-
Name
1,4-Cyclohexanediol
- EINECS 209-126-2
- CAS No. 556-48-9
- Article Data69
- CAS DataBase
- Density 1.156 g/cm3
- Solubility It is highly soluble in water.
- Melting Point 98-100 °C(lit.)
- Formula C6H12O2
- Boiling Point 252.4 °C at 760 mmHg
- Molecular Weight 116.16
- Flash Point 65.6 °C
- Transport Information UN 1325
- Appearance white to off-white crystalline powder
- Safety 24/25
- Risk Codes Xi
-
Molecular Structure
-
Hazard Symbols
 Xi
Xi
- Synonyms 1,4-Dihydroxycyclohexane;NSC 5651;Quinitol;1,4-Cyclohexanediol(Mixture of Cis and Trans);cyclohexane-1,4-diol;
- PSA 40.46000
- LogP 0.28220
Synthetic route

| Conditions | Yield |
|---|---|
| With hydrogen In water at 30℃; under 7500.75 Torr; for 7h; Autoclave; | 99.1% |
| With hydrogen In water at 80℃; under 15001.5 Torr; for 6h; | 99% |
| With potassium hydroxide; samarium diiodide In tetrahydrofuran; water for 0.05h; Ambient temperature; | 98% |

| Conditions | Yield |
|---|---|
| With samarium diiodide; water In tetrahydrofuran at 20℃; | 99% |
| With sodium tetrahydroborate; TiO(acac)2 In tetrahydrofuran for 0.17h; Heating; | 93% |
| With sodium tetrahydroborate; Dowex1-x8 In tetrahydrofuran for 0.34h; Heating; | 90% |

| Conditions | Yield |
|---|---|
| With hydrogen In n-heptane at 140℃; under 750.075 Torr; for 6h; Catalytic behavior; | A 99% B 99% |
-
-
1036648-32-4
(4-hydroxycyclohexyl) 4-methylbenzoate

-
-
556-48-9
1,4-Cyclohexanediol

| Conditions | Yield |
|---|---|
| With methanol; samarium diiodide In tetrahydrofuran; N,N,N,N,N,N-hexamethylphosphoric triamide Reflux; | 98% |
| With methanol; N,N,N,N,N,N-hexamethylphosphoric triamide; samarium diiodide In tetrahydrofuran Inert atmosphere; Reflux; | 98% |
-
-
55724-30-6
1,4-bis-trimethylsiloxycyclohexane

-
-
556-48-9
1,4-Cyclohexanediol

| Conditions | Yield |
|---|---|
| With water; p-toluenesulfonyl chloride at 20℃; for 0.0833333h; | 91% |
| With aminosulfonic acid; water at 20℃; for 4.5h; | 91% |
| With water; boric acid at 20℃; for 24h; | 85% |
-
-
1163693-65-9
4-(4-methylbenzoyloxy)cyclohexyl 4-(trifluoromethyl)benzoate

-
-
556-48-9
1,4-Cyclohexanediol

| Conditions | Yield |
|---|---|
| With tetrabutylammonium tetrafluoroborate In 1-methyl-pyrrolidin-2-one; isopropyl alcohol at 90℃; Electrochemical reaction; | 65% |

| Conditions | Yield |
|---|---|
| In isopropyl alcohol | 53% |
-
-
1965-09-9
4,4'-dihydroxydiphenyl ether

-
A
-
110-82-7
cyclohexane

-
B
-
556-48-9
1,4-Cyclohexanediol

-
C
-
108-93-0
cyclohexanol

| Conditions | Yield |
|---|---|
| With isopropyl alcohol at 150℃; for 6h; Sealed tube; | A 10.5 %Chromat. B 8.4% C 76.2 %Chromat. |


| Conditions | Yield |
|---|---|
| With water; sodium hydrogencarbonate unter Durchleiten von CO2; cis-quinitol; |
-
-
53762-85-9
(1S,4R)-Cyclohex-2-ene-1,4-diol

-
A
-
30182-12-8
4-hydroxy-2-cyclohexenone

-
B
-
556-48-9
1,4-Cyclohexanediol

-
C
-
13482-22-9
4-hydroxycyclohexanone

| Conditions | Yield |
|---|---|
| ruthenium In tetrahydrofuran at 65℃; for 15h; Yield given. Yields of byproduct given; | |
| ruthenium In tetrahydrofuran at 60℃; for 15h; Yield given. Yields of byproduct given; |

-
-
13482-22-9
4-hydroxycyclohexanone

-
A
-
556-48-9
1,4-Cyclohexanediol

-
B
-
108-94-1
cyclohexanone

-
C
-
108-93-0
cyclohexanol

| Conditions | Yield |
|---|---|
| With hydrogen; palladium In tert-butyl alcohol at 30℃; Yield given. Yields of byproduct given; | |
| With hydrogen; palladium In tert-butyl alcohol at 30℃; Product distribution; further catalyst, further 4-substituted cyclohexanone; |

-
-
75-07-0
acetaldehyde

-
-
1165952-91-9
cyclohexa-1,3-diene

-
A
-
931-17-9
1,2-Cyclohexanediol

-
B
-
504-01-8
cyclohexane-1,3-diol

-
C
-
556-48-9
1,4-Cyclohexanediol

-
D
-
40391-19-3
(1R*,1'RS*)-1-cyclohex-2-enylethanol

-
E
-
822-66-2, 72137-22-5
3-cyclohexen-1-ol

| Conditions | Yield |
|---|---|
| With sodium hydroxide; dimethylsulfide borane complex; dihydrogen peroxide Product distribution; other cyclic dienes and hydroborating agents; |


| Conditions | Yield |
|---|---|
| With hydrogen; silica gel; nickel In isopropyl alcohol at 100℃; under 4650.4 Torr; Product distribution; Kinetics; various catalyst, var. temp., var. of hydrogen pressure, var. of substrate concentr.; | A n/a B 87 % Chromat. |
| With hydrogen; palladium/alumina at 130℃; under 22502.3 Torr; Conversion of starting material; | |
| With Zr -F-100 In isopropyl alcohol at 82℃; for 6h; Reagent/catalyst; | |
| With nicotinamide adenine dinucleotide phosphate; isopropyl alcohol In aq. phosphate buffer at 30℃; for 12h; pH=7; Enzymatic reaction; |
-
-
637-88-7
1,4-Cyclohexanedione

-
A
-
556-48-9
1,4-Cyclohexanediol

-
B
-
13482-22-9
4-hydroxycyclohexanone

-
C
-
108-94-1
cyclohexanone

-
D
-
108-93-0
cyclohexanol

| Conditions | Yield |
|---|---|
| With hydrogen; palladium In tert-butyl alcohol at 30℃; Product distribution; further catalyst; | |
| With hydrogen; palladium In tert-butyl alcohol at 30℃; Yield given. Yields of byproduct given; |


| Conditions | Yield |
|---|---|
| With hydrogen; nickel at 170℃; trans-quinitol; |
-
-
108-94-1
cyclohexanone

-
A
-
124-04-9
Adipic acid

-
B
-
110-15-6
succinic acid

-
C
-
556-48-9
1,4-Cyclohexanediol

-
D
-
637-88-7
1,4-Cyclohexanedione

| Conditions | Yield |
|---|---|
| With peracetic acid; Ru-carbon In ethyl acetate at 20℃; for 1h; Further byproducts given; | A 48 % Chromat. B 26 % Chromat. C n/a D 8 % Chromat. |


| Conditions | Yield |
|---|---|
| Multi-step reaction with 2 steps 1: concentrated sulfuric acid View Scheme | |
| Multi-step reaction with 2 steps 1: concentrated sulfuric acid 2: NaHCO3; water / unter Durchleiten von CO2 View Scheme |
-
-
67-56-1
methanol

-
A
-
542-28-9
3,4,5,6-tetrahydro-2H-pyran-2-one

-
B
-
627-93-0
hexanedioic acid dimethyl ester

-
C
-
4547-43-7
methyl 6-hydroxycaproate

-
D
-
1119-40-0
Dimethyl glutarate

-
E
-
14273-92-8
methyl 5-hydroxypentanoate

-
F
-
931-17-9
1,2-Cyclohexanediol

-
G
-
556-48-9
1,4-Cyclohexanediol

-
H
-
106-65-0
Dimethyl succinate

| Conditions | Yield |
|---|---|
| With sulfuric acid |

-
-
123-31-9
hydroquinone

-
A
-
556-48-9
1,4-Cyclohexanediol

-
B
-
13482-22-9
4-hydroxycyclohexanone

-
C
-
637-88-7
1,4-Cyclohexanedione

| Conditions | Yield |
|---|---|
| With hydrogen; Pd/hydrophilic-C at 100℃; under 7500.75 Torr; for 20h; |

-
-
110-82-7
cyclohexane

-
A
-
124-04-9
Adipic acid

-
B
-
1191-25-9
6-Hydroxyhexanoic acid

-
C
-
556-48-9
1,4-Cyclohexanediol

| Conditions | Yield |
|---|---|
| With oxygen at 160℃; under 7500.75 Torr; |


| Conditions | Yield |
|---|---|
| With carbon dioxide; hydrogen at 50℃; under 30003 - 90009 Torr; for 4h; Supercritical conditions; | |
| With hydrogen In water at 25℃; under 750.075 Torr; for 7h; Autoclave; | |
| With hydrogen In dichloromethane at 50℃; under 7500.75 Torr; for 1h; |

| Conditions | Yield |
|---|---|
| With hydrogen In ethanol at 160℃; under 11251.1 Torr; |


| Conditions | Yield |
|---|---|
| With hydrogen In ethanol at 160℃; under 11251.1 Torr; |

| Conditions | Yield |
|---|---|
| With rhodium contaminated with carbon; water; hydrogen at 80℃; for 18h; Supercritical conditions; Autoclave; |

| Conditions | Yield |
|---|---|
| With hydrogen In ethanol at 150℃; under 9000.9 Torr; |

-
-
123-31-9
hydroquinone

-
A
-
556-48-9
1,4-Cyclohexanediol

-
B
-
637-88-7
1,4-Cyclohexanedione

-
C
-
108-95-2
phenol

| Conditions | Yield |
|---|---|
| With hydrogen In water at 119.84℃; under 4500.45 Torr; Kinetics; |

-
-
123-31-9
hydroquinone

-
A
-
556-48-9
1,4-Cyclohexanediol

-
B
-
13482-22-9
4-hydroxycyclohexanone

-
C
-
108-94-1
cyclohexanone

-
D
-
108-93-0
cyclohexanol

| Conditions | Yield |
|---|---|
| With hydrogen In water at 25℃; under 760.051 Torr; for 15h; Schlenk technique; Green chemistry; |


| Conditions | Yield |
|---|---|
| With [Cu3(μ3-5-(4-pyridyl)tetrazolate)4(μ2-N3)2(DMF)2]n·(DMF)2n; dihydrogen peroxide In neat (no solvent) at 20℃; under 760.051 Torr; for 24h; Green chemistry; |
-
-
556-48-9
1,4-Cyclohexanediol

-
-
131832-07-0, 131832-10-5
1,4-cyclohexanediol-O,O-d2

| Conditions | Yield |
|---|---|
| With water-d2 for 6h; Ambient temperature; | 99% |

| Conditions | Yield |
|---|---|
| With triethylamine In dichloromethane | 99% |
| With pyridine; dmap In dichloromethane at 25℃; for 4h; | 30% |
| With pyridine In dichloromethane at 25℃; for 4h; | 30% |
| With pyridine In chloroform at 0 - 20℃; for 6h; |

| Conditions | Yield |
|---|---|
| With MgLa mixed oxides at 129.85℃; for 0.75h; | 98% |
| aluminum oxide; cesium fluoride at 129.85℃; for 0.5h; | 97% |
| With MgLa mixed oxide at 125℃; for 0.75h; | 97% |
| With immobilized 1,5,7-triazabicyclo[4.4.0]dec-5-ene on magnetic γ-Fe2O3 nanoparticles at 125℃; for 10h; | 94% |

| Conditions | Yield |
|---|---|
| With 1-hydroxy-3H-benz[d][1,2]iodoxole-1,3-dione In neat (no solvent) at 20℃; for 1h; Milling; | 97% |
| With ruthenium(IV) oxide; sodium dihydrogenphosphate; sodium chloride In water at 10 - 15℃; electrooxidation; | 94% |
| With m-iodosylbenzoic acid; ruthenium trichloride In water; acetonitrile at 20℃; for 10h; | 84% |
-
-
556-48-9
1,4-Cyclohexanediol

-
-
999-97-3
1,1,1,3,3,3-hexamethyl-disilazane

-
-
55724-30-6
1,4-bis-trimethylsiloxycyclohexane

| Conditions | Yield |
|---|---|
| iodine In dichloromethane at 20℃; for 0.0666667h; Substitution; | 97% |
| With boric acid In acetonitrile at 20℃; for 2h; | 97% |
| With aminosulfonic acid In acetonitrile at 20℃; for 2h; | 95% |
| Stage #1: 1,4-Cyclohexanediol With Iron(III) nitrate nonahydrate; sodium iodide In dichloromethane at 20℃; Stage #2: 1,1,1,3,3,3-hexamethyl-disilazane In dichloromethane at 20℃; for 0.0416667h; | 90% |
| With p-toluenesulfonyl chloride In dichloromethane at 20℃; for 0.5h; | 85% |
-
-
556-48-9
1,4-Cyclohexanediol

-
-
30485-71-3
4-chlorocyclohexanol

| Conditions | Yield |
|---|---|
| With 1,3,5-trichloro-2,4,6-triazine; N,N-dimethyl-formamide In dichloromethane at 25℃; for 0.25h; | 95% |
| With hydrogenchloride at 100℃; im geschlossenen Gefaess; | |
| With hydrogenchloride |
-
-
556-48-9
1,4-Cyclohexanediol

-
-
108-24-7
acetic anhydride

-
-
6289-83-4, 42742-00-7, 19843-75-5
1,4-diacetoxycyclohexane

| Conditions | Yield |
|---|---|
| at 90℃; for 2h; | 95% |
| With nickel dichloride at 20℃; for 0.5h; Neat (no solvent); | 92% |
| With N-Bromosuccinimide In dichloromethane at 20℃; for 12h; | 84% |
-
-
110-87-2
3,4-dihydro-2H-pyran

-
-
556-48-9
1,4-Cyclohexanediol

-
A
-
736-42-5
1,4-Bis--cyclohexan

-
B
-
64230-40-6
4-(2-Tetrahydropyranyloxy)-1-cyclohexanol

| Conditions | Yield |
|---|---|
| With Dowex 50W x 2 (50-100 mesh) In toluene at 30℃; for 1.5h; | A 3% B 95% |
| With iodine In tetrahydrofuran at 67℃; for 0.0472222h; Irradiation; | A 15% B 77% |
| aluminum(III) sulfate; silica gel In hexane for 8h; Ambient temperature; | A 9 % Chromat. B 71 % Chromat. |


| Conditions | Yield |
|---|---|
| With triethylamine In dichloromethane at 10 - 20℃; for 6h; | 94.7% |
-
-
556-48-9
1,4-Cyclohexanediol

| Conditions | Yield |
|---|---|
| With γ-Fe2O3-immobilized 1,5,7-triazabicyclo[4.4.0]dec-5-ene nanoparticles (MNPs-TBD) at 125℃; for 10h; | 94% |
-
-
110-87-2
3,4-dihydro-2H-pyran

-
-
556-48-9
1,4-Cyclohexanediol

-
-
64230-40-6
4-(2-Tetrahydropyranyloxy)-1-cyclohexanol

| Conditions | Yield |
|---|---|
| With pyridinium p-toluenesulfonate In tetrahydrofuran for 6h; Ambient temperature; | 93% |
| With lithium trifluoromethanesulfonate In 1,2-dichloro-ethane for 5.5h; Heating; | 93% |
| With pyridinium p-toluenesulfonate In dichloromethane at 10 - 20℃; for 7h; Inert atmosphere; | 84% |

| Conditions | Yield |
|---|---|
| With sodium bromate; sodium hydrogensulfite In water; acetonitrile at 20℃; for 6h; Oxidation; | A 1% B 93% |
| With sodium bromate; sodium hydrogensulfite In water; acetonitrile at 20℃; for 2h; Oxidation; | A 78% B 10% |
| With 2O34W9Zn(12-)*W(6+)*3Zn(2+)*2H2O; dihydrogen peroxide In water; acetonitrile at 135℃; under 2250.23 Torr; for 0.25h; Microwave irradiation; | A n/a B 58% |

| Conditions | Yield |
|---|---|
| With p-toluenesulfonyl chloride at 20℃; for 0.633333h; neat (no solvent); | 93% |
| With Iron(III) nitrate nonahydrate; sodium iodide at 20℃; for 0.833333h; neat (no solvent); | 92% |
-
-
556-48-9
1,4-Cyclohexanediol

-
-
13482-22-9
4-hydroxycyclohexanone

| Conditions | Yield |
|---|---|
| With sodium bromate; ammonium cerium(IV) nitrate In water; acetonitrile | 92% |
| With sodium bromate; ammonium cerium (IV) nitrate In water; acetonitrile for 2.5h; Reflux; | 91% |
| With calcium hypochlorite; potassium bromide In water; acetic acid at 0℃; for 3h; | 88% |
-
-
556-48-9
1,4-Cyclohexanediol

-
-
1165952-92-0
cyclohexa-1,4-diene

| Conditions | Yield |
|---|---|
| With 1,1,1-trichloro-3,3,3-trifluoro-propan-2-one; toluene-4-sulfonic acid In benzene for 6h; Heating; | 92% |
-
-
5453-80-5, 19926-90-0
5-Norbornene-2-carboxaldehyde

-
-
556-48-9
1,4-Cyclohexanediol

| Conditions | Yield |
|---|---|
| sodium hydride at 20℃; for 10h; | 92% |
-
-
556-48-9
1,4-Cyclohexanediol

-
-
59274-95-2
7-oxa-bicyclo[2.2.1]hept-5-ene-2-carbaldehyde

| Conditions | Yield |
|---|---|
| sodium hydride at 20℃; for 10h; | 92% |

| Conditions | Yield |
|---|---|
| at 160℃; for 5h; Molecular sieve; Inert atmosphere; | 88.4% |

| Conditions | Yield |
|---|---|
| With p-toluenesulfonyl chloride at 20℃; for 0.383333h; neat (no solvent); | 88% |
| With triethylamine In dichloromethane at 0 - 20℃; for 2h; Inert atmosphere; |
-
-
109-80-8
1.3-propanedithiol

-
-
556-48-9
1,4-Cyclohexanediol

-
-
128345-17-5
1,5-dithiaspiro<5.5>undecan-9-ol

| Conditions | Yield |
|---|---|
| Stage #1: 1,4-Cyclohexanediol With sodium bromate; ammonium cerium (IV) nitrate In water; acetonitrile for 1h; Reflux; Stage #2: 1.3-propanedithiol With boron trifluoride diethyl etherate In dichloromethane at 0℃; for 0.25h; | 88% |
-
-
556-48-9
1,4-Cyclohexanediol

-
-
18162-48-6
tert-butyldimethylsilyl chloride

-
-
126931-29-1
4-((tert-butyldimethylsilyl)oxy)cyclohexan-1-ol

| Conditions | Yield |
|---|---|
| With 1H-imidazole In tetrahydrofuran at 20℃; for 16h; | 86.7% |
| With 1H-imidazole In tetrahydrofuran; N,N-dimethyl-formamide at 0℃; | 66% |
| With 1H-imidazole In tetrahydrofuran; N,N-dimethyl-formamide at 0℃; | 62% |

| Conditions | Yield |
|---|---|
| at 150℃; for 4h; Molecular sieve; Inert atmosphere; | 86.6% |

| Conditions | Yield |
|---|---|
| With aluminum (III) chloride In toluene at 150℃; for 4h; Inert atmosphere; | 84.1% |

| Conditions | Yield |
|---|---|
| With dmap; triethylamine In dichloromethane at 40℃; for 18h; Inert atmosphere; | 84% |

| Conditions | Yield |
|---|---|
| With dmap; 1-ethyl-(3-(3-dimethylamino)propyl)-carbodiimide hydrochloride In dichloromethane at 0 - 20℃; for 24h; | 84% |
1,4-Cyclohexanediol Specification
The 1,4-Cyclohexanediol, with the CAS registry number 556-48-9 and EINECS registry number 209-126-2, has the systematic name of cyclohexane-1,4-diol. And the molecular formula of this chemical is C6H12O2. It is a kind of white to off-white crystalline powder, and belongs to the product categories of Aromatic alcohols and diols. What's more, while dealing with this chemical, you should avoid contacting with skin and eyes.
The physical properties of 1,4-Cyclohexanediol are as following: (1)ACD/LogP: -0.09; (2)# of Rule of 5 Violations: 0; (3)ACD/LogD (pH 5.5): -0.09; (4)ACD/LogD (pH 7.4): -0.09; (5)ACD/BCF (pH 5.5): 1; (6)ACD/BCF (pH 7.4): 1; (7)ACD/KOC (pH 5.5): 21.34; (8)ACD/KOC (pH 7.4): 21.34; (9)#H bond acceptors: 2; (10)#H bond donors: 2; (11)#Freely Rotating Bonds: 2; (12)Polar Surface Area: 18.46 Å2; (13)Index of Refraction: 1.526; (14)Molar Refractivity: 30.85 cm3; (15)Molar Volume: 100.4 cm3; (16)Polarizability: 12.23×10-24cm3; (17)Surface Tension: 40.7 dyne/cm; (18)Density: 1.156 g/cm3; (19)Flash Point: 65.6 °C; (20)Enthalpy of Vaporization: 56.9 kJ/mol; (21)Boiling Point: 252.4 °C at 760 mmHg; (22)Vapour Pressure: 0.00301 mmHg at 25°C.
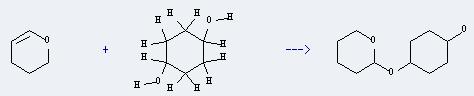
Uses of 1,4-Cyclohexanediol: It can react with 3,4-dihydro-2H-pyran to produce 4-(2-Tetrahydropyranyloxy)-1-cyclohexanol. This reaction will need reagent PPTS, and the solvent tetrahydrofuran. The reaction time is 6 hours with ambient temperature, and the yield is about 93%.
You can still convert the following datas into molecular structure:
(1)SMILES: OC1CCC(O)CC1
(2)InChI: InChI=1/C6H12O2/c7-5-1-2-6(8)4-3-5/h5-8H,1-4H2
(3)InChIKey: VKONPUDBRVKQLM-UHFFFAOYAF
Related Products
- 10,10'-Bis([1,1'-biphenyl]-4-yl)-9,9'-bianthracene
- 10,10'-Dibromo-9,9'-bianthryl
- 10,10-Dimethylanthrone
- 10,10-Oxybisphenoxarsine
- 10-[1,1'-Biphenyl]-4-yl-2-(1-methylethyl)-9-oxo-9H-thioxanthenium hexafluorophosphate
- 10,11-Dehydroimipramine
- 10,11-Dihydro-11-oxodibenzo[b,f][1,4]thiazepine
- 10,11-Dihydro-2-(4-methyl-1-piperazinyl)-11-(2-athiazolyl)-pyridazino(3,4-b)(1,4)benzoxazepine
- 10,11-DIHYDRO-2-(4-METHYL-1-PIPERAZINYL)-11-(3,4-XYLYL)PYRIDAZINO(3,4b)(1,4)-BENZOXAZEPINE MALEATE
- 10,11-Dihydro-5-(3-dimethylamino-2-methylpropyl)-5h-dibenz (b,f)azepine
- 556-50-3
- 55650-58-3
- 556-52-5
- 55652-76-1
- 5565-32-2
- 556-53-6
- 5565-36-6
- 55654-19-8
- 55656-88-7
- 556-56-9
Hot Products
About|Contact|Cas|Product Name|Molecular|Country|Encyclopedia
Message|New Cas|MSDS|Service|Advertisement|CAS DataBase|Article Data|Manufacturers | Chemical Catalog
©2008 LookChem.com,License: ICP
NO.:Zhejiang16009103
complaints:service@lookchem.com Desktop View