-
Name
(S)-4-(4-Aminobenzyl)-2(1H)-oxazolidinone
- EINECS
- CAS No. 152305-23-2
- Article Data6
- CAS DataBase
- Density 1.257 g/cm3
- Solubility
- Melting Point 107-111 °C
- Formula C10H12N2O2
- Boiling Point 479.7 °C at 760 mmHg
- Molecular Weight 192.217
- Flash Point 243.9 °C
- Transport Information
- Appearance white crystalline powder
- Safety 26-36/37
- Risk Codes 22-36/37/38
-
Molecular Structure
-
Hazard Symbols
 Xn
Xn
- Synonyms 2-Oxazolidinone,4-[(4-aminophenyl)methyl]-, (S)-;(4S)-4-(4-Aminobenzyl)-1,3-oxazolidin-2-one;(S)-4-(4-Aminobenzyl)-2-oxazolidinone;
- PSA 64.35000
- LogP 1.82970
Synthetic route
-
-
139264-55-4
(S)-4-(4'-nitrobenzyl)-1,3-oxazolidin-2-one

-
-
152305-23-2
(S)-4-(4-aminobenzyl)-1,3-oxazolidin-2-one

| Conditions | Yield |
|---|---|
| With hydrogenchloride; hydrogen; palladium on activated charcoal In ethanol under 760 Torr; Ambient temperature; | 79% |
| With indium; ammonium chloride In ethanol for 4h; Heating; | 47% |
| With hydrogen; nickel In methanol at 30℃; for 6 - 7h; |
-
-
726134-79-8
(S)-2-amino-3-(4-aminophenyl)propan-1-ol

-
-
105-58-8
Diethyl carbonate

-
-
152305-23-2
(S)-4-(4-aminobenzyl)-1,3-oxazolidin-2-one

| Conditions | Yield |
|---|---|
| potassium carbonate at 115 - 135℃; for 2.5h; | 75% |
-
-
90719-32-7
(S)-4-Benzyl-2-oxazolidinone

-
-
152305-23-2
(S)-4-(4-aminobenzyl)-1,3-oxazolidin-2-one

| Conditions | Yield |
|---|---|
| Multi-step reaction with 2 steps 1: 65 percent / fuming HNO3 / CH2Cl2 / 2 h / 20 °C 2: 47 percent / NH4Cl; In / ethanol / 4 h / Heating View Scheme |

| Conditions | Yield |
|---|---|
| Multi-step reaction with 5 steps 1: 53 percent / conc. H2SO4, conc. HNO3 / 0.5 h / Ambient temperature 2: 76 percent / SOCl2 / 4 h / -10 - 40 °C 3: 65 percent / NaBH4 / ethanol; H2O / 4 h / Heating 4: 75 percent / 0.2M aq. KOH / toluene / 2 h / 0 - 20 °C 5: 79 percent / H2, aq. HCl / 10percent Pd/C / ethanol / 760 Torr / Ambient temperature View Scheme |
-
-
949-99-5
4-nitro-3-phenyl-L-alanine

-
-
152305-23-2
(S)-4-(4-aminobenzyl)-1,3-oxazolidin-2-one

| Conditions | Yield |
|---|---|
| Multi-step reaction with 4 steps 1: 76 percent / SOCl2 / 4 h / -10 - 40 °C 2: 65 percent / NaBH4 / ethanol; H2O / 4 h / Heating 3: 75 percent / 0.2M aq. KOH / toluene / 2 h / 0 - 20 °C 4: 79 percent / H2, aq. HCl / 10percent Pd/C / ethanol / 760 Torr / Ambient temperature View Scheme |
-
-
89288-22-2
(S)-(-)-2-amino-3-(4-nitrophenyl)propan-1-ol

-
-
152305-23-2
(S)-4-(4-aminobenzyl)-1,3-oxazolidin-2-one

| Conditions | Yield |
|---|---|
| Multi-step reaction with 2 steps 1: 75 percent / 0.2M aq. KOH / toluene / 2 h / 0 - 20 °C 2: 79 percent / H2, aq. HCl / 10percent Pd/C / ethanol / 760 Torr / Ambient temperature View Scheme |
-
-
17193-40-7
(S)-4-nitrophenylalanine methyl ester hydrochloride

-
-
152305-23-2
(S)-4-(4-aminobenzyl)-1,3-oxazolidin-2-one

| Conditions | Yield |
|---|---|
| Multi-step reaction with 3 steps 1: 65 percent / NaBH4 / ethanol; H2O / 4 h / Heating 2: 75 percent / 0.2M aq. KOH / toluene / 2 h / 0 - 20 °C 3: 79 percent / H2, aq. HCl / 10percent Pd/C / ethanol / 760 Torr / Ambient temperature View Scheme |
-
-
188404-34-4
(S)-N-butoxycarbonyl-4-aminophenylalaninol

-
-
152305-23-2
(S)-4-(4-aminobenzyl)-1,3-oxazolidin-2-one

| Conditions | Yield |
|---|---|
| With sodium methylate In methanol; butan-1-ol at 5 - 85℃; for 8.5h; |
-
-
2059-76-9
4-iodophenyl isothiocyanate

-
-
152305-23-2
(S)-4-(4-aminobenzyl)-1,3-oxazolidin-2-one

| Conditions | Yield |
|---|---|
| With triethylamine In acetone Reflux; | 90% |
-
-
622-59-3
1-isothiocyanato-4-methylbenzene

-
-
152305-23-2
(S)-4-(4-aminobenzyl)-1,3-oxazolidin-2-one

| Conditions | Yield |
|---|---|
| With triethylamine In acetone Reflux; | 88% |
-
-
2131-61-5
p-nitrophenyl isothiocyanate

-
-
152305-23-2
(S)-4-(4-aminobenzyl)-1,3-oxazolidin-2-one

| Conditions | Yield |
|---|---|
| With triethylamine In acetone Reflux; | 82% |
-
-
152305-23-2
(S)-4-(4-aminobenzyl)-1,3-oxazolidin-2-one

-
-
1985-12-2
4-Bromophenyl isothiocyanate

| Conditions | Yield |
|---|---|
| With triethylamine In acetone Reflux; | 80% |
-
-
152305-23-2
(S)-4-(4-aminobenzyl)-1,3-oxazolidin-2-one

-
-
98-58-8
4-bromobenzenesulfonyl chloride

| Conditions | Yield |
|---|---|
| With triethylamine In dichloromethane at 0℃; | 79% |
-
-
55747-45-0
ethyl 2-acetyl-5-(1,3-dioxo-1,3-dihydro-2-isoindolyl)pentanoate

-
-
152305-23-2
(S)-4-(4-aminobenzyl)-1,3-oxazolidin-2-one

-
-
868622-21-3
ethyl (S)-3-[2-(1,3-dioxoisoindolin-2-yl)ethyl]-5-[(2-oxooxazolidin-4-yl)methyl]-1H-indole-2-carboxylate

| Conditions | Yield |
|---|---|
| Stage #1: (S)-4-(4-aminobenzyl)-1,3-oxazolidin-2-one With hydrogenchloride; sodium nitrite In methanol; water at -5 - 0℃; Industrial scale; Stage #2: ethyl 2-acetyl-5-(1,3-dioxo-1,3-dihydro-2-isoindolyl)pentanoate With sodium acetate In methanol; water at 0 - 30℃; Industrial scale; Stage #3: With hydrogenchloride In methanol for 6h; Fischer Indole Synthesis; Reflux; Industrial scale; | 78% |
-
-
100-52-7
benzaldehyde

-
-
152305-23-2
(S)-4-(4-aminobenzyl)-1,3-oxazolidin-2-one

| Conditions | Yield |
|---|---|
| In methanol at 20℃; for 0.0833333h; | 77.5% |
-
-
98-59-9
p-toluenesulfonyl chloride

-
-
152305-23-2
(S)-4-(4-aminobenzyl)-1,3-oxazolidin-2-one

| Conditions | Yield |
|---|---|
| With triethylamine In dichloromethane at 0℃; | 76% |
-
-
122-01-0
4-chloro-benzoyl chloride

-
-
152305-23-2
(S)-4-(4-aminobenzyl)-1,3-oxazolidin-2-one

| Conditions | Yield |
|---|---|
| With triethylamine In dichloromethane at 20℃; | 75% |
-
-
98-09-9
benzenesulfonyl chloride

-
-
152305-23-2
(S)-4-(4-aminobenzyl)-1,3-oxazolidin-2-one

| Conditions | Yield |
|---|---|
| With triethylamine In dichloromethane at 0℃; | 72% |
-
-
122-04-3
4-nitro-benzoyl chloride

-
-
152305-23-2
(S)-4-(4-aminobenzyl)-1,3-oxazolidin-2-one

| Conditions | Yield |
|---|---|
| With triethylamine In dichloromethane at 20℃; | 58% |
-
-
98-68-0
4-methoxy-phenyl-sulphonyl chloride

-
-
152305-23-2
(S)-4-(4-aminobenzyl)-1,3-oxazolidin-2-one

| Conditions | Yield |
|---|---|
| With triethylamine In dichloromethane at 0℃; | 57% |
-
-
100-07-2
4-methoxy-benzoyl chloride

-
-
152305-23-2
(S)-4-(4-aminobenzyl)-1,3-oxazolidin-2-one

| Conditions | Yield |
|---|---|
| With triethylamine In dichloromethane at 20℃; | 54% |
-
-
349-88-2
4-Fluorobenzenesulfonyl chloride

-
-
152305-23-2
(S)-4-(4-aminobenzyl)-1,3-oxazolidin-2-one

| Conditions | Yield |
|---|---|
| With triethylamine In dichloromethane at 0℃; | 53% |
-
-
403-43-0
4-fluorobenzoyl chloride

-
-
152305-23-2
(S)-4-(4-aminobenzyl)-1,3-oxazolidin-2-one

| Conditions | Yield |
|---|---|
| With triethylamine In dichloromethane at 20℃; | 50% |
-
-
586-75-4
4-chlorobenzoyl chloride

-
-
152305-23-2
(S)-4-(4-aminobenzyl)-1,3-oxazolidin-2-one

| Conditions | Yield |
|---|---|
| With triethylamine In dichloromethane at 20℃; | 48% |
-
-
98-88-4
benzoyl chloride

-
-
152305-23-2
(S)-4-(4-aminobenzyl)-1,3-oxazolidin-2-one

| Conditions | Yield |
|---|---|
| With triethylamine In dichloromethane at 20℃; | 47% |
-
-
152305-23-2
(S)-4-(4-aminobenzyl)-1,3-oxazolidin-2-one

-
-
179534-87-3
(S)-4-(3-iodo-4-aminobenzyl)-1,3-oxazolidin-2-one

| Conditions | Yield |
|---|---|
| With Iodine monochloride; calcium carbonate In methanol; ethyl acetate | 45% |
| With Iodine monochloride; calcium carbonate In methanol for 16h; Ambient temperature; |
-
-
1544-68-9
4-fluorophenyl isothiocyanate

-
-
152305-23-2
(S)-4-(4-aminobenzyl)-1,3-oxazolidin-2-one

| Conditions | Yield |
|---|---|
| With triethylamine In acetone Reflux; | 42% |
-
-
103-72-0
phenyl isothiocyanate

-
-
152305-23-2
(S)-4-(4-aminobenzyl)-1,3-oxazolidin-2-one

| Conditions | Yield |
|---|---|
| With triethylamine In acetone Reflux; | 33% |
-
-
98-60-2
4-chlorobenzenesulfonyl chloride

-
-
152305-23-2
(S)-4-(4-aminobenzyl)-1,3-oxazolidin-2-one

| Conditions | Yield |
|---|---|
| With triethylamine In dichloromethane at 0℃; | 31% |
-
-
152305-23-2
(S)-4-(4-aminobenzyl)-1,3-oxazolidin-2-one

-
-
98-74-8
4-Nitrobenzenesulfonyl chloride

| Conditions | Yield |
|---|---|
| With triethylamine In dichloromethane at 0℃; | 28% |
-
-
2284-20-0
4-Methoxyphenyl isothiocyanate

-
-
152305-23-2
(S)-4-(4-aminobenzyl)-1,3-oxazolidin-2-one

| Conditions | Yield |
|---|---|
| With triethylamine In acetone Reflux; | 27% |
-
-
874-60-2
4-methyl-benzoyl chloride

-
-
152305-23-2
(S)-4-(4-aminobenzyl)-1,3-oxazolidin-2-one

| Conditions | Yield |
|---|---|
| With triethylamine In dichloromethane at 20℃; | 24% |
-
-
2131-55-7
4-Chlorophenyl isothiocyanate

-
-
152305-23-2
(S)-4-(4-aminobenzyl)-1,3-oxazolidin-2-one

| Conditions | Yield |
|---|---|
| With triethylamine In acetone Reflux; | 19% |
-
-
152305-23-2
(S)-4-(4-aminobenzyl)-1,3-oxazolidin-2-one

| Conditions | Yield |
|---|---|
| With hydrogenchloride; sodium nitrite at 0℃; for 0.5h; | |
| With hydrogenchloride; sodium nitrite In water at -10 - 30℃; for 3h; | |
| With hydrogenchloride; sodium nitrite In water at 0 - 5℃; for 0.5h; |
-
-
152305-23-2
(S)-4-(4-aminobenzyl)-1,3-oxazolidin-2-one

-
-
179636-99-8
(S)-2-<5-(2-oxo-1,3-oxazolidin-4-ylmethyl)-1H-indol-3-yl>ethyl alcohol

| Conditions | Yield |
|---|---|
| Multi-step reaction with 3 steps 1: ICl, CaCO3 / methanol / 16 h / Ambient temperature 2: Na2CO3, MgSO4, Pd(OAc)2 / dimethylformamide / 16 h / 110 °C 3: 5 N HCl / methanol / 19 h / Ambient temperature View Scheme |
(S)-4-(4-Aminobenzyl)-2(1H)-oxazolidinone Chemical Properties
Product Name: 2-Oxazolidinone,4-[(4-aminophenyl)methyl]-, (4S)- (CAS NO.152305-23-2)
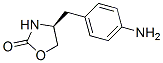
Molecular Formula: C10H12N2O2
Molecular Weight: 192.21g/mol
Mol File: 152305-23-2.mol
Melting Point: 107-111 °C
H bond acceptors: 4
H bond donors: 3
Freely Rotating Bonds: 3
Polar Surface Area: 64.35Å2
Index of Refraction: 1.6
Molar Refractivity: 52.322 cm3
Molar Volume: 152.871 cm3
Surface Tension: 53.826 dyne/cm
Boiling point: 479.7 °C at 760 mmHg
Flash Point: 243.9 °C
Density: 1.257 g/cm3
Surface Tension: 53.8 dyne/cm
Enthalpy of Vaporization: 74.4 kJ/mol
Vapour Pressure: 2.32E-09 mmHg at 25°C
Product Categories: chiral; Chiral Reagents; Asymmetric Synthesis; Chiral Building Blocks; Glycidyl Compounds, etc. (Chiral); Synthetic Organic Chemistry; Intermediates & Fine Chemicals; Pharmaceuticals; API's
(S)-4-(4-Aminobenzyl)-2(1H)-oxazolidinone Safety Profile
Safety Information of 2-Oxazolidinone,4-[(4-aminophenyl)methyl]-, (4S)- (CAS NO.152305-23-2):
Hazard Codes: Xn
Risk Statements: 22-36/37/38
22: Harmful if swallowed
36: Irritating to the eyes
37: Irritating to the respiratory system
38: Irritating to the skin
Safety Statements: 26-36/37
26: In case of contact with eyes, rinse immediately with plenty of water and seek medical advice
36: Wear suitable protective clothing
37: Wear suitable gloves
WGK Germany: 2
(S)-4-(4-Aminobenzyl)-2(1H)-oxazolidinone Specification
2-Oxazolidinone,4-[(4-aminophenyl)methyl]-, (4S)- , its CAS NO. is 152305-23-2, the synonyms are (4s)-4-[(4-Aminophenyl)methyl]-2-oxazolidinone ; (s)-4-(4'-Aminobenzyl)-1,3-oxazolidin-2-one ; (s)-4-(4-Aminobenzyl)-1,3-oxazolidin-2-one ; (s)-4-(4-Aminobenzyl)-2(1h)-oxazolidinone ; (s)-4-(4'-Aminobenzyl)-2-oxazolidinone ; (s)-4-(4-Aminobenzyl)-2-oxazolidinone ; (s)-4-(4-Amino-benzyl)-oxazolidin-2-one ; (s)-4-(4-Amino-benzyl)-oxazolidine-2-one .
Related Products
- (S)-4-(4-Aminobenzyl)-2(1H)-oxazolidinone
- 15230-79-2
- 1523-11-1
- 152312-71-5
- 15231-41-1
- 15231-48-8
- 15231-91-1
- 15233-47-3
- 15233-65-5
- 152338-45-9
- 152340-50-6
Hot Products
About|Contact|Cas|Product Name|Molecular|Country|Encyclopedia
Message|New Cas|MSDS|Service|Advertisement|CAS DataBase|Article Data|Manufacturers | Chemical Catalog
©2008 LookChem.com,License: ICP
NO.:Zhejiang16009103
complaints:service@lookchem.com Desktop View