-
Name
Benzenepropanol, a,a-dimethyl-
- EINECS 203-074-4
- CAS No. 103-05-9
- Article Data77
- CAS DataBase
- Density 0.97 g/cm3
- Solubility 4.569g/L at 20℃
- Melting Point 31-33 °C(lit.)
- Formula C11H16O
- Boiling Point 213.286 °C at 760 mmHg
- Molecular Weight 164.247
- Flash Point 106.667 °C
- Transport Information
- Appearance
- Safety 26-36/37/39
- Risk Codes 41
-
Molecular Structure
-
Hazard Symbols
 Xi
Xi
- Synonyms 2-Butanol,2-methyl-4-phenyl- (6CI,7CI,8CI);1,1-Dimethyl-3-phenyl-1-propanol;1,1-Dimethyl-3-phenylpropanol;1,1-Dimethyl-3-phenylpropyl alcohol;1-Phenyl-3-methylbutan-3-ol;2-(2-Phenylethyl)-2-propanol;2-Hydroxy-2-methyl-4-phenylbutane;2-Methyl-4-phenyl-2-butanol;2-Phenethyl-2-propanol;4-Phenyl-2-hydroxy-2-methylbutane;4-Phenyl-2-methyl-2-butanol;Benzyl-tert-butanol;Dimethylphenethylcarbinol;NSC 62145;Phenylethyl dimethyl carbinol;a,a-Dimethylbenzenepropanol;
- PSA 20.23000
- LogP 2.39010
Synthetic route

-
-
103-05-9
4-phenyl-2-methyl-2-butanol

| Conditions | Yield |
|---|---|
| With 2,3-dicyano-5,6-dichloro-p-benzoquinone In dichloromethane; water at 0 - 20℃; for 1.5h; Reagent/catalyst; | 100% |

| Conditions | Yield |
|---|---|
| With ammonium cerium (IV) nitrate In water; acetone at 0 - 20℃; for 121h; Reagent/catalyst; | A 37% B 100% |

-
-
103-05-9
4-phenyl-2-methyl-2-butanol

| Conditions | Yield |
|---|---|
| Stage #1: 2-methyl-4-phenyl-2-butyl methoxymethyl ether With [2,2]bipyridinyl; trimethylsilyl trifluoromethanesulfonate In dichloromethane at 0℃; for 0.5h; Inert atmosphere; Stage #2: With water In diethyl ether; dichloromethane at 20℃; for 2h; Inert atmosphere; chemoselective reaction; | 99% |
-
-
57132-28-2, 25625-21-2
1,1-dimethyl-3-phenylprop-2-en-1-ol

-
-
103-05-9
4-phenyl-2-methyl-2-butanol

| Conditions | Yield |
|---|---|
| Stage #1: 1,1-dimethyl-3-phenylprop-2-en-1-ol With tetrahydropyrrolo[2,1-c][1,2,4]triazole carbene; diphenylsilane In N,N-dimethyl-formamide; mineral oil at 20℃; Inert atmosphere; Stage #2: With tetrabutyl ammonium fluoride In tetrahydrofuran; N,N-dimethyl-formamide; mineral oil at 20℃; for 0.5h; Inert atmosphere; chemoselective reaction; | 99% |

-
-
103-05-9
4-phenyl-2-methyl-2-butanol

| Conditions | Yield |
|---|---|
| With 2,3-dicyano-5,6-dichloro-p-benzoquinone In dichloromethane; water at 0 - 20℃; for 27h; Reagent/catalyst; Reflux; | 99% |
| With 2,3-dicyano-5,6-dichloro-p-benzoquinone In dichloromethane; water at 0 - 20℃; for 27h; | 99% |
-
-
1402544-07-3
2-methyl-4-phenylbutan-2-yl p-methoxybenzyl ether

-
-
103-05-9
4-phenyl-2-methyl-2-butanol

| Conditions | Yield |
|---|---|
| With water; sodium hydrogencarbonate; bis-[(trifluoroacetoxy)iodo]benzene; meso-2,5-bis(methoxycarbonyl)-2,5-dimethylpyrrolidine-1-oxyl In dichloromethane at 20℃; for 2h; chemoselective reaction; | 99% |
-
-
103-25-3
3-phenylpropanoic acid methyl ester

-
-
74-88-4
methyl iodide

-
-
103-05-9
4-phenyl-2-methyl-2-butanol

| Conditions | Yield |
|---|---|
| Stage #1: methyl iodide With magnesium; ethylene dibromide for 1h; Inert atmosphere; Reflux; Stage #2: 3-phenylpropanoic acid methyl ester at 20℃; for 1h; Inert atmosphere; | 94% |

-
-
103-05-9
4-phenyl-2-methyl-2-butanol

| Conditions | Yield |
|---|---|
| With zinc diacetate In methanol; dichloromethane at 38℃; for 30h; | 92% |
-
-
1296645-18-5
C15H24O3

-
-
103-05-9
4-phenyl-2-methyl-2-butanol

| Conditions | Yield |
|---|---|
| Stage #1: C15H24O3 With [2,2]bipyridinyl; trimethylsilyl trifluoromethanesulfonate In dichloromethane at 0℃; for 0.5h; Inert atmosphere; Stage #2: With water In diethyl ether; dichloromethane at 20℃; for 1h; Inert atmosphere; chemoselective reaction; | 91% |

-
-
103-05-9
4-phenyl-2-methyl-2-butanol

| Conditions | Yield |
|---|---|
| With 2,3-dicyano-5,6-dichloro-p-benzoquinone In aq. phosphate buffer; dichloromethane at 0 - 20℃; for 2h; pH=7; Solvent; Time; | 91% |
| With 2,3-dicyano-5,6-dichloro-p-benzoquinone In aq. phosphate buffer; dichloromethane at 0 - 20℃; for 2h; | 91% |
-
-
2021-28-5
ethyl dihydrocinnamate

-
-
676-58-4
methylmagnesium chloride

-
-
104-88-1
4-chlorobenzaldehyde

-
-
81036-80-8
lithium 2-(N-methyl-N-(2-pyridyl))-amide

-
-
103-05-9
4-phenyl-2-methyl-2-butanol

| Conditions | Yield |
|---|---|
| Product distribution; Multistep reaction; the efficiency of LMPA as in situ carbonyl protecting group of aldehydes were investigated beside carboxylic esters and nitriles against organometallic reagents or LAH; | 90% |

-
-
7065-28-3, 130284-36-5
2-methyl-2-phenethyloxirane

-
-
103-05-9
4-phenyl-2-methyl-2-butanol

| Conditions | Yield |
|---|---|
| With ammonium formate; palladium on activated charcoal In ethanol at 23℃; for 5h; | 90% |

-
-
103-05-9
4-phenyl-2-methyl-2-butanol

| Conditions | Yield |
|---|---|
| With methanol; scandium tris(trifluoromethanesulfonate) In acetonitrile at 20℃; for 2h; | 90% |

| Conditions | Yield |
|---|---|
| With strontium In tetrahydrofuran at 20℃; for 0.5h; Barbier reaction; | 90% |

-
-
103-05-9
4-phenyl-2-methyl-2-butanol

| Conditions | Yield |
|---|---|
| With trimethylaluminum In 1,2-dichloro-ethane; toluene at -30 - 65℃; for 18h; | 89% |
-
-
1296645-32-3
C19H24O2

-
-
103-05-9
4-phenyl-2-methyl-2-butanol

| Conditions | Yield |
|---|---|
| Stage #1: C19H24O2 With [2,2]bipyridinyl; trimethylsilyl trifluoromethanesulfonate In dichloromethane at 0℃; for 0.5h; Inert atmosphere; Stage #2: With water In dichloromethane at 20℃; for 1h; Inert atmosphere; chemoselective reaction; | 88% |
-
-
1296645-33-4
C17H30O2Si

-
-
103-05-9
4-phenyl-2-methyl-2-butanol

| Conditions | Yield |
|---|---|
| Stage #1: C17H30O2Si With [2,2]bipyridinyl; trimethylsilyl trifluoromethanesulfonate In dichloromethane at 0℃; for 0.5h; Inert atmosphere; Stage #2: With water In dichloromethane at 20℃; for 1h; Inert atmosphere; chemoselective reaction; | 87% |
-
-
2550-26-7
4-Phenyl-2-butanone

-
-
676-58-4
methylmagnesium chloride

-
-
103-05-9
4-phenyl-2-methyl-2-butanol

| Conditions | Yield |
|---|---|
| In tetrahydrofuran at 0 - 20℃; for 1h; Inert atmosphere; | 83% |
| In tetrahydrofuran at 0℃; for 0.166667h; Inert atmosphere; | 72% |
-
-
2021-28-5
ethyl dihydrocinnamate

-
-
75-16-1
methylmagnesium bromide

-
-
103-05-9
4-phenyl-2-methyl-2-butanol

| Conditions | Yield |
|---|---|
| In tetrahydrofuran at 0 - 20℃; for 3h; Inert atmosphere; | 83% |

| Conditions | Yield |
|---|---|
| 80% |
-
-
89881-71-0
fluoro(dimethyl)(2-methyl-4-phenylbutan-2-yl)silane

-
-
103-05-9
4-phenyl-2-methyl-2-butanol

| Conditions | Yield |
|---|---|
| With potassium fluoride; 3-chloro-benzenecarboperoxoic acid In N,N-dimethyl acetamide at 60℃; for 6h; | 75% |
| With tert-butyl 4-hydroxy-5-methylphenyl sulfide; 3-chloro-benzenecarboperoxoic acid In N,N-dimethyl acetamide at 60℃; | 62% |

| Conditions | Yield |
|---|---|
| In diethyl ether at 0 - 20℃; Inert atmosphere; | 74% |
-
-
558-30-5
2-methyl-1,2-epoxypropane

-
-
1589-82-8
phenylmagnesium bromide

-
-
103-05-9
4-phenyl-2-methyl-2-butanol

| Conditions | Yield |
|---|---|
| With copper(l) iodide In tetrahydrofuran; toluene at -10 - 0℃; for 3h; | 74% |

| Conditions | Yield |
|---|---|
| Stage #1: benzyl bromide With iodine; magnesium In tetrahydrofuran at 46 - 56℃; Inert atmosphere; Stage #2: 2-methyl-1,2-epoxypropane With copper(l) iodide In tetrahydrofuran at -10 - 0℃; for 2h; | 74% |

| Conditions | Yield |
|---|---|
| With potassium phosphate; tris-(dibenzylideneacetone)dipalladium(0); tetramethylammonium formiate; triphenylphosphine In water; N,N-dimethyl-formamide at 80℃; for 16h; Heck Reaction; regioselective reaction; | 74% |

| Conditions | Yield |
|---|---|
| With strontium In tetrahydrofuran at 20℃; for 1h; | 68% |
-
-
501-52-0
3-Phenylpropionic acid

-
-
74-88-4
methyl iodide

-
A
-
2550-26-7
4-Phenyl-2-butanone

-
B
-
103-05-9
4-phenyl-2-methyl-2-butanol

| Conditions | Yield |
|---|---|
| Stage #1: 3-Phenylpropionic acid With sodium hydride In tetrahydrofuran at 20℃; for 0.5h; Stage #2: methyl iodide With strontium In tetrahydrofuran at 20℃; for 0.75h; | A 64% B 5% |
| With strontium In tetrahydrofuran at 20℃; for 0.5h; | A 63% B 21% |


| Conditions | Yield |
|---|---|
| With hydrogen; copper-palladium; silica gel In ethanol at 25℃; under 760 Torr; Kinetics; | A n/a B 58% |

| Conditions | Yield |
|---|---|
| With methanol; oxo[hexa(trifluoroacetato)]tetrazinc for 96h; Reflux; Inert atmosphere; | 57% |
-
-
103-05-9
4-phenyl-2-methyl-2-butanol

-
-
1079-66-9
chloro-diphenylphosphine

-
-
820961-79-3
1,1-dimethyl-3-phenylpropyl diphenylphosphinite

| Conditions | Yield |
|---|---|
| With dmap; triethylamine In tetrahydrofuran at 20℃; for 2h; | 100% |
| With dmap; triethylamine In tetrahydrofuran at 20℃; for 2h; | 98% |
| With dmap; triethylamine In tetrahydrofuran at 20℃; for 2h; | 95% |
| With dmap; triethylamine In tetrahydrofuran at 20℃; for 2h; | 91% |
| With dmap; triethylamine In tetrahydrofuran at 20℃; for 2h; |

-
-
103-05-9
4-phenyl-2-methyl-2-butanol

| Conditions | Yield |
|---|---|
| With N-Methyldicyclohexylamine; copper diacetate In toluene at 20℃; for 3h; | 100% |
-
-
103-05-9
4-phenyl-2-methyl-2-butanol

-
-
52017-21-7
(3-bromo-3-methyl-n-butyl)benzene

| Conditions | Yield |
|---|---|
| With trimethylsilyl bromide In neat (no solvent) at 20℃; for 5h; Green chemistry; chemoselective reaction; | 99% |
| With hydrogen bromide; lithium bromide In water at 0 - 23℃; | 87% |
| With tetra-(n-butyl)ammonium iodide; ethylene dibromide; triphenylphosphine at 60℃; for 2h; Sealed tube; Inert atmosphere; | 80% |

| Conditions | Yield |
|---|---|
| With chloro-trimethyl-silane In neat (no solvent) at 70 - 75℃; for 24h; Green chemistry; chemoselective reaction; | 99% |
| With hydrogenchloride In water at 25℃; for 24h; | 93% |
| With indium(III) chloride; dimethylmonochlorosilane; benzil In dichloromethane at 20℃; for 12h; Inert atmosphere; | 92% |
-
-
103-05-9
4-phenyl-2-methyl-2-butanol

-
-
616-38-6
carbonic acid dimethyl ester

-
-
1400496-42-5
methyl (2-methyl-4-phenylbutan-2-yl) carbonate

| Conditions | Yield |
|---|---|
| With lanthanum (III) nitrate * water; 1,1,1-trioctyl-1-methylphosphonium methylcarbonate for 1h; Molecular sieve; Reflux; | 99% |

| Conditions | Yield |
|---|---|
| Stage #1: oxalyl dichloride; 4-phenyl-2-methyl-2-butanol In diethyl ether at 0 - 23℃; for 18h; Inert atmosphere; Stage #2: With water In diethyl ether at 0℃; | 99% |
| In diethyl ether at 0 - 20℃; | 92% |
| In diethyl ether at 0 - 20℃; for 18.1667h; Inert atmosphere; |

| Conditions | Yield |
|---|---|
| Stage #1: Dimethoxymethane With trifluorormethanesulfonic acid; acetyl chloride at 0 - 20℃; for 0.166667h; Stage #2: 4-phenyl-2-methyl-2-butanol With N-ethyl-N,N-diisopropylamine In dichloromethane for 0.5h; | 98% |

| Conditions | Yield |
|---|---|
| With N-ethyl-N,N-diisopropylamine; calcium chloride In dichloromethane at 20℃; for 32.5h; Solvent; Reagent/catalyst; Time; Inert atmosphere; | 96% |
| Stage #1: 4-phenyl-2-methyl-2-butanol With N-ethyl-N,N-diisopropylamine; calcium chloride In dichloromethane at 20℃; for 0.5h; Stage #2: 2-((chloromethoxy)methyl)naphthalene In dichloromethane at 20℃; for 32.5h; | 96% |

| Conditions | Yield |
|---|---|
| With N-ethyl-N,N-diisopropylamine In dichloromethane at 20℃; for 19h; Solvent; | 96% |
| With N-ethyl-N,N-diisopropylamine In dichloromethane at 20℃; for 19h; | 96% |
-
-
103-05-9
4-phenyl-2-methyl-2-butanol

-
-
75-05-8
acetonitrile

-
-
79399-13-6
N-[1,1-dimethyl-3-phenylpropyl]acetamide

| Conditions | Yield |
|---|---|
| With sulfuric acid at 0℃; for 0.5h; | 95% |
| With sulfuric acid for 3h; Cooling with ice; | |
| With sulfuric acid for 3h; Ritter Amidation; Cooling with ice; |

| Conditions | Yield |
|---|---|
| With dmap; triethylamine In tetrahydrofuran at 0 - 25℃; for 5h; Inert atmosphere; | 95% |
| With dmap; triethylamine In dichloromethane at 0 - 20℃; for 18h; | 79% |
-
-
103-05-9
4-phenyl-2-methyl-2-butanol

| Conditions | Yield |
|---|---|
| With dmap; triethylamine In tetrahydrofuran at 20℃; | 95% |
-
-
103-05-9
4-phenyl-2-methyl-2-butanol

-
-
74-88-4
methyl iodide

-
-
97231-89-5
3-methoxy-3-methyl-1-phenylbutane

| Conditions | Yield |
|---|---|
| With sodium hydride In tetrahydrofuran at 40℃; for 1.5h; | 94% |
-
-
103-05-9
4-phenyl-2-methyl-2-butanol

-
-
1402544-07-3
2-methyl-4-phenylbutan-2-yl p-methoxybenzyl ether

| Conditions | Yield |
|---|---|
| With (1S)-10-camphorsulfonic acid In tetrahydrofuran at 20℃; for 8h; Reflux; | 94% |
-
-
952057-61-3
N-phenyl-2,2,2-trifluoroacetimidoyl chloride

-
-
103-05-9
4-phenyl-2-methyl-2-butanol

| Conditions | Yield |
|---|---|
| With trimethylsilyl trifluoromethanesulfonate; 5 Angstroem MS In 1,4-dioxane at 20℃; for 12h; | 93% |
-
-
103-05-9
4-phenyl-2-methyl-2-butanol

-
-
19031-66-4
2-fluoro-2-methyl-4-phenylbutane

| Conditions | Yield |
|---|---|
| With 1,2-Diiodoethane; Selectfluor; 1,2-bis-(diphenylphosphino)ethane; zinc dibromide In acetonitrile at 20℃; for 0.25h; Reagent/catalyst; Sealed tube; Inert atmosphere; | 92% |
| With pyridine hydrogenfluoride In tetrahydrofuran at 0℃; for 3h; | 65% |
| With diethylamino-sulfur trifluoride In dichloromethane at -78 - 20℃; |

| Conditions | Yield |
|---|---|
| With N-ethyl-N,N-diisopropylamine In toluene at 20℃; for 12h; | 92% |
| With dmap; N-ethyl-N,N-diisopropylamine In dichloromethane at 0 - 20℃; Inert atmosphere; | |
| With dmap; N-ethyl-N,N-diisopropylamine In dichloromethane at 0 - 20℃; Inert atmosphere; | |
| With N-ethyl-N,N-diisopropylamine In toluene at 5 - 25℃; | |
| With N-ethyl-N,N-diisopropylamine In dichloromethane at 0 - 60℃; Inert atmosphere; Schlenk technique; | 1.2 g |

| Conditions | Yield |
|---|---|
| With dmap; N-ethyl-N,N-diisopropylamine In dichloromethane at 0 - 20℃; Inert atmosphere; | 91% |
-
-
10439-23-3
4-methylbenzenesulfinyl chloride

-
-
103-05-9
4-phenyl-2-methyl-2-butanol

-
-
79399-25-0
2-methyl-4-phenylbut-2-yl toluenesulphinate

| Conditions | Yield |
|---|---|
| With pyridine In diethyl ether at 0℃; for 3h; | 90% |
2-Methyl-4-phenylbutan-2-ol Chemical Properties
IUPAC Name: 2-Methyl-4-phenylbutan-2-ol
Synonyms: 1,1-Dimethyl-3-phenylpropanol ; 1,1-Dimethyl-3-phenylpropyl alcohol ; 1-Propanol, 1,1-dimethyl-3-phenyl-;2-(2-Phenylethyl)-2-propanol ; 2-Butanol, 2-methyl-4-phenyl- ; 2-Methyl-4-phenyl-2-butano ; 2-Methyl-4-phenylbutan ; 2-Methyl-4-phenyl-butan-2-ol
Product Categories: Alcohols;C9 to C30;Oxygen Compounds
CAS NO: 103-05-9
Molecular Formula of Benzyl-tert-butanol (CAS NO.103-05-9) : C11H16O
Molecular Weight of Benzyl-tert-butanol (CAS NO.103-05-9) : 164.24
Molecular Structure of Benzyl-tert-butanol (CAS NO.103-05-9) :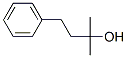
EINECS: 203-074-4
Mol File: 103-05-9.mol
FEMA : 3629
Index of Refraction: 1.516
Surface Tension: 36.1 dyne/cm
Density: 0.969 g/cm3
Flash Point: 106.7 °C
Enthalpy of Vaporization: 47.53 kJ/mol
Boiling Point: 213.3 °C at 760 mmHg
Vapour Pressure: 0.0977 mmHg at 25°C
Melting point: 31-33 °C(lit.)
2-Methyl-4-phenylbutan-2-ol Toxicity Data With Reference
| 1. | orl-rat LD50:2200 mg/kg | FCTXAV Food and Cosmetics Toxicology. 12 (1974),517. | ||
| 2. | skn-rbt LD50:3500 mg/kg | FCTXAV Food and Cosmetics Toxicology. 12 (1974),517. |
2-Methyl-4-phenylbutan-2-ol Consensus Reports
Reported in EPA TSCA Inventory.
2-Methyl-4-phenylbutan-2-ol Safety Profile
Moderately toxic by ingestion and skin contact. When heated to decomposition it emits acrid smoke and irritating fumes.
Hazard Codes Xi
Xi
Risk Statements 41
R41:Risk of serious damage to the eyes.
Safety Statements 26-36/37/39
S26: In case of contact with eyes, rinse immediately with plenty of water and seek medical advice.
S36/37/39:Wear suitable protective clothing, gloves and eye/face protection.
WGK Germany 2
RTECS EL5785000
2-Methyl-4-phenylbutan-2-ol Specification
1. Appearance: Benzyl-tert-butanol (CAS NO.103-05-9) is colorless or pale yellow liquid.
2. Odor: floral, green fragrance, Kusaka.
Related Products
- 2-Methyl-4-phenylbutan-2-ol
- 103-06-0
- 103060-53-3
- 1030613-07-0
- 103065-19-6
- 103068-18-4
- 103068-20-8
- 103068-40-2
- 103068-41-3
- 103068-64-0
- 103069-50-7
Hot Products
About|Contact|Cas|Product Name|Molecular|Country|Encyclopedia
Message|New Cas|MSDS|Service|Advertisement|CAS DataBase|Article Data|Manufacturers | Chemical Catalog
©2008 LookChem.com,License: ICP
NO.:Zhejiang16009103
complaints:service@lookchem.com Desktop View