-
Name
Z-PYR-OH
- EINECS 250-936-0
- CAS No. 32159-21-0
- Article Data9
- CAS DataBase
- Density 1.408 g/cm3
- Solubility
- Melting Point 128-130 °C
- Formula C13H13NO5
- Boiling Point 525.4 °C at 760 mmHg
- Molecular Weight 263.25
- Flash Point 271.5 °C
- Transport Information
- Appearance
- Safety 22-24/25
- Risk Codes 22/22-36/37/38
-
Molecular Structure
- Hazard Symbols
- Synonyms 1,2-Pyrrolidinedicarboxylicacid, 5-oxo-, 1-(phenylmethyl) ester, (S)-;1,2-Pyrrolidinedicarboxylic acid,5-oxo-, 1-benzyl ester, L- (8CI);(2S)-5-Oxopyrrolidine-1,2-dicarboxylic acid1-benzyl ester;(Benzyloxycarbonyl)-L-pyroglutamic acid;Carbobenzoxy-L-pyroglutamic acid;L-(Phenylmethoxycarbonyl)pyroglutamic acid;N-Benzyloxycarbonyl-L-pyroglutamic acid;N-Benzyloxycarbonylpyroglutamic acid;N-Carbobenzoxypyroglutamic acid;Z-Pyr-OH;
- PSA 83.91000
- LogP 1.33670
Synthetic route
-
-
497-19-8
sodium carbonate

-
-
501-53-1
benzyl chloroformate

-
-
98-79-3
L-Pyroglutamic acid

-
-
32159-21-0
Cbz-L-pGlu-OH

| Conditions | Yield |
|---|---|
| In 1,4-dioxane; sodium hydroxide | 87% |


| Conditions | Yield |
|---|---|
| With triethylamine In acetonitrile for 1h; Ambient temperature; | 20% |
| With 2,2,2-trifluoro-N,N-bis(trimethylsilyl)-acetamide; acetonitrile; methyloxirane 1.) r.t., 15 min, 2.) 1 h; Yield given. Multistep reaction; |
-
-
1155-62-0
N-benzyloxycarbonyl-L-glutamic acid

-
-
32159-21-0
Cbz-L-pGlu-OH

| Conditions | Yield |
|---|---|
| With N-cyclohexyl-cyclohexanamine; dicyclohexyl-carbodiimide 1.) THF, 0 deg C; Multistep reaction; | |
| Yield given. Multistep reaction; | |
| Multi-step reaction with 2 steps 1: DCC / tetrahydrofuran / 0 °C 2: dicyclohexylamine (DCHA) / tetrahydrofuran / 0 °C View Scheme |
-
-
4124-76-9
N-(benzyloxycarbonyl)-L-glutamic acid anhydride

-
-
32159-21-0
Cbz-L-pGlu-OH

| Conditions | Yield |
|---|---|
| With N-cyclohexyl-cyclohexanamine In tetrahydrofuran at 0℃; Yield given; |
-
-
1003-09-4
2-bromothiophene

-
-
4124-76-9
N-(benzyloxycarbonyl)-L-glutamic acid anhydride

-
B
-
1155-62-0
N-benzyloxycarbonyl-L-glutamic acid

-
C
-
32159-21-0
Cbz-L-pGlu-OH

| Conditions | Yield |
|---|---|
| Stage #1: 2-bromothiophene With magnesium In tetrahydrofuran at 40℃; for 2h; Stage #2: N-(benzyloxycarbonyl)-L-glutamic acid anhydride In tetrahydrofuran at -78℃; for 4h; Stage #3: With citric acid In tetrahydrofuran at -78 - 20℃; Title compound not separated from byproducts; |

-
-
109-65-9
1-bromo-butane

-
-
4124-76-9
N-(benzyloxycarbonyl)-L-glutamic acid anhydride

-
B
-
1155-62-0
N-benzyloxycarbonyl-L-glutamic acid

-
C
-
32159-21-0
Cbz-L-pGlu-OH

| Conditions | Yield |
|---|---|
| Stage #1: 1-bromo-butane With magnesium In tetrahydrofuran at 40℃; for 2h; Stage #2: N-(benzyloxycarbonyl)-L-glutamic acid anhydride In tetrahydrofuran at -78℃; for 4h; Stage #3: With citric acid In tetrahydrofuran at -78 - 20℃; Title compound not separated from byproducts; |

-
-
2398-37-0
3-methoxyphenyl bromide

-
-
4124-76-9
N-(benzyloxycarbonyl)-L-glutamic acid anhydride

-
B
-
1155-62-0
N-benzyloxycarbonyl-L-glutamic acid

-
C
-
32159-21-0
Cbz-L-pGlu-OH

| Conditions | Yield |
|---|---|
| Stage #1: 3-methoxyphenyl bromide With magnesium In tetrahydrofuran at 40℃; for 2h; Stage #2: N-(benzyloxycarbonyl)-L-glutamic acid anhydride In tetrahydrofuran at -78℃; for 4h; Stage #3: With citric acid In tetrahydrofuran at -78 - 20℃; Title compound not separated from byproducts; |

-
-
111-25-1
1-bromo-hexane

-
-
4124-76-9
N-(benzyloxycarbonyl)-L-glutamic acid anhydride

-
B
-
1155-62-0
N-benzyloxycarbonyl-L-glutamic acid

-
C
-
32159-21-0
Cbz-L-pGlu-OH

| Conditions | Yield |
|---|---|
| Stage #1: 1-bromo-hexane With magnesium In tetrahydrofuran at 40℃; for 2h; Stage #2: N-(benzyloxycarbonyl)-L-glutamic acid anhydride In tetrahydrofuran at -78℃; for 4h; Stage #3: With citric acid In tetrahydrofuran at -78 - 20℃; Title compound not separated from byproducts; |

-
-
100-59-4
phenylmagnesium chloride

-
-
4124-76-9
N-(benzyloxycarbonyl)-L-glutamic acid anhydride

-
B
-
1155-62-0
N-benzyloxycarbonyl-L-glutamic acid

-
C
-
32159-21-0
Cbz-L-pGlu-OH

| Conditions | Yield |
|---|---|
| Stage #1: phenylmagnesium chloride; N-(benzyloxycarbonyl)-L-glutamic acid anhydride In tetrahydrofuran at -78℃; for 4h; Stage #2: With citric acid In tetrahydrofuran |

-
-
591-51-5
phenyllithium

-
-
4124-76-9
N-(benzyloxycarbonyl)-L-glutamic acid anhydride

-
C
-
1155-62-0
N-benzyloxycarbonyl-L-glutamic acid

-
D
-
32159-21-0
Cbz-L-pGlu-OH

| Conditions | Yield |
|---|---|
| Stage #1: phenyllithium; N-(benzyloxycarbonyl)-L-glutamic acid anhydride In tetrahydrofuran at -78℃; for 4h; Stage #2: With citric acid In tetrahydrofuran |

-
-
4124-76-9
N-(benzyloxycarbonyl)-L-glutamic acid anhydride

-
A
-
1155-62-0
N-benzyloxycarbonyl-L-glutamic acid

-
B
-
32159-21-0
Cbz-L-pGlu-OH

| Conditions | Yield |
|---|---|
| Stage #1: N-(benzyloxycarbonyl)-L-glutamic acid anhydride With tert-butylmagnesium chloride In tetrahydrofuran at -78℃; for 4h; Stage #2: With citric acid In tetrahydrofuran | |
| Stage #1: N-(benzyloxycarbonyl)-L-glutamic acid anhydride With phenylzinc chloride In tetrahydrofuran at -78℃; for 2h; Stage #2: With citric acid In tetrahydrofuran |
-
-
4124-76-9
N-(benzyloxycarbonyl)-L-glutamic acid anhydride

-
C
-
1155-62-0
N-benzyloxycarbonyl-L-glutamic acid

-
D
-
32159-21-0
Cbz-L-pGlu-OH

| Conditions | Yield |
|---|---|
| Stage #1: N-(benzyloxycarbonyl)-L-glutamic acid anhydride; Ph3MgLi In tetrahydrofuran at -78 - 20℃; Stage #2: With citric acid In tetrahydrofuran |

-
-
32159-21-0
Cbz-L-pGlu-OH

-
-
74-88-4
methyl iodide

-
-
75857-94-2
(5S)-N-(benzyloxycarbonyl)-5-methoxycarbonyl-2-pyrrolidinone

| Conditions | Yield |
|---|---|
| With N-ethyl-N,N-diisopropylamine In dichloromethane | 98% |
| With N-ethyl-N,N-diisopropylamine In dichloromethane for 6.5h; Heating / reflux; | 98% |
-
-
3398-70-7
(2R,4'R,8'R)-α-tocopheramine

-
-
32159-21-0
Cbz-L-pGlu-OH

-
B
-
3651-67-0
N-methylmorpholine hydrochloride

| Conditions | Yield |
|---|---|
| Stage #1: Cbz-L-pGlu-OH With 4-methyl-morpholine; isobutyl chloroformate In tetrahydrofuran at 0 - 20℃; for 2h; Stage #2: (2R,4'R,8'R)-α-tocopheramine With triethylamine In tetrahydrofuran at 0℃; | A 98% B n/a |


-
-
32159-21-0
Cbz-L-pGlu-OH

-
B
-
3651-67-0
N-methylmorpholine hydrochloride

| Conditions | Yield |
|---|---|
| Stage #1: Cbz-L-pGlu-OH With 4-methyl-morpholine; isobutyl chloroformate In tetrahydrofuran at 0 - 20℃; for 2h; Stage #2: VESA-PEG-NH2 With triethylamine In tetrahydrofuran; water at 0℃; | A 98% B n/a |

-
-
61-54-1
tryptamine

-
-
32159-21-0
Cbz-L-pGlu-OH

| Conditions | Yield |
|---|---|
| In tetrahydrofuran Heating; | 90% |
-
-
32159-21-0
Cbz-L-pGlu-OH

-
-
100-44-7
benzyl chloride

-
-
71389-33-8
dibenzyl (2S)-5-oxotetrahydro-1H-pyrrole-1,2-dicarboxylate

| Conditions | Yield |
|---|---|
| With triethylamine In acetone for 72h; Heating; | 85% |
-
-
32159-21-0
Cbz-L-pGlu-OH

| Conditions | Yield |
|---|---|
| With pyridine; di-tert-butyl dicarbonate; ammonium bicarbonate In 1,4-dioxane 4-16 h; | 84% |
-
-
95-14-7
1,2,3-Benzotriazole

-
-
32159-21-0
Cbz-L-pGlu-OH

-
-
958637-27-9
benzyl (2S)-2-(1H-1,2,3-benzotriazol-1-ylcarbonyl)-5-oxotetrahydro-1H-pyrrole-1-carboxylate

| Conditions | Yield |
|---|---|
| Stage #1: 1,2,3-Benzotriazole With thionyl chloride In dichloromethane at 40 - 50℃; for 0.333333h; Stage #2: Cbz-L-pGlu-OH In dichloromethane at 20℃; for 2h; Further stages.; | 84% |
-
-
32159-21-0
Cbz-L-pGlu-OH

-
-
81470-51-1
tert-butyl N-benzyloxycarbonyl-L-pyroglutamate

| Conditions | Yield |
|---|---|
| With perchloric acid In water at 20℃; for 16h; | 79% |
-
-
32159-21-0
Cbz-L-pGlu-OH

-
-
696-44-6
N-methyl-m-toluidine

| Conditions | Yield |
|---|---|
| With pyridine; 2,4,6-tripropyl-1,3,5,2,4,6-trioxatriphosphinane-2,4,6-trioxide In ethyl acetate at 20℃; for 12h; | 75% |

| Conditions | Yield |
|---|---|
| Stage #1: Cbz-L-pGlu-OH With chloroformic acid ethyl ester; triethylamine In tetrahydrofuran at 0 - 20℃; for 1.5h; Stage #2: 8-amino quinoline In tetrahydrofuran at 20℃; for 6h; | 74% |
-
-
32159-21-0
Cbz-L-pGlu-OH

-
-
104-94-9
4-methoxy-aniline

-
-
339994-37-5
(S)-2-(4-Methoxy-phenylcarbamoyl)-5-oxo-pyrrolidine-1-carboxylic acid benzyl ester

| Conditions | Yield |
|---|---|
| With 4-methyl-morpholine; chloroformic acid ethyl ester In ethyl acetate at -15 - 20℃; for 50h; | 68% |

| Conditions | Yield |
|---|---|
| With chloroformic acid ethyl ester; triethylamine In tetrahydrofuran | 67% |
-
-
32159-21-0
Cbz-L-pGlu-OH

-
-
115-11-7
isobutene

-
-
81470-51-1
tert-butyl N-benzyloxycarbonyl-L-pyroglutamate

| Conditions | Yield |
|---|---|
| With sulfuric acid In dichloromethane | 67% |
| Stage #1: Cbz-L-pGlu-OH; isobutene With sulfuric acid In dichloromethane at 20℃; Cooling with ice; Stage #2: With sodium carbonate In dichloromethane; water | 67% |
| With sulfuric acid In dichloromethane at 20℃; Cooling with ice; | 67% |
| With sulfuric acid In dichloromethane at 20℃; Cooling with ice; | 67% |
-
-
32159-21-0
Cbz-L-pGlu-OH

-
-
2791-84-6
L-glutamic acid dibenzyl ester 4-toluenesulfonate

-
-
122389-55-3
Z-pGlu-Glu(OBzl)-OBzl

| Conditions | Yield |
|---|---|
| With benzotriazol-1-yloxyl-tris-(pyrrolidino)-phosphonium hexafluorophosphate; N-ethyl-N,N-diisopropylamine In dichloromethane | 63% |
-
-
32159-21-0
Cbz-L-pGlu-OH

-
-
153870-19-0
benzyl (2S)-2-(hydroxymethyl)-5-oxopyrrolidine-1-carboxylate

| Conditions | Yield |
|---|---|
| Stage #1: Cbz-L-pGlu-OH With 4-methyl-morpholine; chloroformic acid ethyl ester In tetrahydrofuran at -10℃; Stage #2: With sodium tetrahydroborate In methanol at 0℃; | 61% |
| Multi-step reaction with 2 steps 1: N-methyl morpholine / tetrahydrofuran / 0.08 h / -5 °C 2: NaBH4 / tetrahydrofuran View Scheme |
-
-
95384-14-8
L-histidine methyl ester

-
-
32159-21-0
Cbz-L-pGlu-OH

| Conditions | Yield |
|---|---|
| With dicyclohexyl-carbodiimide In N,N-dimethyl-formamide; acetonitrile 1h at -10 deg C, then 12h at 4 deg C; | 55% |
-
-
32159-21-0
Cbz-L-pGlu-OH

-
-
100-39-0
benzyl bromide

-
-
71389-33-8
dibenzyl (2S)-5-oxotetrahydro-1H-pyrrole-1,2-dicarboxylate

| Conditions | Yield |
|---|---|
| With potassium hydrogencarbonate In N,N-dimethyl-formamide for 18h; Ambient temperature; | 51% |
-
-
32159-21-0
Cbz-L-pGlu-OH

-
-
937-14-4
3-chloro-benzenecarboperoxoic acid

-
-
130654-55-6
(R)-2-(3-Chloro-benzoyloxy)-5-oxo-pyrrolidine-1-carboxylic acid benzyl ester

| Conditions | Yield |
|---|---|
| With dicyclohexyl-carbodiimide In chloroform at 25℃; for 1h; | 42% |

| Conditions | Yield |
|---|---|
| With chloroformic acid ethyl ester; triethylamine In tetrahydrofuran | 40% |
-
-
4530-20-5
BOC-glycine

-
-
15761-38-3
L-N-Boc-Ala

-
-
15260-10-3
N-Boc-O-benzyl-L-threonine

-
-
13836-37-8
Boc-Arg(Tos)-OH

-
-
23680-31-1
BOC-O-benzyl-L-serine

-
-
32159-21-0
Cbz-L-pGlu-OH

-
-
73821-95-1
Boc-Asp(O-cyclohexyl)-OH

-
-
61925-77-7
Boc-cysteine(4-Me-Bn)

-
-
54613-99-9
Boc-(S)-Lys(2-Cl-Z)-OH

-
-
47355-10-2
Nα-tert-butoxycarbonyl-1-formyl-L-tryptophan

-
-
83468-83-1
N(α)-t-butoxycarbonyl-N(ϖ)-benzyloxymethyl-L-histidine

| Conditions | Yield |
|---|---|
| Stage #1: Boc-cysteine(4-Me-Bn) With benzotriazol-1-ol In 1-methyl-pyrrolidin-2-one Automated synthesizer; Stage #2: With trifluoroacetic acid Stage #3: BOC-glycine; L-N-Boc-Ala; N-Boc-O-benzyl-L-threonine; Boc-Arg(Tos)-OH; BOC-O-benzyl-L-serine; Cbz-L-pGlu-OH; Boc-Asp(O-cyclohexyl)-OH; Boc-cysteine(4-Me-Bn); Boc-(S)-Lys(2-Cl-Z)-OH; Nα-tert-butoxycarbonyl-1-formyl-L-tryptophan; N(α)-t-butoxycarbonyl-N(ϖ)-benzyloxymethyl-L-histidine | 22% |

-
-
32159-21-0
Cbz-L-pGlu-OH

-
-
4510-09-2
N-benzyloxycarbonyl-L-glutamic acid-5-hydrazide

| Conditions | Yield |
|---|---|
| With hydrazine hydrate |
-
-
540-88-5
acetic acid tert-butyl ester

-
-
32159-21-0
Cbz-L-pGlu-OH

-
-
81470-51-1
tert-butyl N-benzyloxycarbonyl-L-pyroglutamate

| Conditions | Yield |
|---|---|
| With perchloric acid Yield given. Multistep reaction; | |
| With perchloric acid Ambient temperature; Yield given; |
-
-
54788-35-1
diethyl 1-aminoethylphosphonate

-
-
32159-21-0
Cbz-L-pGlu-OH

| Conditions | Yield |
|---|---|
| With dicyclohexyl-carbodiimide In dichloromethane at 0 - 20℃; for 6.5h; |
-
-
32159-21-0
Cbz-L-pGlu-OH

-
-
2899-28-7, 6519-67-1, 61535-49-7, 67557-18-0, 68401-14-9
L-tryptophan ethyl ester hydrochloride

-
-
87694-59-5
N-carbobenzoxy-L-pyroglutamyl-L-tryptophan ethyl ester

| Conditions | Yield |
|---|---|
| With chloroformic acid ethyl ester; triethylamine 1.) THF, 0 deg C, 30 min, 2.) r.t., 3 h; Yield given. Multistep reaction; |
-
-
32159-21-0
Cbz-L-pGlu-OH

-
-
84983-67-5
tert.-butyloxycarbonylalanyl-Nε-benzyloxycarbonyllysyl-serine methyl ester

-
-
84983-68-6
Z-Pyr-Ala-Lys(Z)-Ser-OMe

| Conditions | Yield |
|---|---|
| With 4-methyl-morpholine; benzotriazol-1-ol; methoxybenzene; trifluoroacetic acid 1.) room temperature, 20 min 2.) DMF, 0 deg C, overnight, 4 deg C; Yield given. Multistep reaction; |

| Conditions | Yield |
|---|---|
| With 4-methyl-morpholine; triethylamine; isobutyl chloroformate 1.) THF, -20 deg C, 10 min, 2.) THF, DMF, H2O, RT, 1 h; Yield given. Multistep reaction; |
(S)-1-(Benzyloxycarbonyl)-5-oxopyrrolidine-2-carboxylic acid Chemical Properties
IUPAC Name: 5-Oxo-1-phenylmethoxycarbonylpyrrolidine-2-carboxylic acid
Synonyms of N(alpha)-Carbobenzoxypyroglutamic acid (CAS NO.32159-21-0): 1-[(Benzyloxy)carbonyl]-5-oxoproline ; 1,2-Pyrrolidinedicarboxylic acid,5-oxo-,1-(phenylmethyl) ester,(S)- ; 1-Benzyl hydrogen (S)-5-oxopyrrolidine-1,2-dicarboxylate ; (S)-1-(Benzyloxycarbonyl)-5-oxopyrrolidine-2-carboxylic acid
CAS NO: 32159-21-0
Molecular Formula: C13H13NO5
Molecular Weight: 263.246
Molecular Structure: 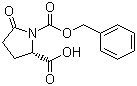
EINECS: 250-936-0
H bond acceptors: 6
H bond donors: 1
Freely Rotating Bonds: 4
Polar Surface Area: 72.91 Å2
Index of Refraction: 1.597
Molar Refractivity: 63.68 cm3
Molar Volume: 186.8 cm3
Surface Tension: 65.6 dyne/cm
Density: 1.408 g/cm3
Flash Point: 271.5 °C
Enthalpy of Vaporization: 84.16 kJ/mol
Boiling Point: 525.4 °C at 760 mmHg
Vapour Pressure: 7.26E-12 mmHg at 25°C
Melting Point: 128-130°C
Storage temp: -20°C
SMILES: O=C2N(C(=O)OCc1ccccc1)C(C(=O)O)CC2
InChI: InChI=1/C13H13NO5/c15-11-7-6-10(12(16)17)14(11)13(18)19-8-9-4-2-1-3-5-9/h1-5,10H,6-8H2,(H,16,17)
InChIKey: VHSFUGXCSGOKJX-UHFFFAOYAB
Std. InChI: InChI=1S/C13H13NO5/c15-11-7-6-10(12(16)17)14(11)13(18)19-8-9-4-2-1-3-5-9/h1-5,10H,6-8H2,(H,16,17)
Std. InChIKey: VHSFUGXCSGOKJX-UHFFFAOYSA-N
Product Categories of N(alpha)-Carbobenzoxypyroglutamic acid (CAS NO.32159-21-0): pharmacetical;Pyroglutamic acid [Pyr, pGu];Unusual Amino Acids;Z-Amino acid series;Amino Acids;I - Z;Modified Amino Acids
(S)-1-(Benzyloxycarbonyl)-5-oxopyrrolidine-2-carboxylic acid Safety Profile
Safety Statements: 22-24/25
S22: Do not breathe dust.
S24/25: When N(alpha)-Carbobenzoxypyroglutamic acid (CAS NO.32159-21-0) is used,avoid contact with skin and eyes.
WGK Germany: 3
Related Products
- (S)-1-(Benzyloxycarbonyl)-5-oxopyrrolidine-2-carboxylic acid
- 32159-22-1
- 321596-51-4
- 321-60-8
- 32161-06-1
- 32161-30-1
- 321-64-2
- 3216-48-6
- 321674-73-1
- 321688-88-4
- 321-69-7
Hot Products
About|Contact|Cas|Product Name|Molecular|Country|Encyclopedia
Message|New Cas|MSDS|Service|Advertisement|CAS DataBase|Article Data|Manufacturers | Chemical Catalog
©2008 LookChem.com,License: ICP
NO.:Zhejiang16009103
complaints:service@lookchem.com Desktop View