-
-
185517-21-9
(R)-(-)-arundic acid

| Conditions | Yield |
|---|---|
| In acetonitrile at 70℃; for 0.25h; | 40% |
-
-
185517-21-9
(R)-(-)-arundic acid

-
-
848899-76-3
(2R)-2-propyloctanoic acid, sodium salt

| Conditions | Yield |
|---|---|
| With sodium hydroxide In ethanol; water at 20℃; for 2h; | |
| With sodium hydroxide In water | |
| Stage #1: (R)-(-)-arundic acid With sodium hydroxide In tetrahydrofuran; water at 25 - 50℃; for 24h; Stage #2: With calcium chloride In tetrahydrofuran; water at 50℃; for 2h; Solvent; Temperature; |
-
-
70-11-1
α-bromoacetophenone

-
-
185517-21-9
(R)-(-)-arundic acid

| Conditions | Yield |
|---|---|
| With triethylamine In dichloromethane at 20℃; for 5h; | n/a |
| With triethylamine In dichloromethane | |
| With potassium carbonate In acetone at 20℃; for 1h; |
-
-
185517-21-9
(R)-(-)-arundic acid

-
-
888038-78-6
lithium (2R)-2-propyloctanoate

| Conditions | Yield |
|---|---|
| With lithium hydroxide In ethanol; water for 0.5h; |
-
-
885020-48-4
(2Z)-4-{[tert-butyl(dimethyl)silyl]oxy}-3-(3,4-difluorophenyl)-2-[4-(methylsulfonyl)phenyl]but-2-en-1-ol

-
-
185517-21-9
(R)-(-)-arundic acid

-
-
888010-86-4
(2Z)-4-{[tert-butyl(dimethyl)silyl]oxy}-3-(3,4-difluorophenyl)-2-[4-(methylsulfonyl)phenyl]but-2-en-1-yl (2R)-2-propyloctanoate

| Conditions | Yield |
|---|---|
| With dmap; dicyclohexyl-carbodiimide In dichloromethane at 20℃; for 3h; |
-
-
2627-86-3
(S)-1-phenyl-ethylamine

-
-
185517-21-9
(R)-(-)-arundic acid

| Conditions | Yield |
|---|---|
| Stage #1: (R)-(-)-arundic acid With oxalyl dichloride In dichloromethane at 0℃; for 1h; Stage #2: (S)-1-phenyl-ethylamine With triethylamine In dichloromethane at 0℃; for 1h; Further stages.; |
(R)-(-)-2-Propyloctanoic acid Chemical Properties
Molecule structure of Arundic acid (CAS NO.185517-21-9):
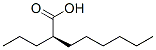
IUPAC Name: (2R)-2-Propyloctanoic acid
Molecular Weight: 186.29118 g/mol
Molecular Formula: C11H22O2
Density: 0.908 g/cm3
Boiling Point: 289.3 °C at 760 mmHg
Flash Point: 154.2 °C
Index of Refraction: 1.444
Molar Refractivity: 54.53 cm3
Molar Volume: 205.1 cm3
Surface Tension: 32.2 dyne/cm
Enthalpy of Vaporization: 58.16 kJ/mol
Vapour Pressure: 0.000557 mmHg at 25 °C
XLogP3: 3.7
H-Bond Donor: 1
H-Bond Acceptor: 2
Rotatable Bond Count: 8
Exact Mass: 186.16198
MonoIsotopic Mass: 186.16198
Topological Polar Surface Area: 37.3
Heavy Atom Count: 13
Canonical SMILES: CCCCCCC(CCC)C(=O)O
Isomeric SMILES: CCCCCC[C@@H](CCC)C(=O)O
InChI: InChI=1S/C11H22O2/c1-3-5-6-7-9-10(8-4-2)11(12)13/h10H,3-9H2,1-2H3,(H,12,13)/t10-/m1/s1
InChIKey: YCYMCMYLORLIJX-SNVBAGLBSA-N
Classification Code of Arundic acid (CAS NO.185517-21-9): Neuroprotective agent
(R)-(-)-2-Propyloctanoic acid Specification
Arundic acid (CAS NO.185517-21-9) is also named as (2R)-2-Propyloctanoic acid ; Acide arundique ; Acide arundique [INN-French] ; Acido arzndico ; Acido arúndico [INN-Spanish] ; Acidum arundicum ; Acidum arundicum [INN-Latin] ; Cereact ; Proglia ; UNII-F2628ZD0FO .
Related Products
- (R)-(-)-2-Propyloctanoic acid
- 185525-49-9
- 185527-11-1
- 18552-90-4
- 18553-11-2
- 185545-90-8
- 18556-27-9
- 1855-63-6
- 18557-22-7
- 18559-59-6
- 18559-94-9
Hot Products
About|Contact|Cas|Product Name|Molecular|Country|Encyclopedia
Message|New Cas|MSDS|Service|Advertisement|CAS DataBase|Article Data|Manufacturers | Chemical Catalog
©2008 LookChem.com,License: ICP
NO.:Zhejiang16009103
complaints:service@lookchem.com Desktop View