-
Name
1,3,5,7-Cyclooctatetraene
- EINECS 211-080-3
- CAS No. 629-20-9
- Article Data90
- CAS DataBase
- Density 0.904 g/cm3
- Solubility Not miscible or difficult to mix in water.
- Melting Point -5 - -3 °C(lit.)
- Formula C8H8
- Boiling Point 140.5 °C at 760 mmHg
- Molecular Weight 104.152
- Flash Point 22.2 °C
- Transport Information UN 2358
- Appearance colourless to light yellow liquid
- Safety 26-62
- Risk Codes 10-36/37/38-65
-
Molecular Structure
-
Hazard Symbols
 Xn,
Xn, Xi
Xi
- Synonyms Cyclooctatetraene;NSC 5093;[8]Annulene;
- PSA 0.00000
- LogP 2.22480
Synthetic route
-
-
34733-74-9
bicyclo[4.2.1]nona-2,4,7-trien-9-one

-
-
629-20-9
1,3,5,7-cyclooctatetraene

| Conditions | Yield |
|---|---|
| In benzene-d6 at 100℃; Kinetics; Rate constant; 125 deg C; | 100% |
-
-
153943-48-7
4-Thia-2,6-diazahexacyclo<5.4.02,6.08,11.09,13.010,12>tridecane-3,5-dione

-
A
-
629-20-9
1,3,5,7-cyclooctatetraene

-
B
-
24046-80-8
Diazabasketene

| Conditions | Yield |
|---|---|
| In [D3]acetonitrile for 0.5h; Ambient temperature; Irradiation; | A 5 % Spectr. B 80% |
| In [D3]acetonitrile for 0.5h; Product distribution; Ambient temperature; Irradiation; other solvents; different reaction conditions; | A 5 % Spectr. B 80% |
-
-
1552-12-1, 111-78-4
1,5-cis,cis-cyclooctadiene

-
-
629-20-9
1,3,5,7-cyclooctatetraene

| Conditions | Yield |
|---|---|
| Stage #1: 1,5-cis,cis-cyclooctadiene With n-butyllithium; N,N,N,N,-tetramethylethylenediamine In hexane; pentane for 24h; Stage #2: With di-tert-butyl peroxide In hexane; pentane for 4h; Heating; Further stages.; | 65% |
| Multi-step reaction with 2 steps 1: K / 120 h / 108 °C 2: azobenzene / tetrahydrofuran / 1.) -78 deg C, 2.) room temp., 3 h further oxidizing agents View Scheme |

| Conditions | Yield |
|---|---|
| With 18-crown-6 ether; potassium tert-butylate In water; pentane | 61% |
-
-
69530-46-7, 69576-52-9
(1R,7S,8R)-8-Chloro-bicyclo[5.1.0]octa-2,4-diene

-
A
-
292638-84-7
styrene

-
B
-
629-20-9
1,3,5,7-cyclooctatetraene

| Conditions | Yield |
|---|---|
| With potassium tert-butylate at 25℃; | A n/a B 56% |
| With potassium tert-butylate at 25℃; Product distribution; Mechanism; other temperature: 90 deg C; other cond.: solvent: tetraglyme, temp: 90 deg C, pressure: 1 Torr; | A n/a B 56% |
| With potassium tert-butylate at 90℃; | A 8% B 8% |
-
-
74-86-2
acetylene

-
A
-
629-20-9
1,3,5,7-cyclooctatetraene

-
B
-
108-88-3
toluene

-
C
-
123-54-6
acetylacetone

-
D
-
71-43-2
benzene

| Conditions | Yield |
|---|---|
| bis(acetylacetonate)nickel(II); calcium carbide In tetrahydrofuran at 85 - 90℃; Further byproducts given; | A 56% B n/a C n/a D 33% |


| Conditions | Yield |
|---|---|
| With calcium carbide; tetrabutylammonium tetrafluoroborate; nickel In tetrahydrofuran at 80℃; under 12751 Torr; for 42h; electrolysis; | A 55% B n/a |
| With tetrabutylammonium tetrafluoroborate; nickel In tetrahydrofuran at 80℃; under 11250.9 Torr; Product distribution; Mechanism; electrolysis, further solvents, further catalysts, different temperatures; |

| Conditions | Yield |
|---|---|
| at 500℃; under 0.1 Torr; | A 51% B 49% |

| Conditions | Yield |
|---|---|
| at 300℃; under 2 Torr; for 0.000555556h; Product distribution; the effect of temperature was investigated; | 50% |
| at 370℃; under 2 Torr; for 0.000555556h; Yield given; |
-
-
20380-30-7
syn-tricyclo{4.2.0.0(2.5)}octa-3,7-diene

-
-
629-20-9
1,3,5,7-cyclooctatetraene

| Conditions | Yield |
|---|---|
| With 1,2,3,5-Tetracyanobenzol In acetonitrile at -40℃; for 10h; Irradiation; | 46% |
-
-
119-61-9
benzophenone

-
-
35434-64-1
tetracyclo<3.3.0.02,4.03,6>oct-7-ene

-
A
-
629-20-9
1,3,5,7-cyclooctatetraene

| Conditions | Yield |
|---|---|
| In acetone for 24h; Product distribution; Irradiation; | A 6% B 43% |

-
-
277-10-1
cubane

-
A
-
629-20-9
1,3,5,7-cyclooctatetraene

-
B
-
20380-30-7
syn-tricyclo{4.2.0.0(2.5)}octa-3,7-diene

| Conditions | Yield |
|---|---|
| With 1,2,3,5-Tetracyanobenzol In acetonitrile at -40℃; for 10h; Irradiation; | A 34% B 17% |
-
-
35434-64-1
tetracyclo<3.3.0.02,4.03,6>oct-7-ene

-
A
-
629-20-9
1,3,5,7-cyclooctatetraene

-
B
-
74-86-2
acetylene

-
C
-
71-43-2
benzene

| Conditions | Yield |
|---|---|
| In Cyclohexane-d12 for 26.6667h; Product distribution; Irradiation; | A 32% B n/a C 6% |

-
-
35434-64-1
tetracyclo<3.3.0.02,4.03,6>oct-7-ene

-
A
-
629-20-9
1,3,5,7-cyclooctatetraene

-
C
-
71-43-2
benzene

| Conditions | Yield |
|---|---|
| In acetone for 85.5833h; Product distribution; Irradiation; | A 13.5% B 3.5% C 5% |


| Conditions | Yield |
|---|---|
| With tetrahydrofuran; nickel(II) cyanide; calcium carbide at 60 - 70℃; under 11032.6 - 14710.2 Torr; unter Stickstoff; | |
| With oxirane; tetrahydrofuran; nickel(II) cyanide at 60 - 70℃; under 11032.6 - 14710.2 Torr; unter Stickstoff; | |
| With nickel | |
| With nickel |

| Conditions | Yield |
|---|---|
| With oxirane; tetrahydrofuran; nickel cyanide at 60 - 130℃; under 11032.6 - 14710.2 Torr; 1c-phenyl-butadiene-(1.3); |
-
-
67-56-1
methanol

-
-
77861-57-5, 92621-14-2
9-phenyl-9-phosphatricyclo<4.2.1.02,5>nona-3,7-diene

-
A
-
2946-61-4
phenylphosphonous acid dimethyl ester

-
B
-
629-20-9
1,3,5,7-cyclooctatetraene

-
C
-
638-21-1
phenylphosphane

| Conditions | Yield |
|---|---|
| at 50℃; for 0.25h; Product distribution; |

-
-
558-37-2
tert-butylethylene

-
-
292-64-8
Cyclooctan

-
A
-
75-83-2
2,2-Dimethylbutane

-
B
-
629-20-9
1,3,5,7-cyclooctatetraene

| Conditions | Yield |
|---|---|
| (iPr3P)2IrH5 at 150℃; for 120h; Product distribution; Mechanism; reaction time; other catalysts: <(p-F-C6H4)3P>2IrH5, <(p-F-C6H4)3P>3RuH4, (Me3P)2IrH5, (Ph3P)3RuH4, (Ar3P)2ReH7; |


| Conditions | Yield |
|---|---|
| With tert-butylethylene; (iPr3P)2IrH5 at 150℃; evacuated sealed tube; Yield given; |

| Conditions | Yield |
|---|---|
| In decalin at 200.3 - 226.5℃; Rate constant; Product distribution; dependence on glass; |
-
-
277-10-1
cubane

-
A
-
292638-84-7
styrene

-
B
-
629-20-9
1,3,5,7-cyclooctatetraene

-
C
-
74-86-2
acetylene

-
D
-
71-43-2
benzene

| Conditions | Yield |
|---|---|
| at 234.8 - 248.6℃; Rate constant; Thermodynamic data; Mechanism; E(a), Σ*, Η*, Γ*; |

-
-
277-10-1
cubane

-
A
-
292638-84-7
styrene

-
B
-
629-20-9
1,3,5,7-cyclooctatetraene

-
C
-
61771-84-4
1,4-dihydropentalene

-
D
-
33284-11-6
1,5-dihydropentalene

-
E
-
74-86-2
acetylene

-
F
-
71-43-2
benzene

| Conditions | Yield |
|---|---|
| Product distribution; pyrolysis as a function of time, temperature and pressure; |

-
-
3332-38-5
<16>annulene

-
-
629-20-9, 17676-32-3, 97590-88-0, 34510-09-3
<8>annulene dianion

-
A
-
629-20-9
1,3,5,7-cyclooctatetraene

-
B
-
3332-38-5, 18446-44-1, 23614-50-8, 83213-58-5
<16>annulene dianion

-
-
3332-38-5
<16>annulene

-
-
629-20-9, 17676-32-3, 97590-88-0
<8>Annulene anion radical

-
A
-
629-20-9
1,3,5,7-cyclooctatetraene

| Conditions | Yield |
|---|---|
| In N,N,N,N,N,N-hexamethylphosphoric triamide at 25℃; Equilibrium constant; Thermodynamic data; ΔH0 (enthalpy of electron transfer), ΔS0 (entropy of electron transfer); |

-
-
68344-46-7
1,5-Cyclooctadien-3-in

-
-
629-20-9
1,3,5,7-cyclooctatetraene

| Conditions | Yield |
|---|---|
| Heating; |
-
-
262-83-9
cyclooctanaphthalene

-
-
629-20-9, 17676-32-3, 97590-88-0
<8>Annulene anion radical

-
A
-
629-20-9
1,3,5,7-cyclooctatetraene

| Conditions | Yield |
|---|---|
| In N,N,N,N,N,N-hexamethylphosphoric triamide at 25℃; Equilibrium constant; Thermodynamic data; ΔH0 (enthalpy of electron transfer), ΔS0 (entropy of electron transfer); |

-
-
17596-57-5
perdeuteriated <8>annulene

-
-
629-20-9, 17676-32-3, 97590-88-0
cyclooctatetraene anion

-
A
-
629-20-9
1,3,5,7-cyclooctatetraene

-
B
-
17596-57-5
C8(2)H8(1-)

| Conditions | Yield |
|---|---|
| In ammonia at -100℃; Equilibrium constant; Thermodynamic data; ΔG0; |


| Conditions | Yield |
|---|---|
| at 180℃; for 1h; Product distribution; Thermodynamic data; Kinetics; gas-phase thermolysis, various temperatures, pressures and times, Arrhenius parameters; |
-
-
4514-70-9
tert-butoxycyclooctatetraene

-
-
629-20-9, 17676-32-3, 97590-88-0
<8>Annulene anion radical

-
A
-
629-20-9
1,3,5,7-cyclooctatetraene

| Conditions | Yield |
|---|---|
| In N,N,N,N,N,N-hexamethylphosphoric triamide at 25℃; Equilibrium constant; Thermodynamic data; ΔH0 (enthalpy of electron transfer), ΔS0 (entropy of electron transfer); |

-
-
13402-35-2
ethylcyclooctatetraene

-
-
629-20-9, 17676-32-3, 97590-88-0
<8>Annulene anion radical

-
A
-
629-20-9
1,3,5,7-cyclooctatetraene

| Conditions | Yield |
|---|---|
| In N,N,N,N,N,N-hexamethylphosphoric triamide at 25℃; Equilibrium constant; Thermodynamic data; ΔH0 (enthalpy of electron transfer), ΔS0 (entropy of electron transfer); |


| Conditions | Yield |
|---|---|
| Stage #1: 1,3,5,7-cyclooctatetraene With bromine In dichloromethane at -70℃; for 1h; Stage #2: With potassium tert-butylate In tetrahydrofuran; dichloromethane at -60℃; for 4h; Concentration; Solvent; Temperature; | 97% |
| Stage #1: 1,3,5,7-cyclooctatetraene With bromine In dichloromethane at -78℃; for 2h; Schlenk technique; Inert atmosphere; Stage #2: With potassium tert-butylate In tetrahydrofuran; dichloromethane at -78℃; for 3h; Schlenk technique; Inert atmosphere; | 85% |
| With potassium tert-butylate; bromine In dichloromethane | 75% |
| (i) Br2, CH2Cl2, (ii) KOtBu; Multistep reaction; | |
| Multi-step reaction with 2 steps 1: Br2 2: t-BuOK View Scheme |
-
-
16453-18-2
3,6-bis(trifluoromethyl)-1,2,4,5-tetrazine

-
-
629-20-9
1,3,5,7-cyclooctatetraene

-
-
137866-82-1
2,4a-dihydro-1,4-bis(trifluoromethyl)cyclooctapyridazine

| Conditions | Yield |
|---|---|
| In dichloromethane for 72h; | 95% |

| Conditions | Yield |
|---|---|
| With zinc(II) iodide; zinc; CoI2(dppe) In 1,2-dichloro-ethane at 40℃; for 20h; | 94% |
-
-
629-20-9
1,3,5,7-cyclooctatetraene

-
-
120546-24-9
(+/-)-7,10-Dithiadinaphtho<2,1-d:1',2'-f>cyclooctene 7,7,10,10-tetraoxide

| Conditions | Yield |
|---|---|
| With hydroquinone In chloroform at 90℃; for 24h; | 93% |
-
-
629-20-9
1,3,5,7-cyclooctatetraene

| Conditions | Yield |
|---|---|
| With bromine at -80 - -60℃; for 0.5h; | 93% |

| Conditions | Yield |
|---|---|
| In toluene | A n/a B n/a C 93% |

-
-
108-31-6
maleic anhydride

-
-
629-20-9
1,3,5,7-cyclooctatetraene

-
-
51447-09-7
endo-tricyclo<4.2.2.02,5>deca-3,9-diene-7,8-dicarboxylate anhydride

| Conditions | Yield |
|---|---|
| With hydroquinone for 2h; Heating; | 90% |
| In chlorobenzene Heating; |

| Conditions | Yield |
|---|---|
| With zinc(II) iodide; zinc; CoI2(dppe) In 1,2-dichloro-ethane at 40℃; for 20h; | 89% |
-
-
14918-21-9
5-hexynonitrile

-
-
629-20-9
1,3,5,7-cyclooctatetraene

| Conditions | Yield |
|---|---|
| With zinc(II) iodide; zinc; CoI2(dppe) In 1,2-dichloro-ethane at 40℃; for 20h; | 88% |

| Conditions | Yield |
|---|---|
| With cobalt acetylacetonate; 1,2-bis-(diphenylphosphino)ethane; zinc(II) iodide; zinc In 1,2-dichloro-ethane at 60℃; for 20h; Sealed tube; Inert atmosphere; | 88% |

| Conditions | Yield |
|---|---|
| Product distribution; Irradiation; with or without benzophenone; | A 13% B 87% |
| Irradiation; | A 13% B 87% |

-
-
629-20-9
1,3,5,7-cyclooctatetraene

-
-
72051-04-8
6-(toluene-4-sulfonyloxy)-hexa-1,2-diene

| Conditions | Yield |
|---|---|
| With 1,2-bis-(diphenylphosphino)ethane; cobalt(II) iodide; zinc(II) iodide; zinc In 1,2-dichloro-ethane at 60℃; for 20h; Inert atmosphere; Sealed tube; stereoselective reaction; | 87% |
-
-
108-31-6
maleic anhydride

-
-
629-20-9
1,3,5,7-cyclooctatetraene

-
-
6295-73-4
3a,4,4a,6a,7,7a-hexahydro-4,7-ethenocyclobutisobenzofuran-1,3-dione

| Conditions | Yield |
|---|---|
| In toluene Heating; | 85% |
-
-
629-20-9
1,3,5,7-cyclooctatetraene

-
-
5660-91-3
1,3-diphenyl-2H-cyclopenta<1>phenanthren-2-one

-
-
78442-58-7
C66H44O2

| Conditions | Yield |
|---|---|
| In benzene at 80℃; for 7h; | 85% |
-
-
629-20-9
1,3,5,7-cyclooctatetraene

-
-
963-16-6
(E)-1,2-bis(phenylsulfonyl)ethylene

-
-
87057-42-9
(1R,6S,9R,10R)-9,10-Bis-benzenesulfonyl-tricyclo[4.2.2.02,5]deca-3,7-diene

| Conditions | Yield |
|---|---|
| In 1,2-dichloro-benzene for 4h; Heating; | 85% |
-
-
16453-18-2
3,6-bis(trifluoromethyl)-1,2,4,5-tetrazine

-
-
629-20-9
1,3,5,7-cyclooctatetraene

-
A
-
137866-82-1
2,4a-dihydro-1,4-bis(trifluoromethyl)cyclooctapyridazine

| Conditions | Yield |
|---|---|
| In dichloromethane for 96h; Heating; | A 85% B 5% |


| Conditions | Yield |
|---|---|
| With 1,2-bis-(diphenylphosphino)ethane; cobalt(II) iodide; zinc(II) iodide; zinc In 1,2-dichloro-ethane at 60℃; for 20h; Inert atmosphere; Sealed tube; stereoselective reaction; | 85% |

| Conditions | Yield |
|---|---|
| With cobalt acetylacetonate; 1,2-bis-(diphenylphosphino)ethane; zinc(II) iodide; zinc In 1,2-dichloro-ethane at 60℃; for 20h; Sealed tube; Inert atmosphere; | 85% |
-
-
629-20-9
1,3,5,7-cyclooctatetraene

-
-
1600-27-7
mercury(II) diacetate

-
-
7698-06-8, 42301-50-8
(+/-)-2-acetyloxy-1,2,2α,6α-tetrahydrocyclobuta[1,2-α]benzenyl acetate

| Conditions | Yield |
|---|---|
| at -5 - 25℃; | 84% |
| With acetic acid at 70 - 80℃; |
-
-
629-20-9
1,3,5,7-cyclooctatetraene

-
-
963-16-6
(E)-1,2-bis(phenylsulfonyl)ethylene

-
-
87057-42-9
5-exo,6-endo-bis(phenylsulfonyl)tricyclo<4.2.2.02,5>deca-3,9-diene

| Conditions | Yield |
|---|---|
| In 1,2-dichloro-benzene at 160℃; for 12h; | 84% |
-
-
629-20-9
1,3,5,7-cyclooctatetraene

-
-
132835-15-5
(Z)-1,4-bis(tert-butyldimethylsilanyloxy)but-2-ene

| Conditions | Yield |
|---|---|
| With tricyclohexylphosphine[1,3-bis(2,4,6-trimethylphenyl)-4,5-dihydroimidazol-2-ylidine][benzylidene]ruthenium(II) dichloride In dichloromethane at 55℃; for 24h; | 83% |
-
-
629-20-9
1,3,5,7-cyclooctatetraene

-
-
22433-39-2
1-methyl-1-phenylallene

| Conditions | Yield |
|---|---|
| With 1,2-bis-(diphenylphosphino)ethane; cobalt(II) iodide; zinc(II) iodide; zinc In 1,2-dichloro-ethane at 60℃; for 20h; Inert atmosphere; Sealed tube; stereoselective reaction; | 83% |
-
-
629-20-9
1,3,5,7-cyclooctatetraene

-
-
40339-20-6
buta-2,3-dienyl-benzene

| Conditions | Yield |
|---|---|
| With 1,2-bis-(diphenylphosphino)ethane; cobalt(II) iodide; zinc(II) iodide; zinc In 1,2-dichloro-ethane at 60℃; for 20h; Inert atmosphere; Sealed tube; stereoselective reaction; | 82% |

| Conditions | Yield |
|---|---|
| With cobalt acetylacetonate; 1,2-bis-(diphenylphosphino)ethane; zinc(II) iodide; zinc In 1,2-dichloro-ethane at 60℃; for 20h; Sealed tube; Inert atmosphere; | 82% |
1,3,5,7-Cyclooctatetraene Specification
The Cyclooctatetraene, also known as [8]Annulene, is an unsaturated derivative of cyclooctane with the formula C8H8. It belongs to the product categories of Alkanes; Cyclic; Organic Building Blocks. Its EINECS registry number is 211-080-3. With the CAS registry number 629-20-9, its IUPAC name is 1,3,5,7-cyclooctatetraene(COT). This polyunsaturated hydrocarbon is a colorless to light yellow flammable liquid at room temperature.
Physical properties of Cyclooctatetraene: (1)ACD/LogP: 2.55; (2)ACD/LogD (pH 5.5): 2.55; (3)ACD/LogD (pH 7.4): 2.55; (4)ACD/BCF (pH 5.5): 51.13; (5)ACD/BCF (pH 7.4): 51.13; (6)ACD/KOC (pH 5.5): 581.68; (7)ACD/KOC (pH 7.4): 581.68; (8)Index of Refraction: 1.525; (9)Molar Refractivity: 35.3 cm3; (10)Molar Volume: 115.1 cm3; (11)Surface Tension: 38 dyne/cm; (12)Density: 0.904 g/cm3; (13)Flash Point: 22.2 °C; (14)Enthalpy of Vaporization: 36.21 kJ/mol; (15)Boiling Point: 140.5 °C at 760 mmHg; (16)Vapour Pressure: 7.64 mmHg at 25°C.
Preparation: Reppe's synthesis of cyclooctatetraene, which involves treating acetylene at high pressure with a warm mixture of nickel cyanide and calcium carbide, was much better, with chemical yields near 90%:
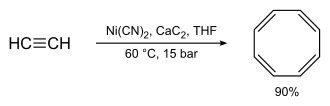
When you are using this chemical, please be cautious about it as the following:
This chemical may cause damage to health and may cause inflammation to the skin or other mucous membranes. It is irritating to eyes, respiratory system and skin. In addition, it may cause lung damage if swallowed. In case of contact with eyes, you should rinse immediately with plenty of water and seek medical advice. If swallowed, do not induce vomiting: seek medical advice immediately and show this container or label.
You can still convert the following datas into molecular structure:
(1)Canonical SMILES: C1=CC=CC=CC=C1
(2)Isomeric SMILES: C\1=C\C=C/C=C\C=C1
(3)InChI: InChI=1S/C8H8/c1-2-4-6-8-7-5-3-1/h1-8H/b2-1-,3-1?,4-2?,5-3-,6-4-,7-5?,8-6?,8-7-
(4)InChIKey: KDUIUFJBNGTBMD-DLMDZQPMSA-N
Related Products
- 10,10'-Bis([1,1'-biphenyl]-4-yl)-9,9'-bianthracene
- 10,10'-Dibromo-9,9'-bianthryl
- 10,10-Dimethylanthrone
- 10,10-Oxybisphenoxarsine
- 10-[1,1'-Biphenyl]-4-yl-2-(1-methylethyl)-9-oxo-9H-thioxanthenium hexafluorophosphate
- 10,11-Dehydroimipramine
- 10,11-Dihydro-11-oxodibenzo[b,f][1,4]thiazepine
- 10,11-Dihydro-2-(4-methyl-1-piperazinyl)-11-(2-athiazolyl)-pyridazino(3,4-b)(1,4)benzoxazepine
- 10,11-DIHYDRO-2-(4-METHYL-1-PIPERAZINYL)-11-(3,4-XYLYL)PYRIDAZINO(3,4b)(1,4)-BENZOXAZEPINE MALEATE
- 10,11-Dihydro-5-(3-dimethylamino-2-methylpropyl)-5h-dibenz (b,f)azepine
- 62922-45-6
- 62922-46-7
- 6292-36-0
- 62924-59-8
- 62924-70-3
- 629-25-4
- 62925-87-5
- 6292-59-7
- 6292-61-1
- 6292-71-3
Hot Products
About|Contact|Cas|Product Name|Molecular|Country|Encyclopedia
Message|New Cas|MSDS|Service|Advertisement|CAS DataBase|Article Data|Manufacturers | Chemical Catalog
©2008 LookChem.com,License: ICP
NO.:Zhejiang16009103
complaints:service@lookchem.com Desktop View