-
Name
5-Bromo-2-chloronicotinic acid
- EINECS
- CAS No. 29241-65-4
- Article Data13
- CAS DataBase
- Density 1.917 g/cm3
- Solubility
- Melting Point 173-176 °C
- Formula C6H3BrClNO2
- Boiling Point 345.1 °C at 760 mmHg
- Molecular Weight 236.452
- Flash Point 162.5 °C
- Transport Information
- Appearance
- Safety 26-36/37/39
- Risk Codes 36/37/38
-
Molecular Structure
-
Hazard Symbols
 Xi
Xi
- Synonyms Nicotinicacid, 5-bromo-2-chloro- (8CI);2-Chloro-5-bromonicotinic acid;
- PSA 50.19000
- LogP 2.19570
Synthetic route
-
-
104612-36-4
5-bromo-2-hydroxynicotinic acid

-
-
29241-65-4
5-bromo-2-chloronicotinic acid

| Conditions | Yield |
|---|---|
| With thionyl chloride In N,N-dimethyl-formamide at 70℃; for 4h; | 99% |
| Stage #1: 5-bromo-2-hydroxynicotinic acid With thionyl chloride In N,N-dimethyl-formamide at 70℃; for 4h; Stage #2: With water In N,N-dimethyl-formamide for 1h; | 99% |
| With thionyl chloride; N,N-dimethyl-formamide | 85% |
-
-
609-71-2
2-hydroxy-3-carboxypyridine

-
-
29241-65-4
5-bromo-2-chloronicotinic acid

| Conditions | Yield |
|---|---|
| Multi-step reaction with 2 steps 1: 78 percent / Br2; aq. NaOH / 18 h / 0 - 50 °C 2: 99 percent / thionyl chloride / dimethylformamide / 4 h / 70 °C View Scheme | |
| Multi-step reaction with 2 steps 1: 63.5 percent / aq. conc. NaOH, aq. NaOBr / 48 h / Ambient temperature 2: 37 percent / SOCl2 / dimethylformamide / 0.5 h / Heating View Scheme | |
| Multi-step reaction with 2 steps 1: 71 percent / Br2, NaOH / H2O / 90 h / 50 °C 2: 82 percent / SO2Cl2 / dimethylformamide / 2 h / Heating View Scheme |
-
-
2942-59-8
2-chloronicotinic acid

-
-
29241-65-4
5-bromo-2-chloronicotinic acid

| Conditions | Yield |
|---|---|
| With bromine In acetic acid |
-
-
67-56-1
methanol

-
-
29241-65-4
5-bromo-2-chloronicotinic acid

-
-
78686-79-0
methyl 2-chloro-5-bromonicotinate

| Conditions | Yield |
|---|---|
| Stage #1: methanol; 5-bromo-2-chloronicotinic acid With sulfuric acid at 60℃; for 36h; Stage #2: With sodium hydrogencarbonate In water | 100% |
| Stage #1: 5-bromo-2-chloronicotinic acid With thionyl chloride at 70℃; Stage #2: methanol In benzene at 65℃; | 95% |
| With sulfuric acid for 15h; Reflux; | 89% |
-
-
29241-65-4
5-bromo-2-chloronicotinic acid

-
-
75-65-0
tert-butyl alcohol

| Conditions | Yield |
|---|---|
| With sulfuric acid; magnesium sulfate In dichloromethane at 25℃; for 16h; | 94% |
-
-
46049-49-4
(S)-(2,3-dihydrobenzo[b][1,4]dioxin-2-yl)methanamine

-
-
29241-65-4
5-bromo-2-chloronicotinic acid

| Conditions | Yield |
|---|---|
| With (benzotriazo-1-yloxy)tris(dimethylamino)phosphonium hexafluorophosphate; N-ethyl-N,N-diisopropylamine In N,N-dimethyl-formamide at 0 - 20℃; for 1h; | 93% |
| With (benzotriazo-1-yloxy)tris(dimethylamino)phosphonium hexafluorophosphate; N-ethyl-N,N-diisopropylamine In N,N-dimethyl-formamide |
-
-
29241-65-4
5-bromo-2-chloronicotinic acid

-
-
1117-97-1
N,0-dimethylhydroxylamine

-
-
885223-63-2
5-bromo-2-chloro-N-methoxy-N-methylnicotinamide

| Conditions | Yield |
|---|---|
| Stage #1: 5-bromo-2-chloronicotinic acid With 1,1'-carbonyldiimidazole In dichloromethane at 20℃; for 1h; Stage #2: N,0-dimethylhydroxylamine In dichloromethane at 20℃; | 90% |
| With 1,1'-carbonyldiimidazole In N,N-dimethyl-formamide at 30℃; for 0.5h; | 82% |
-
-
811-98-3
d(4)-methanol

-
-
29241-65-4
5-bromo-2-chloronicotinic acid

| Conditions | Yield |
|---|---|
| With sulfuric acid In water at 60℃; for 48h; | 90% |
-
-
29241-65-4
5-bromo-2-chloronicotinic acid

-
-
150-76-5
4-methoxy-phenol

-
-
1215864-79-1
5-bromo-2-(4-methoxyphenoxy)nicotinic acid

| Conditions | Yield |
|---|---|
| Stage #1: 4-methoxy-phenol With sodium hydride In N,N-dimethyl-formamide; mineral oil at 0℃; for 0.233333h; Inert atmosphere; Stage #2: 5-bromo-2-chloronicotinic acid In N,N-dimethyl-formamide; mineral oil at 140℃; for 1h; Inert atmosphere; | 83% |
| Stage #1: 4-methoxy-phenol With sodium hydride In N,N-dimethyl-formamide; mineral oil at 0℃; for 0.1h; Stage #2: 5-bromo-2-chloronicotinic acid In N,N-dimethyl-formamide; mineral oil at 20 - 140℃; for 1.16667h; |

| Conditions | Yield |
|---|---|
| Stage #1: 5-bromo-2-chloronicotinic acid With oxalyl dichloride; N,N-dimethyl-formamide In dichloromethane at 20℃; for 3h; Stage #2: ammonia In methanol at -10 - 20℃; for 18h; | 82% |
-
-
6638-79-5
N,O-dimethylhydroxylamine*hydrochloride

-
-
29241-65-4
5-bromo-2-chloronicotinic acid

-
-
885223-63-2
5-bromo-2-chloro-N-methoxy-N-methylnicotinamide

| Conditions | Yield |
|---|---|
| Stage #1: 5-bromo-2-chloronicotinic acid With thionyl chloride at 80℃; for 2h; Stage #2: N,O-dimethylhydroxylamine*hydrochloride With triethylamine In dichloromethane at 0℃; for 1h; | 81% |
| With dmap; 1-ethyl-(3-(3-dimethylamino)propyl)-carbodiimide hydrochloride; N-ethyl-N,N-diisopropylamine In dichloromethane at 0 - 20℃; | 79% |
| With dmap; 1-ethyl-(3-(3-dimethylamino)propyl)-carbodiimide hydrochloride; N-ethyl-N,N-diisopropylamine In dichloromethane at 0 - 20℃; | 79% |
| Stage #1: 5-bromo-2-chloronicotinic acid With 1,1'-carbonyldiimidazole In N,N-dimethyl-formamide for 0.166667h; Stage #2: N,O-dimethylhydroxylamine*hydrochloride In N,N-dimethyl-formamide for 1h; |
-
-
29241-65-4
5-bromo-2-chloronicotinic acid

-
-
100-46-9
benzylamine

-
-
1258846-87-5
2-(benzylamino)-5-bromonicotinic acid

| Conditions | Yield |
|---|---|
| at 120℃; for 2h; | 79% |
-
-
29241-65-4
5-bromo-2-chloronicotinic acid

-
-
62-53-3
aniline

-
-
115891-15-1
5-Bromo-2-phenylamino-nicotinic acid

| Conditions | Yield |
|---|---|
| 76% |
-
-
540-38-5
4-Iodophenol

-
-
29241-65-4
5-bromo-2-chloronicotinic acid

-
-
1335218-37-5
5-bromo-2-(4-iodophenoxy)nicotinic acid

| Conditions | Yield |
|---|---|
| With sodium hydride In N,N-dimethyl-formamide Inert atmosphere; Heating; | 76% |
| Stage #1: p-Iodophenol With sodium hydride In N,N-dimethyl-formamide; mineral oil at 0 - 30℃; for 1h; Inert atmosphere; Stage #2: 5-bromo-2-chloronicotinic acid In N,N-dimethyl-formamide; mineral oil at 115℃; Stage #3: With acetic acid In water; N,N-dimethyl-formamide; mineral oil at 20℃; for 1h; pH=5; | |
| Stage #1: p-Iodophenol With sodium hydride In N,N-dimethyl-formamide at 0 - 30℃; for 1h; Inert atmosphere; Stage #2: 5-bromo-2-chloronicotinic acid In N,N-dimethyl-formamide at 115℃; Inert atmosphere; | 765 g |
| With sodium hydride In N,N-dimethyl-formamide at 115℃; |
-
-
29241-65-4
5-bromo-2-chloronicotinic acid

-
-
742100-75-0
(5-bromo-2-chloro-pyridin-3-yl)-methanol

| Conditions | Yield |
|---|---|
| Stage #1: 5-bromo-2-chloronicotinic acid With triethylamine; isobutyl chloroformate In tetrahydrofuran at 0℃; Stage #2: With sodium tetrahydroborate In tetrahydrofuran at 0 - 23℃; for 18h; Further stages.; | 70% |
| Multi-step reaction with 2 steps 1: triethylamine / tetrahydrofuran / 1.25 h / 0 °C 2: sodium tetrahydroborate / water / 3 h / 0 - 20 °C View Scheme | |
| Stage #1: 5-bromo-2-chloronicotinic acid With triethylamine; isobutyl chloroformate In tetrahydrofuran at 20℃; for 1.5h; Cooling with ice; Stage #2: With sodium tetrahydroborate In water at 20℃; for 20h; |
-
-
29241-65-4
5-bromo-2-chloronicotinic acid

-
-
543-27-1
isobutyl chloroformate

-
-
742100-75-0
(5-bromo-2-chloro-pyridin-3-yl)-methanol

| Conditions | Yield |
|---|---|
| Stage #1: 5-bromo-2-chloronicotinic acid; isobutyl chloroformate With triethylamine In tetrahydrofuran at 0℃; for 1.25h; Stage #2: With sodium tetrahydroborate In tetrahydrofuran; water at 0 - 20℃; for 20h; | 70% |
-
-
29241-65-4
5-bromo-2-chloronicotinic acid

-
-
18107-18-1
diazomethyl-trimethyl-silane

-
-
78686-79-0
methyl 2-chloro-5-bromonicotinate

| Conditions | Yield |
|---|---|
| In methanol; benzene at 0℃; for 1h; | 69% |
-
-
29241-65-4
5-bromo-2-chloronicotinic acid

-
-
109-73-9
N-butylamine

-
-
1247704-98-8
5-bromo-2-(butylamino)nicotinic acid

| Conditions | Yield |
|---|---|
| for 12h; Reflux; | 69% |
-
-
1035689-99-6
2-(4-bromo-2,6-difluorophenylamino)nicotinic acid

-
-
29241-65-4
5-bromo-2-chloronicotinic acid

-
-
1035690-18-6
5-bromo-2-(3'-ethoxy-3,5-difluorobiphenyl-4-ylamino)nicotinic acid

| Conditions | Yield |
|---|---|
| With toluene-4-sulfonic acid In water at 110℃; | 63% |

-
-
29241-65-4
5-bromo-2-chloronicotinic acid

| Conditions | Yield |
|---|---|
| Stage #1: 5-bromo-2-chloronicotinic acid With 1-[(1-(cyano-2-ethoxy-2-oxoethylidenaminooxy)dimethylamino-morpholino)]-uronium hexafluorophosphate; N-ethyl-N,N-diisopropylamine In dichloromethane at 20℃; for 0.5h; Stage #2: {2‐azabicyclo[2.1.1]hexan‐1‐yl}methanamine In dichloromethane at 20℃; for 1h; | 63% |
-
-
14150-95-9
2-aminopyridine N-oxide

-
-
29241-65-4
5-bromo-2-chloronicotinic acid

| Conditions | Yield |
|---|---|
| With Ullmann copper; potassium carbonate In N,N-dimethyl-formamide for 3h; Heating; | 60% |
-
-
29241-65-4
5-bromo-2-chloronicotinic acid

-
-
536-74-3
phenylacetylene

| Conditions | Yield |
|---|---|
| Stage #1: 5-bromo-2-chloronicotinic acid With thionyl chloride for 3h; Reflux; Stage #2: phenylacetylene With bis-triphenylphosphine-palladium(II) chloride; copper(l) iodide; triethylamine In tetrahydrofuran at 0 - 20℃; for 16h; Inert atmosphere; Sealed tube; | 60% |
-
-
66362-95-6
2-amino-5-methylpyridine 1-oxide

-
-
29241-65-4
5-bromo-2-chloronicotinic acid

| Conditions | Yield |
|---|---|
| With Ullmann copper; potassium carbonate In N,N-dimethyl-formamide for 3h; Heating; | 52% |
-
-
186581-53-3, 908094-01-9
diazomethane

-
-
29241-65-4
5-bromo-2-chloronicotinic acid

-
-
78686-79-0
methyl 2-chloro-5-bromonicotinate

| Conditions | Yield |
|---|---|
| In diethyl ether Ambient temperature; | 49% |
-
-
25063-84-7
2-amino-6-methylpyridine 1-oxide

-
-
29241-65-4
5-bromo-2-chloronicotinic acid

| Conditions | Yield |
|---|---|
| With Ullmann copper; potassium carbonate In N,N-dimethyl-formamide for 3h; Heating; | 41% |
-
-
83700-78-1
2-amino-4-methylpyridine-1-oxide

-
-
29241-65-4
5-bromo-2-chloronicotinic acid

| Conditions | Yield |
|---|---|
| With Ullmann copper; potassium carbonate In N,N-dimethyl-formamide for 3h; Heating; | 41% |

| Conditions | Yield |
|---|---|
| at 130℃; | 38% |
-
-
27329-70-0
5-formylfurane-2-boronic acid

-
-
29241-65-4
5-bromo-2-chloronicotinic acid

-
-
1190223-48-3
2-chloro-5-(5-formylfuran-2-yl)nicotinic acid

| Conditions | Yield |
|---|---|
| With bis-triphenylphosphine-palladium(II) chloride; sodium carbonate In 1,2-dimethoxyethane; ethanol; water at 50℃; for 28h; Suzuki-Miyaura coupling; Inert atmosphere; | 28% |
-
-
29241-65-4
5-bromo-2-chloronicotinic acid

-
-
62-53-3
aniline

-
-
143094-44-4
5-bromo-2-chloro-N-phenylnicotinamide

| Conditions | Yield |
|---|---|
| With 1-ethyl-(3-(3-dimethylamino)propyl)-carbodiimide hydrochloride; triethylamine In dichloromethane at 20℃; | 26% |
-
-
828-27-3
4-Trifluoromethoxyphenol

-
-
29241-65-4
5-bromo-2-chloronicotinic acid

-
-
473256-18-7
5-Bromo-2-[4-(trifluoromethoxy)phenoxy]nicotinic acid

| Conditions | Yield |
|---|---|
| With potassium carbonate In N,N-dimethyl-formamide; pentane | 24% |
-
-
13220-33-2
1-methyl-3-pyrrolidinol

-
-
29241-65-4
5-bromo-2-chloronicotinic acid

| Conditions | Yield |
|---|---|
| With sodium hydride In tetrahydrofuran for 1.5h; Heating; | 190 g |
-
-
29241-65-4
5-bromo-2-chloronicotinic acid

-
-
106-47-8
4-chloro-aniline

| Conditions | Yield |
|---|---|
| With pyridine; toluene-4-sulfonic acid In water Heating; |
5-Bromo-2-chloronicotinic acid Chemical Properties
IUPAC Name: 5-Bromo-2-chloropyridine-3-carboxylic acid
Molecular Formula: C6H3BrClNO2
Molecular Weight: 236.450520 g/mol
Melting Point: 173-176 °C
Density: 1.917 g/cm3
Flash Point: 162.5 °C
Storage temp.: Keep cold
Enthalpy of Vaporization: 62.18 kJ/mol
Boiling Point: 345.1 °C at 760 mmHg
Vapour Pressure: 2.39E-05 mmHg at 25 °C
Water Solubility: 699.3 mg/L at 25 °C
Structure of 5-Bromo-2-chloronicotinic acid (CAS NO.29241-65-4):
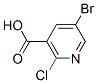
Categories of 5-Bromo-2-chloronicotinic acid (CAS NO.29241-65-4): Blocks;Bromides;Carboxes;Pyridines;Acids and Derivatives;Heterocycles;pharmacetical;Carboxylic Acids;Pyridine;Carboxylic Acids
5-Bromo-2-chloronicotinic acid Safety Profile
Safety Information of 5-Bromo-2-chloronicotinic acid (CAS NO.29241-65-4):
Hazard Codes: Xi 
Risk Statements: 36/37/38
36/37/38:Irritating to eyes, respiratory system and skin
Safety Statements: 26-36/37/39
26:In case of contact with eyes, rinse immediately with plenty of water and seek medical advice
36/37/39:Wear suitable protective clothing, gloves and eye/face protection
Hazard Note: Irritant/Keep Cold
5-Bromo-2-chloronicotinic acid Specification
5-Bromo-2-chloronicotinic acid , its cas register number is 29241-65-4. It also can be called Acide 5-bromo-2-chloronicotinique ; 5-Bromo-2-chloropyridine-3-carboxylic acid ; and 3-Pyridinecarboxylic acid, 5-bromo-2-chloro- .
Related Products
- 5-Bromo-2-chloronicotinic acid
- 29241-66-5
- 29242-84-0
- 292-46-6
- 29247-15-2
- 2925-00-0
- 2925-42-0
- 29256-81-3
- 292606-35-0
- 29261-33-4
- 292621-45-5
Hot Products
About|Contact|Cas|Product Name|Molecular|Country|Encyclopedia
Message|New Cas|MSDS|Service|Advertisement|CAS DataBase|Article Data|Manufacturers | Chemical Catalog
©2008 LookChem.com,License: ICP
NO.:Zhejiang16009103
complaints:service@lookchem.com Desktop View