-
Name
4-Bromoisoquinoline
- EINECS 216-244-8
- CAS No. 1532-97-4
- Article Data32
- CAS DataBase
- Density 1.564 g/cm3
- Solubility
- Melting Point 40-43 °C(lit.)
- Formula C9H6BrN
- Boiling Point 282.5 °C at 760 mmHg
- Molecular Weight 208.057
- Flash Point 133.3 °C
- Transport Information
- Appearance pale yellow solid
- Safety 26-37/39-36/37/39-22-36
- Risk Codes 36/37/38-21/22-20/21/22
-
Molecular Structure
-
Hazard Symbols
 Xi,
Xi, Xn
Xn
- Synonyms 4-Isoquinolinyl bromide;NSC 56333;4-Bromo isoquinoline;
- PSA 12.89000
- LogP 2.99730
Synthetic route
-
-
126798-69-4
2-(2-Benzenesulfonyl-4-bromo-1,2-dihydro-isoquinolin-1-yl)-malonic acid diethyl ester

-
-
1532-97-4
4-bromoisoquinoline

| Conditions | Yield |
|---|---|
| With hydrogenchloride; acetic acid for 0.25h; Heating; | 94% |
-
-
126798-68-3
2-(2-Benzoyl-4-bromo-1,2-dihydro-isoquinolin-1-yl)-malonic acid diethyl ester

-
-
1532-97-4
4-bromoisoquinoline

| Conditions | Yield |
|---|---|
| With hydrogenchloride; acetic acid for 0.25h; Heating; | 92% |

| Conditions | Yield |
|---|---|
| With acetic acid for 0.25h; Heating; | A 70% B 92% |

| Conditions | Yield |
|---|---|
| With sodium hydroxide; Bakers'yeast In ethanol; water for 3h; Heating; | 86% |

| Conditions | Yield |
|---|---|
| With [bis(acetoxy)iodo]benzene; potassium bromide In 1,2-dichloro-ethane at 50℃; for 14h; regioselective reaction; | 70% |
| With N-Bromosuccinimide; acetic acid at 100℃; | 23.8% |
| With bromine Erhitzen des Additionsprodukts auf 180-200grad; |
-
-
126824-87-1
[4-Bromo-1-(2,4-dinitro-benzyl)-1H-isoquinolin-2-yl]-phenyl-methanone

-
-
1532-97-4
4-bromoisoquinoline

| Conditions | Yield |
|---|---|
| With sodium hydroxide In ethanol for 3h; Heating; | 62% |
-
-
126798-67-2
2-(2-Benzoyl-4-bromo-1,2-dihydro-isoquinolin-1-yl)-2-bromo-1-phenyl-ethanone

-
-
1532-97-4
4-bromoisoquinoline

| Conditions | Yield |
|---|---|
| With hydrogenchloride; acetic acid for 2h; Heating; | 60% |
-
-
100-52-7
benzaldehyde

-
-
53600-04-7
2-benzoyl-4-bromo-1,2-dihydro-isoquinoline-1-carbonitrile

-
A
-
1532-97-4
4-bromoisoquinoline

-
B
-
117908-30-2
Benzoic acid (4-bromo-isoquinolin-1-yl)-phenyl-methyl ester

| Conditions | Yield |
|---|---|
| With sodium hydride In N,N-dimethyl-formamide for 0.25h; | A 17% B 51% |

-
-
108133-31-9
N-benzoyl-4-bromo-1-(2',4',6'-trinitrobenzyl)-1,2-dihydroisoquinoline

-
-
1532-97-4
4-bromoisoquinoline

| Conditions | Yield |
|---|---|
| With hydrogenchloride; acetic acid for 10h; Heating; | 20% |
-
-
21364-46-5
isoquinoline hydrochloride

-
-
1532-97-4
4-bromoisoquinoline

| Conditions | Yield |
|---|---|
| With bromine at 180℃; im Rohr; |
-
-
87777-05-7
4-bromo-2-xylosylisoquinolinium bromide

-
A
-
1532-97-4
4-bromoisoquinoline

-
B
-
87-72-9, 608-45-7, 608-46-8, 608-47-9, 2460-44-8, 6748-95-4, 6763-34-4, 7261-26-9, 7283-06-9, 7283-07-0, 7296-55-1, 7296-56-2, 7296-58-4, 7296-59-5, 7296-60-8, 7296-61-9, 7296-62-0, 7322-30-7, 10257-31-5, 10257-32-6, 10257-33-7, 10257-34-8, 10257-35-9, 19982-83-3, 20242-88-0, 28697-53-2, 36562-42-2, 41546-41-2, 89299-64-9, 107655-34-5, 115794-06-4, 115794-07-5, 130550-15-1, 130606-21-2
D-Xylose

| Conditions | Yield |
|---|---|
| With ethylenediaminetetraacetic acid; sodium phosphate buffer at 25℃; Rate constant; Bacillus pumilus β-D-xylosidase; |
-
-
75705-26-9
4-bromo-2-(β-D-glucopyranosyl)isoquinolinium bromide

-
A
-
1532-97-4
4-bromoisoquinoline

-
B
-
2280-44-6
D-Glucose

| Conditions | Yield |
|---|---|
| With sodium phosphate buffer at 25℃; Rate constant; (1->3)-β-D-glucanase of Sporotrichum dimorphosporum; |
-
-
118798-87-1
N-(2-Cyanoethyl)-4-bromoisoquinolinium cation

-
A
-
1532-97-4
4-bromoisoquinoline

-
B
-
107-13-1
acrylonitrile

| Conditions | Yield |
|---|---|
| In water at 25℃; Rate constant; ionic strength 0.1, KOH+KCl solutions (pH 10.7-13); |

-
A
-
1532-97-4
4-bromoisoquinoline

| Conditions | Yield |
|---|---|
| With water at 65℃; Rate constant; Mechanism; solvent deuterium kinetic isotope effect; acetate buffer pH=4.41, also with added var. sodium salts, other pH; |

-
A
-
1532-97-4
4-bromoisoquinoline

-
B
-
13299-15-5
2-deoxy-D-glucose

-
C
-
13299-16-6
β-D-2-deoxy-glucopyranose

| Conditions | Yield |
|---|---|
| With phosphate buffer; sodium perchlorate at 65℃; Rate constant; other pyridinium and isoquinolinium derivatives; other anions; |


-
-
1532-97-4
4-bromoisoquinoline

| Conditions | Yield |
|---|---|
| With bromine |

| Conditions | Yield |
|---|---|
| With 4-methyl-morpholine; water at 65℃; Kinetics; Hydrolysis; |

| Conditions | Yield |
|---|---|
| With 4-methyl-morpholine; water at 65℃; Kinetics; Hydrolysis; |
-
-
34950-15-7
2-benzoyl-1-(2-oxo-2-phenyl-ethyl)-1,2-dihydro-isoquinoline

-
-
1532-97-4
4-bromoisoquinoline

| Conditions | Yield |
|---|---|
| Multi-step reaction with 2 steps 1: 52 percent / Br2 / CHCl3 / 20 - 30 °C 2: 60 percent / glacial acetic acid, concd. hydrochloric acid / 2 h / Heating View Scheme |
-
-
37009-02-2
(2-benzoyl-1,2-dihydro-isoquinolin-1-yl)-malonic acid diethyl ester

-
-
1532-97-4
4-bromoisoquinoline

| Conditions | Yield |
|---|---|
| Multi-step reaction with 2 steps 1: 60 percent / Br2 / CHCl3 / 20 - 30 °C 2: 92 percent / glacial acetic acid, concd. hydrochloric acid / 0.25 h / Heating View Scheme |
-
-
94170-02-2
[1-(2,4-Dinitro-benzyl)-1H-isoquinolin-2-yl]-phenyl-methanone

-
-
1532-97-4
4-bromoisoquinoline

| Conditions | Yield |
|---|---|
| Multi-step reaction with 2 steps 1: 94 percent / Br2 / CHCl3 / 20 - 30 °C 2: 62 percent / aq. NaOH / ethanol / 3 h / Heating View Scheme |
-
-
126798-64-9
N-benzenesulfonyl-1-di(ethoxycarbonyl)methyl-1,2-dihydroisoquinoline

-
-
1532-97-4
4-bromoisoquinoline

| Conditions | Yield |
|---|---|
| Multi-step reaction with 2 steps 1: 85 percent / Br2 / CHCl3 / 20 - 30 °C 2: 94 percent / glacial acetic acid, concd. hydrochloric acid / 0.25 h / Heating View Scheme |
-
-
94169-93-4
2-benzoyl-1-(2',4',6'-trinitrobenzyl)-1,2-dihydroisoquinoline

-
-
1532-97-4
4-bromoisoquinoline

| Conditions | Yield |
|---|---|
| Multi-step reaction with 2 steps 1: Br2 / CHCl3 / 20 - 30 °C 2: 20 percent / glacial acetic acid, concd. hydrochloric acid / 10 h / Heating View Scheme |
-
-
1532-97-4
4-bromoisoquinoline

-
-
64-17-5
ethanol

-
-
201230-82-2
carbon monoxide

-
-
50741-47-4
isoquinoline-4-carboxylic acid ethyl ester

| Conditions | Yield |
|---|---|
| With triethylamine; bis-triphenylphosphine-palladium(II) chloride at 100℃; under 5171.5 Torr; | 100% |
| With palladium diacetate; triethylamine; 4,5-bis(diphenylphos4,5-bis(diphenylphosphino)-9,9-dimethylxanthenephino)-9,9-dimethylxanthene for 12h; | 98% |

-
-
1532-97-4
4-bromoisoquinoline

-
-
31469-15-5
1-methoxy-2-methyl-1-trimethylsiloxy-1-propene

-
-
541-41-3
chloroformic acid ethyl ester

| Conditions | Yield |
|---|---|
| In dichloromethane at 0℃; for 0.5h; | 100% |

-
-
1532-97-4
4-bromoisoquinoline

-
-
34880-70-1
[1-methoxyprop-1-enyloxy]trimethylsilane

-
-
541-41-3
chloroformic acid ethyl ester

| Conditions | Yield |
|---|---|
| In dichloromethane at 0℃; for 0.5h; | 100% |

-
-
1532-97-4
4-bromoisoquinoline

-
-
541-41-3
chloroformic acid ethyl ester

-
-
19980-43-9
1-(trimethylsilyloxy)cyclopentene

| Conditions | Yield |
|---|---|
| In dichloromethane at 0℃; for 0.5h; | 100% |

-
-
1532-97-4
4-bromoisoquinoline

-
-
74-88-4
methyl iodide

-
-
54931-73-6
4-bromo-2-methylisoquinolin-2-ium iodide

| Conditions | Yield |
|---|---|
| In isopropyl alcohol at 90℃; for 12h; Inert atmosphere; | 99% |
| In acetonitrile for 14h; Reflux; | 88% |

| Conditions | Yield |
|---|---|
| With 3-chloro-benzenecarboperoxoic acid In dichloromethane at 0 - 20℃; for 76h; | 99% |
| With 3-chloro-benzenecarboperoxoic acid In chloroform | 94% |
| With 3-chloro-benzenecarboperoxoic acid In chloroform | 94% |

| Conditions | Yield |
|---|---|
| With 2F6P(1-)*C36H26N8Pd2(2+); potassium carbonate In ethanol at 100℃; for 24h; Suzuki Coupling; | 99% |
| With tetrakis(triphenylphosphine) palladium(0); sodium carbonate In ethanol; benzene for 12h; Heating; | 95.6% |
| With dichloro[N-hydroxy-1-(1-methyl-1H-benzimidazol-2-yl-κN3)ethanamine-κN]palladium; tetrabutylammomium bromide; potassium hydroxide In water at 160℃; for 0.15h; Suzuki coupling; Microwave irradiation; | 90% |
-
-
1532-97-4
4-bromoisoquinoline

-
-
1833-53-0
2-(Trimethylsilyloxy)propene

-
-
541-41-3
chloroformic acid ethyl ester

| Conditions | Yield |
|---|---|
| In dichloromethane at 0℃; for 0.5h; | 99% |

-
-
1532-97-4
4-bromoisoquinoline

-
-
31469-15-5
1-methoxy-2-methyl-1-trimethylsiloxy-1-propene

-
-
98-88-4
benzoyl chloride

| Conditions | Yield |
|---|---|
| In dichloromethane at 0℃; for 0.5h; | 99% |

-
-
1532-97-4
4-bromoisoquinoline

-
-
31469-15-5
1-methoxy-2-methyl-1-trimethylsiloxy-1-propene

-
-
1885-14-9
phenyl chloroformate

| Conditions | Yield |
|---|---|
| In dichloromethane at 0℃; for 0.5h; | 99% |

-
-
1532-97-4
4-bromoisoquinoline

-
-
33966-50-6
SEC-BUTYLAMINE

-
-
1056627-86-1
N-sec-butyl-4-amino-isoquinoline

| Conditions | Yield |
|---|---|
| With [(CyPF-tBu)PdCl2]; sodium t-butanolate In 1,2-dimethoxyethane at 100℃; for 24h; Inert atmosphere; Sealed vial; | 99% |
-
-
183158-33-0
acenaphthene-5-boronic acid

-
-
1532-97-4
4-bromoisoquinoline

-
-
1092384-66-1
4-(1,2-dihydroacenaphthylen-5-yl)isoquinoline

| Conditions | Yield |
|---|---|
| With dichloro[1,1'-bis(di-t-butylphosphino)ferrocene]palladium(II); triethylamine In water at 40℃; for 4h; Suzuki-Miyaura coupling; Inert atmosphere; | 99% |
-
-
1532-97-4
4-bromoisoquinoline

-
-
109674-45-5
4-methyl-2,6-dioxo-8-phenylhexahydro-[1,3,2]oxazaborolo[2,3-b][1,3,2]oxazaborol-4-ium-8-uide

-
-
19571-30-3
4-phenylisoquinoline

| Conditions | Yield |
|---|---|
| With potassium phosphate; [Pd(η(5)-C5H5)Fe(η(5)-C5H3-C(CH3)=N-C6H4-4-CH3)Cl(P(C6H5)3)] In ethanol; water at 90℃; for 12h; Suzuki-Miyaura Coupling; Inert atmosphere; Schlenk technique; Green chemistry; | 99% |

| Conditions | Yield |
|---|---|
| With potassium phosphate; (DPEPhos)Ni(mesityl)Br; water In 1,4-dioxane; benzene at 25℃; for 16h; Suzuki-Miyaura Coupling; Inert atmosphere; Sealed tube; | 99% |

| Conditions | Yield |
|---|---|
| Stage #1: 3-Sulfolene With o-phenylenebis(diphenylphosphine); potassium methanolate; palladium diacetate; potassium carbonate In tetrahydrofuran at 20℃; for 0.0833333h; Sealed tube; Inert atmosphere; Stage #2: 4-bromoisoquinoline In tetrahydrofuran at 20 - 110℃; for 17.3333h; Sealed tube; stereoselective reaction; | 99% |

| Conditions | Yield |
|---|---|
| Stage #1: 4-bromoisoquinoline; phenyl chloroformate With trimethylamine-borane In acetonitrile for 0.0833333h; Cooling; Stage #2: With trimethylamine-borane In acetonitrile at 20℃; for 0.166667h; Cooling; regioselective reaction; | 99% |
-
-
1532-97-4
4-bromoisoquinoline

-
-
23687-25-4
4-aminoisoquinoline

| Conditions | Yield |
|---|---|
| With ammonium hydroxide; copper(II) sulfate In water at 165 - 170℃; for 24h; | 98% |
| With lithium amide; (CyPF-t-Bu)PdCl2 In 1,2-dimethoxyethane at 80℃; for 20h; | 82% |
| With bis(1,5-cyclooctadiene)nickel (0); (R)-1-[(Sp)-2-(dicyclohexylphosphanyl)ferrocenyl]ethyldicyclohexylphosphane; ammonia; lithium tert-butoxide In 1,4-dioxane; toluene at 110℃; for 16h; Sealed tube; | 82% |

| Conditions | Yield |
|---|---|
| In dichloromethane at 0℃; | 98% |

-
-
1532-97-4
4-bromoisoquinoline

-
-
146140-95-6
2-pivaloylaminophenyl boronic acid

| Conditions | Yield |
|---|---|
| With tetrakis(triphenylphosphine) palladium(0); sodium carbonate In 1,2-dimethoxyethane; water at 110℃; for 2h; Suzuki coupling; Inert atmosphere; | 98% |

| Conditions | Yield |
|---|---|
| With {N,N-(1,2-diphenylethane-1,2-diylidene)bis(2-benzhydryl-4,6-dimthylaniline)}dichloropalladium; potassium carbonate; Trimethylacetic acid In N,N-dimethyl acetamide at 130℃; for 12h; Catalytic behavior; | 98% |
| With C40H36N2O2Pd(2+)*2C2H4O2; potassium carbonate; Trimethylacetic acid In N,N-dimethyl acetamide at 100℃; for 24h; Inert atmosphere; Schlenk technique; regioselective reaction; | 85% |

| Conditions | Yield |
|---|---|
| With {N,N-(1,2-diphenylethane-1,2-diylidene)bis(2-benzhydryl-4,6-dimthylaniline)}dichloropalladium; potassium carbonate; Trimethylacetic acid In N,N-dimethyl acetamide at 130℃; for 12h; Catalytic behavior; | 98% |
-
-
1532-97-4
4-bromoisoquinoline

-
-
24850-33-7
allyltributylstanane

-
-
79-22-1
methyl chloroformate

-
-
115119-31-8
1-allyl-4-bromo-2-(methoxycarbonyl)-1,2-dihydroisoquinoline

| Conditions | Yield |
|---|---|
| In dichloromethane | 97% |


| Conditions | Yield |
|---|---|
| With copper(l) iodide; potassium carbonate; bis(η3-allyl-μ-chloropalladium(II)); (1RS,2RS,3SR,4SR)-1,2,3,4-tetrakis((diphenylphosphanyl)methyl)cyclopentane In N,N-dimethyl-formamide at 100℃; for 20h; | 97% |
-
-
1532-97-4
4-bromoisoquinoline

-
-
127972-02-5
5-formyl-2-methoxyphenyl boronic acid

-
-
1254163-65-9
3-isoquinolin-4-yl-4-methoxy-benzaldehyde

| Conditions | Yield |
|---|---|
| With potassium phosphate; tetrakis(triphenylphosphine) palladium(0) In 1,4-dioxane at 120℃; for 10h; | 97% |
-
-
1532-97-4
4-bromoisoquinoline

-
-
126213-50-1
3,4-(ethylenedioxy)thiophene

| Conditions | Yield |
|---|---|
| With {N,N-(1,2-diphenylethane-1,2-diylidene)bis(2-benzhydryl-4,6-dimthylaniline)}dichloropalladium; potassium carbonate; Trimethylacetic acid In N,N-dimethyl acetamide at 130℃; for 12h; Catalytic behavior; | 97% |

| Conditions | Yield |
|---|---|
| With {N,N-(1,2-diphenylethane-1,2-diylidene)bis(2-benzhydryl-4,6-dimthylaniline)}dichloropalladium; potassium carbonate; Trimethylacetic acid In N,N-dimethyl acetamide at 130℃; for 12h; Catalytic behavior; | 97% |
| With [Pd(IPr*IMe)An(3-Cl-pyridinyl)Cl2]; potassium carbonate; Trimethylacetic acid In N,N-dimethyl acetamide at 130℃; for 12h; regioselective reaction; | 46% |

| Conditions | Yield |
|---|---|
| With {N,N-(1,2-diphenylethane-1,2-diylidene)bis(2-benzhydryl-4,6-dimthylaniline)}dichloropalladium; potassium carbonate; Trimethylacetic acid In N,N-dimethyl acetamide at 130℃; for 12h; Catalytic behavior; | 97% |
| With C82H77Cl2N3O2Pd; potassium carbonate; Trimethylacetic acid In N,N-dimethyl acetamide at 130℃; for 4h; | 94% |
| With C34H48Cl2N2Pd; potassium carbonate; Trimethylacetic acid In N,N-dimethyl acetamide at 100℃; for 8h; | 72% |

| Conditions | Yield |
|---|---|
| With C82H77Cl2N3O2Pd; potassium carbonate; Trimethylacetic acid In N,N-dimethyl acetamide at 130℃; for 4h; | 97% |
-
-
1532-97-4
4-bromoisoquinoline

-
-
17573-94-3
4-ethynyl-N,N-dimethylaniline

| Conditions | Yield |
|---|---|
| With copper(l) iodide; TEA; bis-triphenylphosphine-palladium(II) chloride In tetrahydrofuran for 24h; Heating; | 96% |
-
-
1532-97-4
4-bromoisoquinoline

-
-
94839-07-3
3,4-(methylenedioxy)-benzeneboronic acid

| Conditions | Yield |
|---|---|
| With potassium hydroxide; 2-pyridinealdoxime-based Pd(II)-complex on glass; tetrabutylammomium bromide; Polymer In water at 100℃; for 7h; Suzuki-Miyaura coupling; | 96% |
4-Bromoisoquinoline Chemical Properties
IUPAC Name: 4-Bromoisoquinoline
Molecular Formula: C9H6BrN
Molecular Weight: 208.06g/mol
EINECS: 216-244-8
Melting Point: 40 - 43 °C
Polar Surface Area: 12.89 Å2
Index of Refraction: 1.673
Molar Refractivity: 49.87 cm3
Molar Volume: 132.9 cm3
Polarizability: 19.77 ×10-24cm3
Surface Tension: 51.2 dyne/cm
Density: 1.564 g/cm3
Flash Point: 133.3 °C
Enthalpy of Vaporization: 50.04 kJ/mol
Boiling Point: 282.5 °C at 760 mmHg
Vapour Pressure: 0.00571 mmHg at 25°C
The Cas Register Number of 4-Bromoisoquinoline is 1532-97-4 .The chemical synonyms of 4-Bromoisoquinoline (CAS No.1532-97-4) are 4-Bromoisoquinoline ; Labotest-bb lt01143353 ; 4-Bromoisoqunoline ; 4-Bromoisquinoline;4-Isoquinolinyl bromide ; Nsc 56333 ; 4-Bromoisoquinoline ,98% .Product categories of 4-Bromoisoquinoline (CAS No.1532-97-4) are Halides ; Heterocycles ; Quinolines, quinazolines and derivatives ; Quinolines, isoquinolines & Quinoxalines ; Quinoline&isoquinoline ; Isoquinoline derivertives ; Aromatics compounds ; Aromatics ; Inhibitors ; Quinolines, isoquinolines & Quinoxalines ; Halogenated heterocycles ; Heterocyclic building blocks ; Isoquinolines ; Isoquinolinesbuilding blocks ; Isoquinoline ; Organohalides .The molecular structure of 4-Bromoisoquinoline (CAS No.1532-97-4) is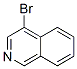 .
.
4-Bromoisoquinoline Uses
It can be used in organic synthesis.
4-Bromoisoquinoline Safety Profile
Hazard Codes:  Xi
Xi  Xn
Xn
Risk Statements: 36/37/38-21/22-20/21/22
R36/37/38: Irritating to eyes, respiratory system and skin.
R21/22: Harmful in contact with skin and if swallowed.
R20/21/22: Harmful by inhalation, in contact with skin and if swallowed.
Safety Statements: 26-37/39-36/37/39-22-36
S26: In case of contact with eyes, rinse immediately with plenty of water and seek medical advice.
S37/39: Wear suitable gloves and eye/face protection.
S36/37/39: Wear suitable protective clothing, gloves and eye/face protection.
S22: Do not breathe dust. S36:Wear suitable protective clothing.
WGK Germany: 3
About|Contact|Cas|Product Name|Molecular|Country|Encyclopedia
Message|New Cas|MSDS|Service|Advertisement|CAS DataBase|Article Data|Manufacturers | Chemical Catalog
©2008 LookChem.com,License: ICP
NO.:Zhejiang16009103
complaints:service@lookchem.com Desktop View