Henan Allgreen Chemical Co.,Ltd
T he company has advanced technology, as well as a large number of excellent R & D team, to provide customers from the grams to one hundred kilograms and tons of high-quality products, competitive prices and quality service Appearance:White or
Cas:7664-41-7
Min.Order:1 Kilogram
Negotiable
Type:Manufacturers
inquiryAlity Chemical Corporation
The above product is Ality Chemical's strong item with best price, good quality and fast supply. Ality Chemical has been focusing on the research and production of this field for over 14 years. At the same time, we are always committed to providi
Hebei yanxi chemical co.,LTD.
hebei yanxi chemical co., LTD who registered capital of 10 million yuan, nearly to $2 million, we have a pharmaceutical raw materials factory production of pharmaceutical raw materials, and a reagent r&d center, and we do research and developm
Cas:7664-41-7
Min.Order:1 Kilogram
FOB Price: $1.0 / 3.0
Type:Trading Company
inquiryHenan Tianfu Chemical Co., Ltd.
Our company engages in Sodium Tripolyphosphate (STPP) and Sodium Hexametabphosphate (SHMP) production; development of noble metal catalysts, synthesis of electronic chemical materials and general chemicals Imp&Exp trading business. The company
Cas:7664-41-7
Min.Order:1 Kilogram
Negotiable
Type:Lab/Research institutions
inquiryHenan Wentao Chemical Product Co., Ltd.
Henan Wentao Chemical Product Co.,Ltd is Located in Zhengzhou High-tech Development Zone with import and export license, We passed ISO 9001:2008 as well, Henan Wentao has developed more than 1000 compounds, which are widely used in the fields of prod
Changchun Artel lmport and Export trade company
Minimum Order Qty. 10 Gram Supply Ability 500 Kilograms/Month Storage store in cool, dry, ventilated place 20℃ Delivery Time 3 business days after payment Payment Term TT,western union,Paypal,MoneyGram Package 10g,20g,50g,100g,500g,1KGS,
Cas:7664-41-7
Min.Order:10 Gram
Negotiable
Type:Trading Company
inquiryHangzhou Keyingchem Co.,Ltd
Hangzhou KeyingChem Co., Ltd. exported this product to many countries and regions at best price. If you are looking for the material’s manufacturer or supplier in China, KeyingChem is your best choice. Pls contact with us freely for getting det
Hangzhou J&H Chemical Co., Ltd.
J&H CHEM R&D center can offer custom synthesis according to the contract research and development services for the fine chemicals, pharmaceutical, biotechnique and some of the other chemicals. J&H CHEM has some Manufacturing base in Jia
Zibo Hangyu Biotechnology Development Co., Ltd
Zibo Hangyu Biotechnology Development Co., Ltd is a leading manufacturer and supplier of chemicals in China. We develop produce and distribute high quality pharmaceuticals, intermediates, special chemicals and OLED intermediates and other fine chemi
Cas:7664-41-7
Min.Order:10 Gram
FOB Price: $100.0
Type:Lab/Research institutions
inquiryHANGZHOU YUNUO CHEMICAL CO.,LTD
Superior quality, moderate price & quick delivery. Appearance:colourless gas Storage:Well-sealed and put in dry and cool conditions. Protected from direct sunlight or high temperature. Package:as per your request Application:Ammonia is a
Zibo Dorne chemical technology co. LTD
Product Details Grade: pharmaceutical grade Purity:99%+ ProductionCapacity: 1000 Kilogram/Month Scope of use: For scientific research only(The product must be used legally) Our Advantage 1. Best quality with competitive price. 2. Quick shipping,
Cas:7664-41-7
Min.Order:1 Metric Ton
Negotiable
Type:Lab/Research institutions
inquiryKono Chem Co.,Ltd
high purity lowest priceAppearance:solid or liquid Storage:in sealed air resistant place Package:drum and bag Application:for pharma use Transportation:by sea or air Port:Beijing or Guangzhou
Hangzhou Dingyan Chem Co., Ltd
R & D enterprises have their own stock in stock Package:1kg Application:pharmaceutical intermediates
Zhuozhou Wenxi import and Export Co., Ltd
Product Description Description & Specification Category Pharmaceutical Raw Materials, Fine Chemicals, Bulk drug Standard Medical standard
Cas:7664-41-7
Min.Order:1 Kilogram
FOB Price: $112.0
Type:Trading Company
inquiryAntimex Chemical Limied
Ansciep Chemical is a professional enterprise manufacturing and distributing fine chemicals and speciality chemicals. We have been dedicated to heterocycle compounds and phenyl rings for tens of years. This is our mature product for export. Our quali
Hubei Langyou International Trading Co., Ltd
Liquid Anhydrous Ammonia for Nitrogen Fetilizer CAS NO.7664-41-7 CAS NO.7664-41-7 Application:Liquid Anhydrous Ammonia for Nitrogen Fetilizer CAS NO.7664-41-7 CAS NO.7664-41-7
Cas:7664-41-7
Min.Order:0
Negotiable
Type:Other
inquiryWuhan ZeShanCheng Biomedical Technology Co., Ltd.
1,we produce and sell good chemicals around the world. 2,our success rate is about 95%. this means, if customer order is accepted, the probability that the customer will obtain the ordered substances, is 95%. 3,our staff consists of highl
Hunan Longxianng Runhui Trading Co.,Ltd
Liquid Anhydrous Ammonia for Nitrogen Fetilizer CAS NO.7664-41-7 CAS NO.7664-41-7Appearance:powder Storage:Store at RT. Package:bag Application:Industrial Transportation:By express (Door to door) such as FEDEX, DHL, EMS for small amount. By air(airpo
Cas:7664-41-7
Min.Order:0
Negotiable
Type:Lab/Research institutions
inquiryHENAN SUNLAKE ENTERPRISE CORPORATION
Ammonia Basic information Product Name: Ammonia Synonyms: am-fol;a
Theta biotechnology CO.,LTD
FAQ 1. Can I get a free sample Yes, but you have to pay the courier fee. 2. How to pay We accept payments in the form of Western Union, MoneyGram, Bitcoin, etc. 3. What should I do if we don’t ship after payment We are a formal c
Cas:7664-41-7
Min.Order:1 Gram
FOB Price: $1.0
Type:Lab/Research institutions
inquiryChangzhou Extraordinary Pharmatech co.,LTD
Changzhou Extraordinary Pharmatech co., LTD. As a leading chemical manufacturer and supplier in China.DAS authentication is passed.We can provide the popular precursor chemicals, we have our own strong R & D team, have our own laboratories and fa
Shaanxi Mingqi Chemical Co., Ltd
Known for its best quality and competitve price, this chemicals we offered is widely appreciated by our customers. Our advantages:1, High quality with competitive price:1) Standard:BP/USP/EP/Enterprise standard2) All Purity≥99%3) We are manufacturer
Qingdao Sigma Chemical Co., Ltd.
We promise our customer following items s 1.Reasonable pre price: We provide high quality products with competitive price in china, , 2.Low moq moq: No worry about the low moq, our moq is 1 gram or lower. . 3
Cas:7664-41-7
Min.Order:1 Kilogram
FOB Price: $1.0
Type:Trading Company
inquiryQuzhou Youngtime Chemicl Co.,ltd
Product Chinese Name: 液氨(氨水) Product English Name: Liquid ammonia (anhydrous ammonia) CAS number: 7664-41-7 Molecular formula: NH3 Molecular weight: 17.04 HS number: 28141000 Tax refund rate: 0 UN number: 1005 Dangerous cargo number
Cas:7664-41-7
Min.Order:6 Metric Ton
Negotiable
Type:Trading Company
inquiryShandong Everlast AC Chemical Co.,Ltd
1, ISO 9001:2008 Approved 2, 18 years production experience 3, vendor of China State Owned Enterprise 4, Ammonia Supplier for Pride-chem, National Traders.... 5, largest NH3/Ammonia manufacturer and Exporter in North China Appearance:Colorl
Cas:7664-41-7
Min.Order:7 Metric Ton
FOB Price: $970.0
Type:Trading Company
inquiryShanghai Wechem Chemical Co Ltd
Ammonia is colorless , pungent , non-flammable gas at atmospheric pressure and temperature . It is irritating to the mucous membranes and toxic in high concentrations. Ammonia is shipped as a liquefied gas under its own vapor pressure . Applicatio
Nanjing Chemlin Chemical Co., Ltd.
Compound sourcing ServicesAgency of testing serviceFactory audit serviceFounded in Dec. 1999, Nanjing Chemlin Chemical Industrial Co.,Ltd(CHEMLIN) has been covering the business scope from trading of chemicals to custom-synthesis & Manufacturing. Co
shandong everlast ac chemical co.,ltd.
1, ISO 9001:2008 Approved 2, 18 years production experience 3, vendor of China State Owned Enterprise 4, Ammonia Supplier for Pride-chem, National Traders.... 5, largest NH3/Ammonia manufacturer and Exporter in North China Appearance:liquid
Cas:7664-41-7
Min.Order:7 Metric Ton
FOB Price: $345.0 / 1800.0
Type:
inquiryAngel Beauty Holdings, Inc.
WE HAVE THIS PRODUCT TOGETHER WITH OTHER TOP CLASS BEAUTY PRODUCTS OF HIGH STANDARD THAT WE SELL AT VERY AFFORDABLE PRICES. WE GUARANTEE YOU A 100% TOP CLASS PRODUCT QUALITY, ON TIME DELIVERY OF YOUR PRODUCT TO ANY PART OF THE WORLD. CONTAC
U-Chemo Holding Co.,Limited
U-CHEMO is a high-tech enterprise taking pharmaceutical intermediates and fine chemicals as main products. We pay high attention on product quality and apply quality management in the whole manufacturing process from research to production. In additi
Synthetic route

| Conditions | Yield |
|---|---|
| In water Electrolysis; Cu-cathode, in presence of H2SO4;; | 100% |
| With aluminium In water at elevated pressure;; | 0% |
| With aluminium In water only small amounts of NH3 in dild. HNO3 (5%-20%) at atmospheric pressure;; |

| Conditions | Yield |
|---|---|
| With water byproducts: CO; heating with H2O vapour to 300°C; | A 100% B n/a |
| With H2O |
-
-
180893-21-4
cis,trans-[WCl2(NNC5H2Me3-2,4,6)(C2H4)(PMe2Ph)2][BF4]

-
-
7664-41-7
ammonia

| Conditions | Yield |
|---|---|
| With KOH In methanol byproducts: 2,4,6-trimethylpyridine; N2-atmosphere; excess KOH, stirring at room temp. for 1 h; collection of pyridine derivative (cold trap), colorimetry of NH3; | 100% |

-
-
7664-41-7
ammonia

| Conditions | Yield |
|---|---|
| With KOH In methanol byproducts: 4-methoxypyridine; N2-atmosphere; excess KOH, stirring at room temp. for 1 h; collection of pyridine derivative (cold trap), colorimetry of NH3; | 100% |

-
-
7664-41-7
ammonia

| Conditions | Yield |
|---|---|
| Alkaline conditions; | 100% |
-
-
16712-16-6, 64165-14-6, 65387-22-6, 70179-79-2, 540-69-2
ammonium formate

-
A
-
124-38-9
carbon dioxide

-
B
-
7664-41-7
ammonia

| Conditions | Yield |
|---|---|
| With oxygen In water at 200℃; Catalytic behavior; Temperature; Flow reactor; Inert atmosphere; | A 100% B 100% |

| Conditions | Yield |
|---|---|
| at 130.0°C, 74.1 Torr equilibrium; | A 99.6% B 99.6% |
| at 130.0°C, 74.1 Torr equilibrium; | A 99.6% B 99.6% |
| at 54.8°C, 57.6 Torr equilibrium; | A 90.8% B 90.8% |
| at 54.8°C, 57.6 Torr equilibrium; | A 90.8% B 90.8% |

| Conditions | Yield |
|---|---|
| With [(C5H5)Mo(S2CH2)(S)(SH)Mo(C5H5)](OSO2CF3) In tetrahydrofuran Schlenk techniques; 10 equiv. of Mo complex in THF stirred at 25°C for 5 min under N2; N2 replaced by 1 atm of H2; W complex added portionwise; stirred at 25°C for 24 h and then at 55°C for 24 h; evapd. under reduced pressure; distillate trapped in dilute H2SO4 soln.;residue extd. with H2O, treated with activated charcoal, filtered throu gh Celite; | A 99% B 1% |


| Conditions | Yield |
|---|---|
| With catalyst: Ni(2+)Y zeolite In neat (no solvent) reduction of very dild. mixt. of NO/NO2 in gas mixt. of N2/H2 on zeolite catalyst (300°C reaction temp.); gas chromy. (dimethylsulfolane coated diatomite); | 99% |
| With catalyst: industrial nickel methanation catalyst In neat (no solvent) reduction of very dild. mixt. of NO/NO2 in gas mixt. of N2/H2 on zeolite catalyst (300°C reaction temp.); gas chromy. (dimethylsulfolane coated diatomite); | 99% |
| With catalyst: phthalocyanineNiY zeolite In neat (no solvent) reduction of very dild. mixt. of NO/NO2 in gas mixt. of N2/H2 on zeolite catalyst (230°C reaction temp.); gas chromy. (dimethylsulfolane coated diatomite); | 94% |
| With catalyst: NiY zeolite In neat (no solvent) reduction of very dild. mixt. of NO/NO2 in gas mixt. of N2/H2 on zeolite catalyst (450°C reaction temp.); gas chromy. (dimethylsulfolane coated diatomite); | 84% |


| Conditions | Yield |
|---|---|
| In tetrahydrofuran Schlenk techniques; 2 equiv. of Mo complex in THF stirred at 25°Cfor 5 min under N2; N2 replaced by 1 atm of H2; W complex added portion wise; stirred at 25°C for 1 h; solvent removed under vac.; dissolved in THF-d8; not isolated; detd. by NMR spectra; | A 84% B 99% C 0% |


| Conditions | Yield |
|---|---|
| In neat (no solvent) heated at 373 K for 1 h; XRD; | A 99% B n/a C n/a |


| Conditions | Yield |
|---|---|
| In neat (no solvent) heated at 373 K for 1 h; XRD; | A 99% B n/a C n/a |


| Conditions | Yield |
|---|---|
| With samarium diiodide bis(tetrahydrofuran) In tetrahydrofuran at 20℃; under 760.051 Torr; for 2h; Inert atmosphere; Schlenk technique; Glovebox; | A 99% B 47% |


| Conditions | Yield |
|---|---|
| Casale method; at 450-500°C; space velocity 16000-25000; contact time 13.5-19 sec; | 98.7% |
| With catalsyt: Fe-Al-cyanides Mont-Cenis method; very pure reactants used; at 90-100 atm, 350-430°C; deep cooling; | 98% |
| Casale method; at 450-500°C; space velocity 16000-25000; contact time 13.5-19 sec; | 98.7% |

| Conditions | Yield |
|---|---|
| (WC5(CH3)5(CH3)3NH2NH2)(1+) In tetrahydrofuran room temp.; N2 atm., 2 equiv of N2H4;; dependence of yield from aded equiv of N2H4;; | 98% |
| (MoC5(CH3)5(CH3)3NH2NH2)(1+) In tetrahydrofuran room temp.; N2 atm., 2 equiv of N2H4;; dependence of yield from added equiv of N2H4;; | 95% |
| W(η5-C5Me5)Me3(NNH2) In tetrahydrofuran room temp.; N2 atm., 3 equiv of N2H4;; dependence of yield from added equiv of N2H4;; | 84% |

-
-
7664-41-7
ammonia

| Conditions | Yield |
|---|---|
| With cobaltocene; water In tetrahydrofuran at 20℃; for 12h; Reagent/catalyst; | 98% |
-
-
70458-96-7
1-ethyl-6-fluoro-1,4-dihydro-4-oxo-7-(1-piperazinyl)-3-quinoline carboxylic acid

-
A
-
50-00-0
formaldehyd

-
B
-
7664-41-7
ammonia

-
C
-
75001-63-7
6-fluoro-7-amino-1-ethyl-1,4-dihydro-4-oxo-3-quinolinecarboxylic acid

| Conditions | Yield |
|---|---|
| With potassium permanganate; cetyltrimethylammonim bromide; acetic acid In water; acetonitrile at 24.84℃; Kinetics; Catalytic behavior; Mechanism; Thermodynamic data; Activation energy; Temperature; Concentration; Solvent; UV-irradiation; | A n/a B n/a C 98% |

-
-
1333-74-0
hydrogen

-
-
10102-43-9
nitrogen(II) oxide

-
-
10102-44-0
Nitrogen dioxide

-
A
-
7727-37-9
nitrogen

-
B
-
7664-41-7
ammonia

| Conditions | Yield |
|---|---|
| With catalyst: industrial nickel methanation catalyst In neat (no solvent) reduction of mixt. of NO/NO2 in gas mixt. of N2/H2 on zeolite catalyst (pretreated in H2 at 550°C, 200°C reaction temp.); gas chromy. (dimethylsulfolane coated diatomite); | A 96% B 0% |
| With catalyst: industrial nickel methanation catalyst In neat (no solvent) reduction of mixt. of NO/NO2 in gas mixt. of N2/H2 on zeolite catalyst (pretreated in H2 at 300°C, 200°C reaction temp.); gas chromy. (dimethylsulfolane coated diatomite); | A 92.5% B 7.5% |
| With catalyst: industrial nickel methanation catalyst In neat (no solvent) reduction of mixt. of NO/NO2 in gas mixt. of N2/H2 on zeolite catalyst (pretreated in H2 at 300°C, 150°C reaction temp.); gas chromy. (dimethylsulfolane coated diatomite); | A 91% B 0% |


| Conditions | Yield |
|---|---|
| In tetrahydrofuran at -96 - 20℃; | A n/a B 96% |


| Conditions | Yield |
|---|---|
| With hydrogenchloride; tin(ll) chloride In water | A n/a B 94% C 0% |
| With HCl; SnCl2 In water | A n/a B 94% C 0% |

-
-
10102-43-9
nitrogen(II) oxide

-
-
7664-41-7
ammonia

| Conditions | Yield |
|---|---|
| With hydrogen at 700°C, with 3% Pd; | 94% |
| With hydrogen at 400°C, with 3% Pd; | 83% |
| With hydrogen at 600°C, with 3% Pd; | 78% |
-
-
225245-55-6
cis,trans-[WCl2(NNC5H4OMe-4)(CO)(PMe2Ph)2][ClO4]

-
-
7664-41-7
ammonia

| Conditions | Yield |
|---|---|
| With KOH In methanol byproducts: 4-methoxypyridine; N2-atmosphere; excess KOH, stirring at room temp. for 1 h; collection of pyridine derivative (cold trap), colorimetry of NH3; | 94% |
-
-
180893-19-0
cis,trans-[WCl2(NNC5H2Me3-2,4,6)(CO)(PMe2Ph)2][BF4]

-
-
7664-41-7
ammonia

| Conditions | Yield |
|---|---|
| With KOH In methanol byproducts: 2,4,6-trimethylpyridine; N2-atmosphere; excess KOH, stirring at room temp. for 1 h; collection of pyridine derivative (cold trap), colorimetry of NH3; | 94% |

| Conditions | Yield |
|---|---|
| In neat (no solvent) High Pressure; mixed, sealed in autoclave under Ar, heated at 450, 500, 550, and 600 °C for 10 h; washed with water, dried in vac. at 70 °C for 12 h; powder XRD; | A 93% B n/a C n/a D n/a |


-
-
7664-41-7
ammonia

| Conditions | Yield |
|---|---|
| With CoCp; 2,6-lutidine hydrochloride In tetrahydrofuran THF, room temp., N2 atm.; 12 equiv of CoCp2 and 16 equiv of lutidine hydrichloride; mixt. was stirred for approx. 15 h;; The ammonia was quantified by the indophenol method;; | 92% |

-
-
7664-41-7
ammonia

| Conditions | Yield |
|---|---|
| With zinc amalgam; 2,6-lutidinium chloride; HCl In tetrahydrofuran inert atmosphere; cold (-40°C) THF addn. to mixt. of W-complex, zinc amalgam and proton source in Schlenk flask, stirring vigorously, soln. allowing to stand ca. 20 h at 25°C; HCl addn., solvent removal (vac.), residue treating with NaOH soln. in closed system under argon and soln. distn. into H2SO4 soln., or residue extn. with H2O, soln. filtration (Millipore); chem. anal.; | 92% |
| With Zn#Hg; H2; HCl In tetrahydrofuran nitrogen atmosphere; cold (-40°C) THF addn. to mixt. of W-complex, Zn#Hg and proton source in Schlenk flask, stirring vigorously, soln. allowing to stand ca. 20 h at 25°C; HCl addn., solvent removal (vac.), residue treating with NaOH soln. in closed system under argon and soln. distn. into H2SO4 soln., or residue extn. with H2O, soln. filtration (Millipore); chem. anal.; | 71% |
| With zinc amalgam; phenol; HCl In tetrahydrofuran inert atmosphere; cold (-40°C) THF addn. to mixt. of W-complex, zinc amalgam and proton source in Schlenk flask, stirring vigorously, soln. allowing to stand ca. 20 h at 25°C; HCl addn., solvent removal (vac.), residue treating with NaOH soln. in closed system under argon and soln. distn. into H2SO4 soln., or residue extn. with H2O, soln. filtration (Millipore); chem. anal.; | 65% |
| With zinc amalgam; 2,3,5-triisopropylbenzenethiol; HCl In tetrahydrofuran inert atmosphere; cold (-40°C) THF addn. to mixt. of W-complex, zinc amalgam and proton source in Schlenk flask, stirring vigorously, soln. allowing to stand ca. 20 h at 25°C; HCl addn., solvent removal (vac.), residue treating with NaOH soln. in closed system under argon and soln. distn. into H2SO4 soln., or residue extn. with H2O, soln. filtration (Millipore); chem. anal.; | 60% |

-
-
7664-41-7
ammonia

| Conditions | Yield |
|---|---|
| With cobaltocene; 2,6-lutidinium chloride; HCl In tetrahydrofuran inert atmosphere; cold (-40°C) THF addn. to mixt. of W-complex, cobaltocene and 2,6-lutidinium chloride in Schlenk flask, stirring vigorously, soln. allowing to stand ca. 20 h at 25°C; HCl addn., solvent removal (vac.), residue treating with NaOH soln. in closed system under argon and soln. distn. into H2SO4 soln., or residue extn. with H2O, soln. filtration (Millipore); chem. anal.; | 92% |
| With zinc amalgam; 2,6-lutidinium chloride; HCl In tetrahydrofuran inert atmosphere; cold (-40°C) THF addn. to mixt. of W-complex, zinc amalgam and 2,6-lutidinium chloride in Schlenk flask, stirring vigorously, soln. allowing to stand ca. 20 h at 25°C; HCl addn., solvent removal (vac.), residue treating with NaOH soln. in closed system under argon and soln. distn. into H2SO4 soln., or residue extn. with H2O, soln. filtration (Millipore); chem. anal.; | 91% |
| With zinc amalgam; phenol; HCl In tetrahydrofuran inert atmosphere; cold (-40°C) THF addn. to mixt. of W-complex, zinc amalgam and phenol in Schlenk flask, stirring vigorously, soln. allowing to stand ca. 20 h at 25°C; HCl addn., solvent removal (vac.), residue treating with NaOH soln. in closed system under argon and soln. distn. into H2SO4 soln., or residue extn. with H2O, soln. filtration (Millipore); chem. anal.; | 88% |
| With zinc amalgam; H2; HCl In tetrahydrofuran inert atmosphere; cold (-40°C) THF addn. to mixt. of W-complex, zinc amalgam and hydrogen as proton source in Schlenk flask, stirring vigorously, soln. allowing to stand ca. 20 h at 25°C; HCl addn., solvent removal (vac.), residue treating with NaOH soln. in closed system under argon and soln. distn. into H2SO4 soln., or residue extn. with H2O, soln. filtration (Millipore); chem. anal.; | 57% |
| With SnCl2; 2,6-lutidinium chloride; HCl In tetrahydrofuran inert atmosphere; cold (-40°C) THF addn. to mixt. of W-complex, SnCl2 and 2,6-lutidinium chloride in Schlenk flask, stirring vigorously, soln. allowing to stand ca. 20 h at 25°C; HCl addn., solvent removal (vac.), residue treating with NaOH soln. in closed system under argon and soln. distn. into H2SO4 soln., or residue extn. with H2O, soln. filtration (Millipore); chem. anal.; | 45% |
-
-
225245-35-2
cis,mer-[WBr2(NNC5H4OMe-4)(PMe2Ph)3][PF6]

-
-
7664-41-7
ammonia

| Conditions | Yield |
|---|---|
| With KOH In methanol byproducts: 4-methoxypyridine; N2-atmosphere; excess KOH, stirring at room temp. for 1 h; collection of pyridine derivative (cold trap), colorimetry of NH3; | 92% |

| Conditions | Yield |
|---|---|
| With bis(pentamethylcyclopentadienyl)cobalt(II); [Mo(N)Cl(bis(di-tert-butylphosphinoethyl)phenylphosphine)] In toluene at 20℃; under 760.051 Torr; for 20h; Schlenk technique; | 92% |
| With bis(pentamethylcyclopentadienyl)cobalt(II); C25H44I3MoN2P2 In toluene at 20℃; under 760.051 Torr; for 20h; Catalytic behavior; Reagent/catalyst; Time; Inert atmosphere; Glovebox; Schlenk technique; | 82% |
| With bis(pentamethylcyclopentadienyl)cobalt(II); C40H65FeMoN7P2*C4H10O In toluene at 20℃; under 760.051 Torr; for 20h; Glovebox; Schlenk technique; | |
| With bis(pentamethylcyclopentadienyl)cobalt(II); [molybdenum(iodide)3(2,6-bis(di-tert-butylphosphinomethyl)pyridine)] In toluene at 20℃; under 760.051 Torr; for 20h; Reagent/catalyst; Solvent; Concentration; Glovebox; Schlenk technique; | 91 %Spectr. |
| With bis(pentamethylcyclopentadienyl)chromium; C21H41Cl3MoN3P2 In toluene at 20℃; for 19h; |

| Conditions | Yield |
|---|---|
| With (η5-C5Me5)Rh(2-pyridylphenyl)H In tetrahydrofuran at 23℃; under 3040.2 Torr; for 120h; Catalytic behavior; Reagent/catalyst; Inert atmosphere; Schlenk technique; | 92% |
-
-
7664-41-7
ammonia

| Conditions | Yield |
|---|---|
| Product distribution / selectivity; | 100% |

| Conditions | Yield |
|---|---|
| In water at -11 - -8℃; pH=~ 10; | 100% |
| In water at -15 - -7℃; pH=~ 10; Product distribution / selectivity; | 100% |
| In diethyl ether; water at -20 - -10℃; for 0.5h; |


| Conditions | Yield |
|---|---|
| In water NH3 passed into a soln. of (NH4)2CO3-NaCl until satn.; product free of Cl and NH3; | 100% |
| In water NH3 passed into a soln. of (NH4)2CO3-NaCl until satn.; product free of Cl and NH3; | 100% |


| Conditions | Yield |
|---|---|
| With catalyst: Pt-oxide at 1000°C; | 100% |
| platinum at 1000°C; | 100% |
| platinum at 800°C; | 62.8% |
| With catalyst: Pt-oxide at 800°C; | 62.8% |


| Conditions | Yield |
|---|---|
| In ammonia byproducts: H2; react. of Rb in liq. NH3 at room temp. for 6-10 h;; | 100% |
| In ammonia byproducts: H2; NH3 (liquid); react. of Rb in liq. NH3 at room temp. for 6-10 h;; | 100% |

| Conditions | Yield |
|---|---|
| Autoclave; Glovebox; Inert atmosphere; Schlenk technique; | 100% |
| at 50℃; for 72h; Autoclave; High pressure; | 56% |
| In neat (no solvent) Yb dissolved in liq. ammonia; soln. left to stand at 273 K for 1-12 h; ppt.; | |
| In ammonia NH3 (liquid); (N2); Yb dissolved in liquid NH3;; soln. stand at 273 K; ppt.; ammonia removed; XRD; |

| Conditions | Yield |
|---|---|
| Prepd. by laser chemical vapor pptn. at atmospheric pressure.; | 100% |
| In gas under Ar, in a low-pressure flow reactor; | |
| thin film deposited on SiO2 substrate at 500-900 K, 1-10 Torr; AFM; |

| Conditions | Yield |
|---|---|
| Prepd. by laser chemical vapor pptn. at atmospheric pressure.; | 100% |
| In neat (no solvent) Kinetics; under Ar, in a low-pressure flow reactor at various condns.; | |
| low pressure chemical vapor deposition at 820 °C; |
-
-
7664-41-7
ammonia

-
-
80937-33-3
oxygen

-
A
-
7727-37-9
nitrogen

-
B
-
10102-43-9
nitrogen(II) oxide

-
C
-
10024-97-2
dinitrogen monoxide

| Conditions | Yield |
|---|---|
| With oxygen In neat (no solvent) Fe-ZSM-5 catalyst prepared by ion exchange and heat-treated at 400, 425or 450 °C, 100 % NH3 conversion, 100 % N2 selectivity, 1000 ppm NH3 in 2 % O2-contg. He; | A 100% B 0% C 0% |
| With catalyst:Fe-mordenite In neat (no solvent) Fe-mordenite catalyst prepared by ion exchange and heat-treated at 425 °C, 92 % NH3 conversion, 99 % N2 selectivity, 1000 ppm NH3 in 2 %O2-contg. He; | A 92% B n/a C 0% |
| With catalyst:Fe-ZSM-5 In neat (no solvent) Fe-ZSM-5 catalyst prepared by ion exchange and heat-treated at 375 °C, 90 % NH3 conversion, 99 % N2 selectivity, 1000 ppm NH3 in 2 % O2-contg. He; | A 90% B n/a C 0% |


| Conditions | Yield |
|---|---|
| byproducts: N2; red heat; | 100% |
| decompn., heated porcelain pipe, 1100.degreeC; | 75.7% |
| With catalyst: Ru/SiC In gas Kinetics; byproducts: N2; NH3 decompd. in integrated ceramic microreactor at 450-1000°C; analyzed by gas chromatograph (Porapak N, TCD detector); |

| Conditions | Yield |
|---|---|
| In neat (no solvent) 550°C, p(NH3)=6 kbar, 14 d; elem. anal.; | 100% |
| In neat (no solvent) heating (10-250 atm NH3, 870°C, several days); |

| Conditions | Yield |
|---|---|
| In water by evapn. soln. of 3 mol HIO3 + 1 mol NH3; | 100% |
| In water by evapn. soln. of 3 mol HIO3 + 1 mol NH3; | 100% |
| In water concd. HIO3 soln. (50%); | |
| In water concd. HIO3 soln. (50%); |

| Conditions | Yield |
|---|---|
| In ammonia byproducts: H2; pressure: 1 atm (min.);; | 100% |
| In ammonia byproducts: H2; NH3 (liquid); pressure: 1 atm (min.);; | 100% |
| In neat (no solvent) byproducts: H2; react. of RbH and gaseous NH3 at ambient temp.;; |
-
-
19615-74-8, 30759-83-2, 15274-43-8, 16610-41-6
{NiCl2(Tri-{n-butyl}-phosphin)2}

-
-
7664-41-7
ammonia

| Conditions | Yield |
|---|---|
| In diethyl ether; ammonia byproducts: PBu3; absence of moisture; condensation of liquid NH3 into Ni-complex soln. (in ether), stirring (2 h); evapn. of NH3, filtration, distn. off of ether; | 100% |
-
-
10544-50-0
sulfur

-
-
16940-66-2
sodium tetrahydroborate

-
-
7664-41-7
ammonia

-
-
10043-11-5
ammonia borane complex

| Conditions | Yield |
|---|---|
| In ammonia to NaBH4 in a flask at -40°C NH3 is condensed, then slowly S8 isadded (5 h), to the mixt. (after 3 h) CH2Cl2 is added, then the mixt. is warmed to room temp.; residue is extd. with CH2Cl2, the soln. is evapd., elem. anal.; | 100% |


| Conditions | Yield |
|---|---|
| In neat (no solvent) to triisopropyl borane added NH3 with vigorous stirring and cooling under dry Ar; mixt. stirred for 3 h; NMR; | 100% |

| Conditions | Yield |
|---|---|
| In diethyl ether; ammonia byproducts: PPh3; absence of moisture; condensation of liquid NH3 into Ni-complex soln. (in ether), stirring (2 h); evapn. of NH3, filtration, distn. off of ether; | 100% |

| Conditions | Yield |
|---|---|
| In benzene byproducts: H2O; | 100% |
| With sodium hydroxide In neat (no solvent) byproducts: H2O; keeping in dry NH3 atmosphere in the presence of NaOH to remove H2O; |
-
-
36673-36-6, 111408-20-9, 14126-37-5, 54053-52-0
dibromobis(triphenylphosphine)nickel(II)

-
-
7664-41-7
ammonia

| Conditions | Yield |
|---|---|
| In diethyl ether; ammonia byproducts: PPh3; absence of moisture; condensation of liquid NH3 into Ni-complex soln. (in ether), stirring (2 h); evapn. of NH3, filtration, distn. off of ether; | 100% |
-
-
787624-20-8, 14057-03-5
bis(triphenylphosphine)nickel(II) diiodide

-
-
7664-41-7
ammonia

| Conditions | Yield |
|---|---|
| In diethyl ether; ammonia byproducts: PPh3; absence of moisture; condensation of liquid NH3 into Ni-complex soln. (in ether), stirring (2 h); evapn. of NH3, filtration, distn. off of ether; | 100% |
-
-
14264-16-5, 53996-95-5, 62075-39-2, 39716-73-9
bis(triphenylphosphine)nickel(II) chloride

-
-
7664-41-7
ammonia

| Conditions | Yield |
|---|---|
| In diethyl ether; ammonia byproducts: PPh3; absence of moisture; condensation of liquid NH3 into Ni-complex soln. (in ether), stirring (2 h); evapn. of NH3, filtration, distn. off of ether; | 100% |

| Conditions | Yield |
|---|---|
| With ammonium chloride; mercury dichloride In ammonia room temp.; | 100% |
| With HgCl2; NH4Cl In ammonia aq. ammonia=NH3; room temp.; | 100% |

| Conditions | Yield |
|---|---|
| In petroleum ether 709mg Co2(CO)8 in 20ml petroleum ether are treated with NH3;; | 100% |
| In not given | |
| In water byproducts: H2O; NH3 reacts with intermediates;; |
-
-
75732-01-3
mesitylcopper(I)

-
-
7664-41-7
ammonia

-
A
-
77590-45-5
amino-copper

-
B
-
108-67-8
1,3,5-trimethyl-benzene

| Conditions | Yield |
|---|---|
| In tetrahydrofuran THF, ambient temp., excess of NH3;; evapd. or filtered; elem. anal.;; | A n/a B 100% |


| Conditions | Yield |
|---|---|
| In neat (no solvent) byproducts: (CH3)3SiH, (C2H5)2O; evacuating Schlenk vessel loaded with ((CH3)3Si)3Al*(C2H5)2O (glovebag, N2-atmosphere); condensing NH3 into flask at -196°C; warming slowly to room temp. (vigorous react.); stirring at 25°C for 48 h;; pptn.; removing volatile byproducts into trap cooled to -196°C; elem. anal.;; | 100% |
-
-
133869-39-3, 65702-94-5
undecacarbonyl(acetonitrile)triosmium

-
-
7664-41-7
ammonia

-
-
170212-37-0, 74344-99-3
Os3(CO)11(NH3)

| Conditions | Yield |
|---|---|
| In neat (no solvent) NH3-atmosphere; 80°C (24 h); | 100% |

| Conditions | Yield |
|---|---|
| In neat (no solvent) NH3-atmosphere; 80°C (24 h); | 100% |
Related products
Raw Materials
Hot Products
About|Contact|Cas|Product Name|Molecular|Country|Encyclopedia
Message|New Cas|MSDS|Service|Advertisement|CAS DataBase|Article Data|Manufacturers | Chemical Catalog
©2008 LookChem.com,License: ICP
NO.:Zhejiang16009103
complaints:service@lookchem.com Desktop View


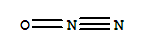












 T;
T;  N
N