-
Name
Abacavir
- EINECS 620-487-9
- CAS No. 136470-78-5
- Article Data23
- CAS DataBase
- Density 1.7 g/cm3
- Solubility
- Melting Point 165°
- Formula C14H18N6O
- Boiling Point 636 °C at 760 mmHg
- Molecular Weight 286.337
- Flash Point 338.4 °C
- Transport Information
- Appearance white powder
- Safety
- Risk Codes R21; R23/25; R43; R50/53
-
Molecular Structure
-
Hazard Symbols
 T,
T, N
N
- Synonyms {(1S,4R)-4-[2-amino-6-(cyclopropylamino)-9H-purin-9-yl]cyclopent-2-en-1-yl}methanol;Ziagen;2-Cyclopentene-1-methanol, 4-(2-amino-6-(cyclopropylamino)-9H-purin-9-yl)-, (1S-cis)-;4-Amino-5-chloro-2-ethoxy benzoic acid;Trizivir;2-Cyclopentene-1-methanol, 4-(2-amino-6-(cyclopropylamino)-9H-purin-9-yl)-, (1S,4R)-;Abacavir (Ziagen);
- PSA 101.88000
- LogP 1.74650
Synthetic route
-
-
136522-33-3
(1S,4R)-4-[2-amino-6-chloro-9H-purin-9-yl]-2-cyclopentene-1-methanol

-
-
765-30-0
Cyclopropylamine

-
-
136470-78-5
abacavir

| Conditions | Yield |
|---|---|
| In ethanol at 70℃; | 98% |
| In ethanol Heating; | 72% |
| Stage #1: (1S,4R)-4-[2-amino-6-chloro-9H-purin-9-yl]-2-cyclopentene-1-methanol; Cyclopropylamine In butan-1-ol at 40 - 70℃; for 5h; Stage #2: With sodium hydrogencarbonate In butan-1-ol at 20 - 25℃; for 1h; Product distribution / selectivity; | |
| In butan-1-ol at 45 - 90℃; for 2h; Product distribution / selectivity; | |
| With sodium hydrogencarbonate In ethanol; chloroform | 0.43 g (80%) |
-
-
765-30-0
Cyclopropylamine

-
-
172015-79-1
(1S,cis)-4-(2-amino-6-chloro-9-H-purin-9-yl)-2-cyclopentene-1-methanol hydrochloride

-
-
136470-78-5
abacavir

| Conditions | Yield |
|---|---|
| In methanol at 70℃; for 12h; Substitution; | 90% |
| Stage #1: (1S,cis)-4-(2-amino-6-chloro-9-H-purin-9-yl)-2-cyclopentene-1-methanol hydrochloride With triethylamine In water Stage #2: Cyclopropylamine In water at 70 - 75℃; Concentration; Solvent; Temperature; | 90% |
| In isopropyl alcohol at 90 - 95℃; for 12h; sealed reactor; | |
| In ethanol at 25 - 78℃; for 6h; | |
| With sodium carbonate In ethanol at 70 - 80℃; | 25 g |
-
-
136470-88-7
(-)-N-{6-(cyclopropylamino)-9-[(1R,4S)-4-(hydroxymethyl)cyclopent-2-enyl]-9H-purin-2-yl}isobutyramide

-
-
136470-78-5
abacavir

| Conditions | Yield |
|---|---|
| Stage #1: (-)-N-{6-(cyclopropylamino)-9-[(1R,4S)-4-(hydroxymethyl)cyclopent-2-enyl]-9H-purin-2-yl}isobutyramide With isopropyl alcohol; sodium hydroxide for 1h; Reflux; Stage #2: With hydrogenchloride In water; isopropyl alcohol at 20 - 25℃; pH=7.0 - 7.5; Product distribution / selectivity; | 90% |
| With sodium hydroxide; water; isopropyl alcohol for 1h; Heating / reflux; | 77% |
| With sodium hydroxide; water; isopropyl alcohol for 1h; Product distribution / selectivity; Heating / reflux; | 43% |
-
-
7440-44-0
pyrographite

-
-
765-30-0
Cyclopropylamine

-
-
172015-79-1
(1S,cis)-4-(2-amino-6-chloro-9-H-purin-9-yl)-2-cyclopentene-1-methanol hydrochloride

-
-
136470-78-5
abacavir

| Conditions | Yield |
|---|---|
| With sodium hydroxide In acetone | 90% |

-
-
1067882-78-3
6-cyclopropylamine-9-((1R,4S)-4-((triisopropylsilyloxy)methyl)cyclopent-2-enyl)-9H-purin-2-amine

-
-
136470-78-5
abacavir

| Conditions | Yield |
|---|---|
| With tetrabutyl ammonium fluoride; water In tetrahydrofuran at 20℃; for 1h; Inert atmosphere; | 89% |

-
-
136470-78-5
abacavir

| Conditions | Yield |
|---|---|
| With sodium hydroxide In water; ethyl acetate at 25 - 45℃; pH=6 - 6.1; | 78% |
-
-
1360538-03-9
((1S,4R)-4-(6-(cyclopropylamino)-2-((4-methoxybenzyl)amino)-9H-purin-9-yl)cyclopent-2-en-1-yl)methanol

-
-
136470-78-5
abacavir

| Conditions | Yield |
|---|---|
| With trifluoroacetic acid In chloroform at 50℃; for 72h; Inert atmosphere; | 73% |

-
-
136470-78-5
abacavir

| Conditions | Yield |
|---|---|
| With trifluoroacetic acid In dichloromethane at 60℃; for 60h; Schlenk technique; | 70% |
-
-
1108600-46-9
((1S,4R)-4-(2-chloro-6-(cyclopropylamino)-9H-purin-9-yl)cyclopent-2-en-1-yl)methanol

-
-
136470-78-5
abacavir

| Conditions | Yield |
|---|---|
| With ammonium hydroxide; copper(l) iodide In ethanol at 150℃; for 40h; Schlenk technique; Sealed tube; | 63% |
| Stage #1: ((1S,4R)-4-(2-chloro-6-(cyclopropylamino)-9H-purin-9-yl)cyclopent-2-en-1-yl)methanol With hydrazine In methanol; water at 50℃; Stage #2: With acetic acid; sodium nitrite In water at 0℃; for 1h; Stage #3: With tin(ll) chloride In ethanol for 2h; Product distribution / selectivity; Heating / reflux; | |
| Stage #1: ((1S,4R)-4-(2-chloro-6-(cyclopropylamino)-9H-purin-9-yl)cyclopent-2-en-1-yl)methanol With hydrazine In methanol at 50℃; Stage #2: With acetic acid; sodium nitrite In water at 0℃; for 1h; Stage #3: With ammonia; triphenylphosphine In 1,4-dioxane; water for 5h; Product distribution / selectivity; Heating / reflux; | |
| Multi-step reaction with 2 steps 1: dimethyl sulfoxide / 150 °C 2: trifluoroacetic acid / chloroform / 72 h / 50 °C / Inert atmosphere View Scheme | |
| Multi-step reaction with 3 steps 1: hydrazine hydrate / methanol / 50 °C 2: acetic acid; sodium nitrite / water / 1 h / Cooling with ice 3: stannous chloride dihydrate / ethanol / 2 h / Reflux View Scheme |
-
-
765-30-0
Cyclopropylamine

-
-
171887-04-0
(1R,4S)-1-[(2-amino-6-chloro-5-formamido-4-pyrimidinyl)amino]-4-(hydroxymethyl)-2-cyclopentene

-
A
-
141271-12-7
(1S, 4R)-(4-(2,5-diamino-6-chloro-4-pyrimidinyl)amino)-2-cyclopenten-1-methanol

-
B
-
136470-78-5
abacavir

| Conditions | Yield |
|---|---|
| In butan-1-ol at 130℃; for 21h; Substitution; hydrolysis; | A 16% B 60% |

-
-
10310-21-1
2-Amino-6-chloropurin

-
-
765-30-0
Cyclopropylamine

-
-
268737-86-6
cis-3-benzoyloxy-4-(hydroxymethyl)cyclopentene

-
-
136470-78-5
abacavir

| Conditions | Yield |
|---|---|
| Stage #1: 2-Amino-6-chloropurin; cis-3-benzoyloxy-4-(hydroxymethyl)cyclopentene With 1,2,2,6,6-pentamethylpiperidine; tris(dibenzylideneacetone)dipalladium (0); triphenylphosphine In tetrahydrofuran; dimethyl sulfoxide at 45℃; for 16h; Solid phase reaction; addition; elimination; Stage #2: Cyclopropylamine With N-ethyl-N,N-diisopropylamine In butan-1-ol at 80℃; for 4h; Solid phase reaction; amination; Further stages.; | 60% |


-
-
594-45-6
ethanesulfonic acid

-
-
122-51-0
orthoformic acid triethyl ester

-
-
765-30-0
Cyclopropylamine

-
-
136470-78-5
abacavir

| Conditions | Yield |
|---|---|
| In hydrogenchloride; methanol; N,N-dimethyl-formamide | 19% |

-
-
178456-36-5
((1S,4R)-4-(2-amino-6-(cyclopropylamino)-9H-purin-9-yl)cyclopent-2-en-1-yl)methyl acetate

-
-
136470-78-5
abacavir

| Conditions | Yield |
|---|---|
| With sodium hydroxide; water Yield given; | |
| With sodium hydroxide at 20℃; | 0.027 g |
| With sodium hydroxide |
-
-
143395-28-2
(1R,5R)-5-hydroxymethyl-2-cyclopenten-1-ol

-
-
136470-78-5
abacavir

| Conditions | Yield |
|---|---|
| Multi-step reaction with 3 steps 1: 90 percent / Et3N; DMAP / CH2Cl2 / 0 °C 2: 62 percent / NaH; Pd(PPh3)4 / dimethylsulfoxide; tetrahydrofuran / 45 °C 3: aq. NaOH View Scheme | |
| Multi-step reaction with 4 steps 1: 90 percent / Et3N; DMAP / CH2Cl2 / 0 °C 2: 0.298 g / NaH; Pd(PPh3)4 / dimethylsulfoxide; tetrahydrofuran / 45 °C 3: ethanol / 5 h / Heating 4: aq. NaOH View Scheme | |
| Multi-step reaction with 4 steps 1.1: Et3N 2.1: Et3N; 4-DMAP 3.1: aq. HF / acetonitrile 4.1: Pd2(dba)3; PPh3; 1,2,2,6,6-pentamethylpiperidine (pempidine) / tetrahydrofuran; dimethylsulfoxide / 16 h / 45 °C 4.2: 60 percent / EtN(i-Pr)2 / butan-1-ol / 4 h / 80 °C View Scheme |
-
-
120503-69-7
2-amino-6-(cyclopropylamino)purine

-
-
136470-78-5
abacavir

| Conditions | Yield |
|---|---|
| Multi-step reaction with 2 steps 1: 62 percent / NaH; Pd(PPh3)4 / dimethylsulfoxide; tetrahydrofuran / 45 °C 2: aq. NaOH View Scheme |
-
-
10310-21-1
2-Amino-6-chloropurin

-
-
136470-78-5
abacavir

| Conditions | Yield |
|---|---|
| Multi-step reaction with 3 steps 1: dimethylsulfoxide / 16 h / 55 °C 2: 62 percent / NaH; Pd(PPh3)4 / dimethylsulfoxide; tetrahydrofuran / 45 °C 3: aq. NaOH View Scheme |
-
-
162992-44-1
9-[cis-(1'R,4'S)-4'-acetoxymethyl-2'-cyclopenten-1'-yl]-9H-2-amino-6-chloropurine

-
-
136470-78-5
abacavir

| Conditions | Yield |
|---|---|
| Multi-step reaction with 2 steps 1: ethanol / 5 h / Heating 2: aq. NaOH View Scheme | |
| Multi-step reaction with 2 steps 1: ethanol 2: NaOH, H2O View Scheme |
-
-
178456-34-3
(1R,2R)-2-acetoxy-1-(acetoxymethyl)-3-cyclopentene

-
-
136470-78-5
abacavir

| Conditions | Yield |
|---|---|
| Multi-step reaction with 2 steps 1: 62 percent / NaH; Pd(PPh3)4 / dimethylsulfoxide; tetrahydrofuran / 45 °C 2: aq. NaOH View Scheme | |
| Multi-step reaction with 3 steps 1: 0.298 g / NaH; Pd(PPh3)4 / dimethylsulfoxide; tetrahydrofuran / 45 °C 2: ethanol / 5 h / Heating 3: aq. NaOH View Scheme | |
| Multi-step reaction with 2 steps 1: 62 percent / NaH, Pd(PPh3)4 / tetrahydrofuran; dimethylsulfoxide 2: NaOH, H2O View Scheme | |
| Multi-step reaction with 3 steps 1: 65 percent / NaH, Pd(PPh3)4 / tetrahydrofuran; dimethylsulfoxide 2: ethanol 3: NaOH, H2O View Scheme |
-
-
178327-18-9
[3(1R,2R),4S]-4-benzyl-3-(2-hydroxycyclopent-3-enecarbonyl)oxazolidin-2-one

-
-
136470-78-5
abacavir

| Conditions | Yield |
|---|---|
| Multi-step reaction with 4 steps 1: 78 percent / LiBH4 / tetrahydrofuran; methanol / 1 h / 0 °C 2: 90 percent / Et3N; DMAP / CH2Cl2 / 0 °C 3: 62 percent / NaH; Pd(PPh3)4 / dimethylsulfoxide; tetrahydrofuran / 45 °C 4: aq. NaOH View Scheme | |
| Multi-step reaction with 5 steps 1: 78 percent / LiBH4 / tetrahydrofuran; methanol / 1 h / 0 °C 2: 90 percent / Et3N; DMAP / CH2Cl2 / 0 °C 3: 0.298 g / NaH; Pd(PPh3)4 / dimethylsulfoxide; tetrahydrofuran / 45 °C 4: ethanol / 5 h / Heating 5: aq. NaOH View Scheme | |
| Multi-step reaction with 4 steps 1: 78 percent / LiBH4 / tetrahydrofuran; methanol 2: 90 percent / Et3N, DMAP / CH2Cl2 3: 62 percent / NaH, Pd(PPh3)4 / tetrahydrofuran; dimethylsulfoxide 4: NaOH, H2O View Scheme | |
| Multi-step reaction with 5 steps 1: 78 percent / LiBH4 / tetrahydrofuran; methanol 2: 90 percent / Et3N, DMAP / CH2Cl2 3: 65 percent / NaH, Pd(PPh3)4 / tetrahydrofuran; dimethylsulfoxide 4: ethanol 5: NaOH, H2O View Scheme |
-
-
178327-17-8
[3(2S,4R),4S]-3-(2-allyl-3-hydroxypent-4-enoyl)-4-benzyloxazolidin-2-one

-
-
136470-78-5
abacavir

| Conditions | Yield |
|---|---|
| Multi-step reaction with 5 steps 1: 97 percent / (Cy3P)2Cl2Ru=CHPh / CH2Cl2 / 0.5 h / 25 °C 2: 78 percent / LiBH4 / tetrahydrofuran; methanol / 1 h / 0 °C 3: 90 percent / Et3N; DMAP / CH2Cl2 / 0 °C 4: 62 percent / NaH; Pd(PPh3)4 / dimethylsulfoxide; tetrahydrofuran / 45 °C 5: aq. NaOH View Scheme | |
| Multi-step reaction with 6 steps 1: 97 percent / (Cy3P)2Cl2Ru=CHPh / CH2Cl2 / 0.5 h / 25 °C 2: 78 percent / LiBH4 / tetrahydrofuran; methanol / 1 h / 0 °C 3: 90 percent / Et3N; DMAP / CH2Cl2 / 0 °C 4: 0.298 g / NaH; Pd(PPh3)4 / dimethylsulfoxide; tetrahydrofuran / 45 °C 5: ethanol / 5 h / Heating 6: aq. NaOH View Scheme | |
| Multi-step reaction with 5 steps 1: 97 percent / Grubbs catalyst / CH2Cl2 2: 78 percent / LiBH4 / tetrahydrofuran; methanol 3: 90 percent / Et3N, DMAP / CH2Cl2 4: 62 percent / NaH, Pd(PPh3)4 / tetrahydrofuran; dimethylsulfoxide 5: NaOH, H2O View Scheme | |
| Multi-step reaction with 6 steps 1: 97 percent / Grubbs catalyst / CH2Cl2 2: 78 percent / LiBH4 / tetrahydrofuran; methanol 3: 90 percent / Et3N, DMAP / CH2Cl2 4: 65 percent / NaH, Pd(PPh3)4 / tetrahydrofuran; dimethylsulfoxide 5: ethanol 6: NaOH, H2O View Scheme |
-
-
268737-90-2
[4S,2R,3R]-(4-benzyl-2-thioxooxazolidin-3-yl)(2-hydroxycyclopent-3-enyl)methanone

-
-
136470-78-5
abacavir

| Conditions | Yield |
|---|---|
| Multi-step reaction with 4 steps 1: 85 percent / LiBH4 / tetrahydrofuran; methanol 2: 90 percent / Et3N; DMAP / CH2Cl2 / 0 °C 3: 62 percent / NaH; Pd(PPh3)4 / dimethylsulfoxide; tetrahydrofuran / 45 °C 4: aq. NaOH View Scheme | |
| Multi-step reaction with 5 steps 1: 85 percent / LiBH4 / tetrahydrofuran; methanol 2: 90 percent / Et3N; DMAP / CH2Cl2 / 0 °C 3: 0.298 g / NaH; Pd(PPh3)4 / dimethylsulfoxide; tetrahydrofuran / 45 °C 4: ethanol / 5 h / Heating 5: aq. NaOH View Scheme | |
| Multi-step reaction with 5 steps 1.1: 78 percent / LiBH4 / methanol 2.1: Et3N 3.1: Et3N; 4-DMAP 4.1: aq. HF / acetonitrile 5.1: Pd2(dba)3; PPh3; 1,2,2,6,6-pentamethylpiperidine (pempidine) / tetrahydrofuran; dimethylsulfoxide / 16 h / 45 °C 5.2: 60 percent / EtN(i-Pr)2 / butan-1-ol / 4 h / 80 °C View Scheme |
-
-
318488-34-5
(E)-(2R,3R)-2-Allyl-hex-4-ene-1,3-diol

-
-
136470-78-5
abacavir

| Conditions | Yield |
|---|---|
| Multi-step reaction with 4 steps 1: Et3N / CH2Cl2 2: (Cy3P)2Cl2Ru=CHPh / CH2Cl2 / 25 °C 3: 62 percent / NaH; Pd(PPh3)4 / dimethylsulfoxide; tetrahydrofuran / 45 °C 4: aq. NaOH View Scheme | |
| Multi-step reaction with 5 steps 1: Et3N / CH2Cl2 2: (Cy3P)2Cl2Ru=CHPh / CH2Cl2 / 25 °C 3: 0.298 g / NaH; Pd(PPh3)4 / dimethylsulfoxide; tetrahydrofuran / 45 °C 4: ethanol / 5 h / Heating 5: aq. NaOH View Scheme |

-
-
136470-78-5
abacavir

| Conditions | Yield |
|---|---|
| Multi-step reaction with 3 steps 1: (Cy3P)2Cl2Ru=CHPh / CH2Cl2 / 25 °C 2: 62 percent / NaH; Pd(PPh3)4 / dimethylsulfoxide; tetrahydrofuran / 45 °C 3: aq. NaOH View Scheme | |
| Multi-step reaction with 4 steps 1: (Cy3P)2Cl2Ru=CHPh / CH2Cl2 / 25 °C 2: 0.298 g / NaH; Pd(PPh3)4 / dimethylsulfoxide; tetrahydrofuran / 45 °C 3: ethanol / 5 h / Heating 4: aq. NaOH View Scheme |
-
-
318488-32-3
(E)-(2S,3R)-2-Allyl-1-((S)-4-benzyl-2-thioxo-oxazolidin-3-yl)-3-hydroxy-hex-4-en-1-one

-
-
136470-78-5
abacavir

| Conditions | Yield |
|---|---|
| Multi-step reaction with 5 steps 1: 77 percent / NaBH4 / tetrahydrofuran; H2O 2: Et3N / CH2Cl2 3: (Cy3P)2Cl2Ru=CHPh / CH2Cl2 / 25 °C 4: 62 percent / NaH; Pd(PPh3)4 / dimethylsulfoxide; tetrahydrofuran / 45 °C 5: aq. NaOH View Scheme | |
| Multi-step reaction with 6 steps 1: 77 percent / NaBH4 / tetrahydrofuran; H2O 2: Et3N / CH2Cl2 3: (Cy3P)2Cl2Ru=CHPh / CH2Cl2 / 25 °C 4: 0.298 g / NaH; Pd(PPh3)4 / dimethylsulfoxide; tetrahydrofuran / 45 °C 5: ethanol / 5 h / Heating 6: aq. NaOH View Scheme |
-
-
318488-35-6
(E)-(2S,3R)-2-Allyl-1-((R)-4-benzyl-2-thioxo-oxazolidin-3-yl)-3-hydroxy-hex-4-en-1-one

-
-
136470-78-5
abacavir

| Conditions | Yield |
|---|---|
| Multi-step reaction with 5 steps 1: 74 percent / (Cy3P)2Cl2Ru=CHPh / CH2Cl2 / 1 h / 40 °C 2: 85 percent / LiBH4 / tetrahydrofuran; methanol 3: 90 percent / Et3N; DMAP / CH2Cl2 / 0 °C 4: 62 percent / NaH; Pd(PPh3)4 / dimethylsulfoxide; tetrahydrofuran / 45 °C 5: aq. NaOH View Scheme | |
| Multi-step reaction with 6 steps 1: 74 percent / (Cy3P)2Cl2Ru=CHPh / CH2Cl2 / 1 h / 40 °C 2: 85 percent / LiBH4 / tetrahydrofuran; methanol 3: 90 percent / Et3N; DMAP / CH2Cl2 / 0 °C 4: 0.298 g / NaH; Pd(PPh3)4 / dimethylsulfoxide; tetrahydrofuran / 45 °C 5: ethanol / 5 h / Heating 6: aq. NaOH View Scheme |
-
-
268737-91-3
(1R,5R)-5-(tert-Butyl-dimethyl-silanyloxymethyl)-cyclopent-2-enol

-
-
136470-78-5
abacavir

| Conditions | Yield |
|---|---|
| Multi-step reaction with 3 steps 1.1: Et3N; 4-DMAP 2.1: aq. HF / acetonitrile 3.1: Pd2(dba)3; PPh3; 1,2,2,6,6-pentamethylpiperidine (pempidine) / tetrahydrofuran; dimethylsulfoxide / 16 h / 45 °C 3.2: 60 percent / EtN(i-Pr)2 / butan-1-ol / 4 h / 80 °C View Scheme |
-
-
268737-92-4
Benzoic acid (1R,5R)-5-(tert-butyl-dimethyl-silanyloxymethyl)-cyclopent-2-enyl ester

-
-
136470-78-5
abacavir

| Conditions | Yield |
|---|---|
| Multi-step reaction with 2 steps 1.1: aq. HF / acetonitrile 2.1: Pd2(dba)3; PPh3; 1,2,2,6,6-pentamethylpiperidine (pempidine) / tetrahydrofuran; dimethylsulfoxide / 16 h / 45 °C 2.2: 60 percent / EtN(i-Pr)2 / butan-1-ol / 4 h / 80 °C View Scheme |
-
-
268737-89-9
(2S,3R)-2-Allyl-1-((R)-4-benzyl-2-thioxo-oxazolidin-3-yl)-3-hydroxy-pent-4-en-1-one

-
-
136470-78-5
abacavir

| Conditions | Yield |
|---|---|
| Multi-step reaction with 6 steps 1.1: 97 percent / catalytic ring-closing metathesis (RCM) 2.1: 78 percent / LiBH4 / methanol 3.1: Et3N 4.1: Et3N; 4-DMAP 5.1: aq. HF / acetonitrile 6.1: Pd2(dba)3; PPh3; 1,2,2,6,6-pentamethylpiperidine (pempidine) / tetrahydrofuran; dimethylsulfoxide / 16 h / 45 °C 6.2: 60 percent / EtN(i-Pr)2 / butan-1-ol / 4 h / 80 °C View Scheme |
-
-
79200-56-9
(-)-2-azabicyclo[2.2.1]hept-5-en-3-one

-
-
136470-78-5
abacavir

| Conditions | Yield |
|---|---|
| Multi-step reaction with 4 steps 1: 1.264 g / tetrahydrofuran; H2O / 18 h / 20 - 60 °C 2: 90.5 percent / LiAlH4 / tetrahydrofuran / 19.5 h / 0 °C / Heating 3: 83 percent / Et3N / ethanol / 7 h / Heating 4: 60 percent / butan-1-ol / 21 h / 130 °C View Scheme | |
| Multi-step reaction with 5 steps 1: 1.264 g / tetrahydrofuran; H2O / 18 h / 20 - 60 °C 2: 90.5 percent / LiAlH4 / tetrahydrofuran / 19.5 h / 0 °C / Heating 3: 83 percent / Et3N / ethanol / 7 h / Heating 4: 92 percent / triethylorthoformate; HCl / H2O / 4 h 5: 90 percent / methanol / 12 h / 70 °C View Scheme |
-
-
136470-78-5
abacavir

| Conditions | Yield |
|---|---|
| With hydrogen; palladium on activated charcoal In ethanol under 2585.74 Torr; for 1.5h; Catalytic hydrogenation; | 98% |
-
-
141-78-6
ethyl acetate

-
-
136470-78-5
abacavir

-
-
178456-36-5
((1S,4R)-4-(2-amino-6-(cyclopropylamino)-9H-purin-9-yl)cyclopent-2-en-1-yl)methyl acetate

| Conditions | Yield |
|---|---|
| With heterogeneous zinc/imidazole catalyst for 6.5h; Inert atmosphere; Schlenk technique; Reflux; chemoselective reaction; | 98% |
-
-
136470-78-5
abacavir

| Conditions | Yield |
|---|---|
| With sulfuric acid In water; isopropyl alcohol at 50 - 55℃; for 2h; Concentration; Temperature; Solvent; | 95% |
-
-
110-16-7
maleic acid

-
-
136470-78-5
abacavir

-
-
1280629-56-2
(1S,4R)-4-[2-amino-6-(cyclopropylamino)-9H-purin-9-yl]-2-cyclopentene-1-methanol maleate

| Conditions | Yield |
|---|---|
| In ethanol at 25 - 30℃; for 0.25h; | 94.3% |
-
-
100-10-7
4-dimethylamino-benzaldehyde

-
-
136470-78-5
abacavir

| Conditions | Yield |
|---|---|
| With acetic acid microwave irradiation; | 89% |
-
-
141-82-2
malonic acid

-
-
136470-78-5
abacavir

-
-
1280629-49-3
(1S,4R)-4-[2-amino-6-(cyclopropylamino)-9H-purin-9-yl]-2-cyclopentene-1-methanol malonate

| Conditions | Yield |
|---|---|
| In ethanol at 25 - 70℃; for 0.75h; Product distribution / selectivity; | 88% |
-
-
443-69-6
5-fluoro-1H-indole-2,3-dione

-
-
136470-78-5
abacavir

| Conditions | Yield |
|---|---|
| With acetic acid microwave irradiation; | 87% |
-
-
1579-72-2
2,2,2-trifluoroethyl benzoate

-
-
136470-78-5
abacavir

| Conditions | Yield |
|---|---|
| With (μ-oxo)bis[(1,2-ethanediamino-N,N'-bis(salicylidene))iron(III)] In toluene at 120℃; for 5h; Inert atmosphere; Schlenk technique; chemoselective reaction; | 87% |

| Conditions | Yield |
|---|---|
| With dmap; 1-ethyl-(3-(3-dimethylamino)propyl)-carbodiimide hydrochloride In N,N-dimethyl-formamide at 0℃; for 5h; | 85% |
-
-
66089-45-0
1-ethenesulfonyl-piperidine

-
-
136470-78-5
abacavir

| Conditions | Yield |
|---|---|
| Stage #1: abacavir With silver(I) acetate; 1,2-bis-(diphenylphosphino)ethane In N,N-dimethyl-formamide at -20℃; Inert atmosphere; Stage #2: 1-ethenesulfonyl-piperidine With potassium hexamethylsilazane In tetrahydrofuran; N,N-dimethyl-formamide at -20℃; for 21h; Inert atmosphere; regioselective reaction; | 85% |
-
-
136470-78-5
abacavir

| Conditions | Yield |
|---|---|
| With hydrogen bromide In ethanol; water at 25 - 30℃; for 0.75h; Product distribution / selectivity; | 83% |
-
-
91-56-5
indole-2,3-dione

-
-
136470-78-5
abacavir

| Conditions | Yield |
|---|---|
| With acetic acid microwave irradiation; | 82% |
-
-
136470-78-5
abacavir

-
-
1280727-16-3
(1S,4R)-4-[2-amino-6-(cyclopropylamino)-9H-purin-9-yl]-2-cyclopentene-1-methanol hydrobromide

| Conditions | Yield |
|---|---|
| With hydrogen bromide In ethanol; water at 25 - 30℃; for 0.75h; Product distribution / selectivity; | 82% |
-
-
608-05-9
5-methyl-indole-2,3-dione

-
-
136470-78-5
abacavir

| Conditions | Yield |
|---|---|
| With acetic acid microwave irradiation; | 78% |

-
-
136470-78-5
abacavir

-
-
261909-26-6
(S)-2-{[(1S,4R)-4-(2-Amino-6-cyclopropylamino-purin-9-yl)-cyclopent-2-enylmethoxy]-phenoxy-phosphorylamino}-propionic acid methyl ester

| Conditions | Yield |
|---|---|
| Stage #1: abacavir With tert-butylmagnesium chloride In tetrahydrofuran at -78℃; Inert atmosphere; Stage #2: C13H18ClN2O5P In tetrahydrofuran at 20℃; Inert atmosphere; | 76% |
-
-
13211-32-0
tert-butoxyacetic acid

-
-
136470-78-5
abacavir

| Conditions | Yield |
|---|---|
| With dmap; 1-ethyl-(3-(3-dimethylamino)propyl)-carbodiimide hydrochloride In dichloromethane at 25 - 30℃; | 74% |

| Conditions | Yield |
|---|---|
| Stage #1: abacavir With pyridine; water; trichlorophosphate In acetonitrile at 0℃; Stage #2: With water; ammonium bicarbonate In acetonitrile at 0℃; pH=8; Stage #3: tetra(n-butyl)ammonium hydroxide In water; acetonitrile | 72% |
-
-
136470-78-5
abacavir

-
-
99-93-4
4-Hydroxyacetophenone

| Conditions | Yield |
|---|---|
| With acetic acid microwave irradiation; | 71% |
-
-
2052-49-5
tetra(n-butyl)ammonium hydroxide

-
-
136470-78-5
abacavir

| Conditions | Yield |
|---|---|
| Stage #1: abacavir With trichlorophosphate at 0℃; for 1h; Inert atmosphere; Stage #2: With water for 1h; Cooling with ice; Stage #3: tetra(n-butyl)ammonium hydroxide In water pH=7.0; | 71% |
-
-
123-11-5
4-methoxy-benzaldehyde

-
-
136470-78-5
abacavir

| Conditions | Yield |
|---|---|
| With acetic acid microwave irradiation; | 70% |

| Conditions | Yield |
|---|---|
| Stage #1: 1,10-decanedioic acid With dmap; benzotriazol-1-yloxyl-tris-(pyrrolidino)-phosphonium hexafluorophosphate; diisopropylamine In N,N-dimethyl-formamide at -20℃; for 0.333333h; Stage #2: abacavir In N,N-dimethyl-formamide at -20 - 20℃; for 16h; | 70% |
Abacavir Specification
1. Introduction of Abacavir
Abacavir is one kind of white powder. Its IUPAC name is called [(1S,4R)-4-[2-amino-6-(cyclopropylamino)purin-9-yl]cyclopent-2-en-1-yl]methanol. It belongs to classification codes which are Anti-HIV Agents, Anti-Infective Agents, Anti-Retroviral Agents, Antiviral Agents, Enzyme Inhibitors, Nucleic Acid Synthesis Inhibitors, Reverse Transcriptase Inhibitors. Besides, its Product Categories is Bases & Related Reagents;Inhibitors;Intermediates & Fine Chemicals;Nucleotides;Pharmaceuticals. It should be store at refrigerator. Abacavir can be soluble in water. Its Classification Codes is Anti-HIV Agents; Anti-Infective Agents; Anti-Retroviral Agents; Antiviral Agents; Enzyme Inhibitors; Nucleic Acid Synthesis Inhibitors; Reverse Transcriptase Inhibitors.
2. Properties of Abacavir
Physical properties about Abacavir are:
(1) ACD/LogP: -0.78 ; (2) # of Rule of 5 Violations: 0 ; (3) ACD/LogD (pH 7.4): 0.66 ; (4) ACD/BCF (pH 5.5): 1 ; (5) ACD/BCF (pH 7.4): 1.8 ; (6) ACD/KOC (pH 5.5): 4.47 ; (7) ACD/KOC (pH 7.4): 50.77 ; (8) #H bond acceptors: 7 ; (9) #H bond donors: 4 ; (10) #Freely Rotating Bonds: 4 ; (11) Polar Surface Area: 59.31 Å2 ; (12) Index of Refraction: 1.864 ; (13) Molar Refractivity: 75.8 cm3 ; (14) Molar Volume: 167.6 cm3 ; (15) Surface Tension: 80.1 dyne/cm ; (16) Density: 1.7 g/cm3 ; (17) Flash Point: 338.4 °C ; (18) Enthalpy of Vaporization: 98.77 kJ/mol ; (19) Boiling Point: 636 °C at 760 mmHg ; (20) Vapour Pressure: 4.77E-17 mmHg at 25°C.
3. Structure Descriptors of Abacavir
(1) SMILES:n3c1c(ncn1[C@H]2/C=C\[C@@H](CO)C2)c(nc3N)NC4CC4;
(2) InChI:InChI=1/C14H18N6O/c15-14-18-12(17-9-2-3-9)11-13(19-14)20(7-16-11)10-4-1-8(5-10)6-21/h1,4,7-10,21H,2-3,5-6H2,(H3,15,17,18,19)/t8-,10+/m1/s1;
(3) InChIKey:MCGSCOLBFJQGHM-SCZZXKLOBP
4. Uses of Abacavir
Abacavir (ABC) is a powerful nucleoside analog reverse transcriptase inhibitor (NRTI) used to treat HIV and AIDS. This compound has been used as a drug with the trade name of Ziagen. It is a kind of antiretroviral drugs approved by the Food and Drug Administration (FDA). It has tablets and oral solution. This drug is used to treat HIV and AIDS in combination with other antiretroviral agents. It can be used for research and manufacture Epzicom tablet.
5. Production of Abacavir
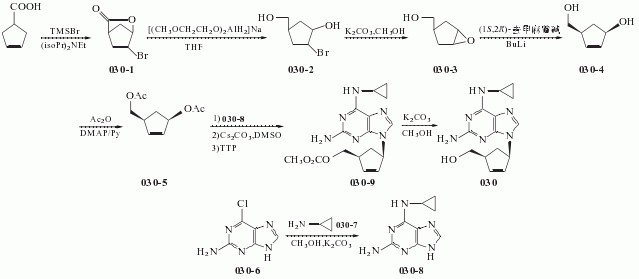
030-8 1.0g (0.0053mol), in the reaction flask was added cesium carbonate 1.75 g (0.0054 mol) and dry DMSO 50ml, stirred under N2 protection, the temperature was raised to 60 °C and stirred at this temperature for 2 h the mixture wascooled to room temperature, then add tetrakis (triphenylphosphine) combined palladium (TTP) [0.85 (0.00074mol)] and compound 030-5 [0.79g (0.0034 mol), DMSO (10 ml) solution was stirred and heated to 65 °C held 65 °C and stirred reaction 2.25h. The you can get the mixture containing compounds 030-9.
To the mixture was added methanol 100ml and K2CO3 is 2.10g, the mixture reaction was stirred for 45min at 40 °C, a solid precipitate which was filtered through a Celite layer and the filtrate was evaporated to a small volume under vacuum at 90 °C, and the remaining gum pounding mill was extracted with dichloromethane (100ml * 2) to give a brown solid residue was purified by silica gel (Merck 9385) column chromatography [eluent: dichloromethane / methanol (volume ratio 9:1)] to give a yellow foam was 030 0.26 g, yield 26.8.
Related Products
- Abacavir
- Abacavir sulfate
- 13647-35-3
- 136480-53-0
- 136483-17-5
- 136496-72-5
- 13649-88-2
- 136499-31-5
- 13650-38-9
- 136504-87-5
- 13650-49-2
- 136505-00-5
Hot Products
About|Contact|Cas|Product Name|Molecular|Country|Encyclopedia
Message|New Cas|MSDS|Service|Advertisement|CAS DataBase|Article Data|Manufacturers | Chemical Catalog
©2008 LookChem.com,License: ICP
NO.:Zhejiang16009103
complaints:service@lookchem.com Desktop View