-
Name
Benazepril hydrochloride
- EINECS 630-414-2
- CAS No. 86541-74-4
- Article Data13
- CAS DataBase
- Density
- Solubility DMSO: ~34 mg/mL, soluble
- Melting Point 188-190 °C
- Formula C24H29ClN2O5
- Boiling Point 691.2 °C at 760 mmHg
- Molecular Weight 460.958
- Flash Point 371.8 °C
- Transport Information
- Appearance crystalline solid
- Safety 22-24/25
- Risk Codes
-
Molecular Structure
- Hazard Symbols
- Synonyms 1H-1-Benzazepine-1-aceticacid,3-[[(1S)-1-(ethoxycarbonyl)-3-phenylpropyl]amino]-2,3,4,5-tetrahydro-2-oxo-,monohydrochloride, (3S)- (9CI);1H-1-Benzazepine-1-acetic acid,3-[[1-(ethoxycarbonyl)-3-phenylpropyl]amino]-2,3,4,5-tetrahydro-2-oxo-,monohydrochloride, [S-(R*,R*)]-;CGS 14824A;CGS14824A HCl;Lotensin;Lotension;
- PSA 95.94000
- LogP 3.83100
Synthetic route
-
-
109010-61-9
(2S,3'S)-2-(1-tert-butoxycarbonylmethyl-2-oxo-2,3,4,5-tetrahydro-1H-benzo[b]azepin-3-ylamino)-4-phenylbutyric acid ethyl ester

-
-
86541-74-4
benazepril hydrochloride

| Conditions | Yield |
|---|---|
| With hydrogenchloride In ethyl acetate | 91.6% |
| With hydrogenchloride In toluene at 0 - 20℃; for 1.5h; | 90% |
| With hydrogenchloride In ethyl acetate at -10 - 25℃; for 16h; Industry scale; | |
| With hydrogenchloride In ethyl acetate at 10℃; under 760.051 Torr; Solvent; Reflux; | Ca. 114 g |
| With hydrogenchloride In Isopropyl acetate at 10℃; | 99.6 g |

-
-
86541-74-4
benazepril hydrochloride

| Conditions | Yield |
|---|---|
| Stage #1: ethyl (S)-2-(((S)-1-(2-(benzyloxy)-2-oxoethyl)-2-oxo-2,3,4,5-tetrahydro-1H-benzo[b]azepin-3- yl)amino)-4-phenylbutanoate With palladium 10% on activated carbon; hydrogen In ethanol at 20℃; for 3h; Stage #2: With hydrogenchloride In ethyl acetate; acetone Reflux; | 91% |
-
-
64920-29-2
2-oxo-4-phenylbutanoic acid ethyl ester

-
-
86499-53-8
(3S)-3-amino-1-(carboxymethyl)-2,3,4,5-tetrahydro-1H-<1>benzazepin-2-one sodium salt

-
A
-
86541-74-4
benazepril hydrochloride

-
B
-
86541-77-7
(3S)-1-(carboxymethyl)-<<(1S)-1-(ethoxycarbonyl)-3-phenylpropyl>amino>-2,3,4,5-tetrahydro-1H-<1>benzazepin-2-one hydrochloride

| Conditions | Yield |
|---|---|
| With sodium cyanoborohydride In methanol; acetic acid Ambient temperature; | A 12% B 25% |

-
-
577-59-3
2-acetylnitrobenzene

-
-
86541-74-4
benazepril hydrochloride

| Conditions | Yield |
|---|---|
| Multi-step reaction with 7 steps 1.1: 99 percent / sodium ethoxide / tetrahydrofuran / 2 h / 0 °C 2.1: phenacyl chloride; baker's yeast / diethyl ether; H2O / 24 h / 30 °C 3.1: H2; HCl / Pd/C / methanol / 20 °C 3.2: 42 percent / AcOH / toluene / 80 °C 4.1: Et3N / tetrahydrofuran / 16 h / 20 °C 5.1: 78 percent / 1,2-dimethoxy-ethane / 60 h / 50 °C 6.1: tetrabutylammonium bromide; KOH / tetrahydrofuran / 0.5 h / 0 °C 6.2: 98 percent / tetrahydrofuran / 5 h / 20 °C 7.1: 90 percent / HCl / toluene / 1.5 h / 0 - 20 °C View Scheme | |
| Multi-step reaction with 8 steps 1.1: 99 percent / sodium ethoxide / tetrahydrofuran / 2 h / 0 °C 2.1: phenacyl chloride; baker's yeast / diethyl ether; H2O / 24 h / 30 °C 3.1: NaBH4; acetic acid / 2 h / 0 °C 4.1: H2 / Pd/C / methanol / 24 h / 20 °C 4.2: H2; hydrochloric acid / Pd/C / methanol / 36 h / 20 °C 4.3: 74 percent / AcOH / toluene / 16 h / 80 °C 5.1: Et3N / tetrahydrofuran / 16 h / 20 °C 6.1: 78 percent / 1,2-dimethoxy-ethane / 60 h / 50 °C 7.1: tetrabutylammonium bromide; KOH / tetrahydrofuran / 0.5 h / 0 °C 7.2: 98 percent / tetrahydrofuran / 5 h / 20 °C 8.1: 90 percent / HCl / toluene / 1.5 h / 0 - 20 °C View Scheme |
-
-
178114-28-8
4-(2-nitrophenyl)-2,4-dioxobutanoic acid ethyl ester

-
-
86541-74-4
benazepril hydrochloride

| Conditions | Yield |
|---|---|
| Multi-step reaction with 6 steps 1.1: phenacyl chloride; baker's yeast / diethyl ether; H2O / 24 h / 30 °C 2.1: H2; HCl / Pd/C / methanol / 20 °C 2.2: 42 percent / AcOH / toluene / 80 °C 3.1: Et3N / tetrahydrofuran / 16 h / 20 °C 4.1: 78 percent / 1,2-dimethoxy-ethane / 60 h / 50 °C 5.1: tetrabutylammonium bromide; KOH / tetrahydrofuran / 0.5 h / 0 °C 5.2: 98 percent / tetrahydrofuran / 5 h / 20 °C 6.1: 90 percent / HCl / toluene / 1.5 h / 0 - 20 °C View Scheme | |
| Multi-step reaction with 7 steps 1.1: phenacyl chloride; baker's yeast / diethyl ether; H2O / 24 h / 30 °C 2.1: NaBH4; acetic acid / 2 h / 0 °C 3.1: H2 / Pd/C / methanol / 24 h / 20 °C 3.2: H2; hydrochloric acid / Pd/C / methanol / 36 h / 20 °C 3.3: 74 percent / AcOH / toluene / 16 h / 80 °C 4.1: Et3N / tetrahydrofuran / 16 h / 20 °C 5.1: 78 percent / 1,2-dimethoxy-ethane / 60 h / 50 °C 6.1: tetrabutylammonium bromide; KOH / tetrahydrofuran / 0.5 h / 0 °C 6.2: 98 percent / tetrahydrofuran / 5 h / 20 °C 7.1: 90 percent / HCl / toluene / 1.5 h / 0 - 20 °C View Scheme |
-
-
46460-23-5
ethyl 2-amino-4-phenyl-(2S)-butyrate

-
-
86541-74-4
benazepril hydrochloride

| Conditions | Yield |
|---|---|
| Multi-step reaction with 3 steps 1.1: 78 percent / 1,2-dimethoxy-ethane / 60 h / 50 °C 2.1: tetrabutylammonium bromide; KOH / tetrahydrofuran / 0.5 h / 0 °C 2.2: 98 percent / tetrahydrofuran / 5 h / 20 °C 3.1: 90 percent / HCl / toluene / 1.5 h / 0 - 20 °C View Scheme |
-
-
608148-60-3
(3R)-3-hydroxy-1,3,4,5-tetrahydrobenzo[b]azepin-2-one

-
-
86541-74-4
benazepril hydrochloride

| Conditions | Yield |
|---|---|
| Multi-step reaction with 4 steps 1.1: Et3N / tetrahydrofuran / 16 h / 20 °C 2.1: 78 percent / 1,2-dimethoxy-ethane / 60 h / 50 °C 3.1: tetrabutylammonium bromide; KOH / tetrahydrofuran / 0.5 h / 0 °C 3.2: 98 percent / tetrahydrofuran / 5 h / 20 °C 4.1: 90 percent / HCl / toluene / 1.5 h / 0 - 20 °C View Scheme |
-
-
608148-58-9
(2R)-2-hydroxy-4-(2-nitrophenyl)-4-oxobutyric acid ethyl ester

-
-
86541-74-4
benazepril hydrochloride

| Conditions | Yield |
|---|---|
| Multi-step reaction with 5 steps 1.1: H2; HCl / Pd/C / methanol / 20 °C 1.2: 42 percent / AcOH / toluene / 80 °C 2.1: Et3N / tetrahydrofuran / 16 h / 20 °C 3.1: 78 percent / 1,2-dimethoxy-ethane / 60 h / 50 °C 4.1: tetrabutylammonium bromide; KOH / tetrahydrofuran / 0.5 h / 0 °C 4.2: 98 percent / tetrahydrofuran / 5 h / 20 °C 5.1: 90 percent / HCl / toluene / 1.5 h / 0 - 20 °C View Scheme | |
| Multi-step reaction with 6 steps 1.1: NaBH4; acetic acid / 2 h / 0 °C 2.1: H2 / Pd/C / methanol / 24 h / 20 °C 2.2: H2; hydrochloric acid / Pd/C / methanol / 36 h / 20 °C 2.3: 74 percent / AcOH / toluene / 16 h / 80 °C 3.1: Et3N / tetrahydrofuran / 16 h / 20 °C 4.1: 78 percent / 1,2-dimethoxy-ethane / 60 h / 50 °C 5.1: tetrabutylammonium bromide; KOH / tetrahydrofuran / 0.5 h / 0 °C 5.2: 98 percent / tetrahydrofuran / 5 h / 20 °C 6.1: 90 percent / HCl / toluene / 1.5 h / 0 - 20 °C View Scheme |
-
-
656830-87-4
(R)-2,4-Dihydroxy-4-(2-nitro-phenyl)-butyric acid ethyl ester

-
-
86541-74-4
benazepril hydrochloride

| Conditions | Yield |
|---|---|
| Multi-step reaction with 5 steps 1.1: H2 / Pd/C / methanol / 24 h / 20 °C 1.2: H2; hydrochloric acid / Pd/C / methanol / 36 h / 20 °C 1.3: 74 percent / AcOH / toluene / 16 h / 80 °C 2.1: Et3N / tetrahydrofuran / 16 h / 20 °C 3.1: 78 percent / 1,2-dimethoxy-ethane / 60 h / 50 °C 4.1: tetrabutylammonium bromide; KOH / tetrahydrofuran / 0.5 h / 0 °C 4.2: 98 percent / tetrahydrofuran / 5 h / 20 °C 5.1: 90 percent / HCl / toluene / 1.5 h / 0 - 20 °C View Scheme |

-
-
86541-74-4
benazepril hydrochloride

| Conditions | Yield |
|---|---|
| Multi-step reaction with 5 steps 1.1: phenacyl chloride; baker's yeast / diethyl ether; H2O / 24 h / 30 °C 1.2: H2; hydrochloric acid / Pd/C / methanol / 20 °C 1.3: AcOH / toluene / 80 °C 2.1: Et3N / tetrahydrofuran / 16 h / 20 °C 3.1: 78 percent / 1,2-dimethoxy-ethane / 60 h / 50 °C 4.1: tetrabutylammonium bromide; KOH / tetrahydrofuran / 0.5 h / 0 °C 4.2: 98 percent / tetrahydrofuran / 5 h / 20 °C 5.1: 90 percent / HCl / toluene / 1.5 h / 0 - 20 °C View Scheme |
-
-
608148-63-6
4-nitrobenzenesulfonic acid (3R)-2-oxo-2,3,4,5-tetrahydro-1H-benzo[b]azepin-3-yl ester

-
-
86541-74-4
benazepril hydrochloride

| Conditions | Yield |
|---|---|
| Multi-step reaction with 3 steps 1.1: 78 percent / 1,2-dimethoxy-ethane / 60 h / 50 °C 2.1: tetrabutylammonium bromide; KOH / tetrahydrofuran / 0.5 h / 0 °C 2.2: 98 percent / tetrahydrofuran / 5 h / 20 °C 3.1: 90 percent / HCl / toluene / 1.5 h / 0 - 20 °C View Scheme |
-
-
367909-45-3
(2S,3'S)-2-(2'-oxo-2',3',4',5'-tetrahydro-1H-benzo[b]azepin-3'-ylamino)-4-phenylbutyric acid ethyl ester

-
-
86541-74-4
benazepril hydrochloride

| Conditions | Yield |
|---|---|
| Multi-step reaction with 2 steps 1.1: tetrabutylammonium bromide; KOH / tetrahydrofuran / 0.5 h / 0 °C 1.2: 98 percent / tetrahydrofuran / 5 h / 20 °C 2.1: 90 percent / HCl / toluene / 1.5 h / 0 - 20 °C View Scheme |
-
-
4424-80-0
2,3,4,5-tetrahydro-1H-1-benzo[b]azepin-2-one

-
-
86541-74-4
benazepril hydrochloride

| Conditions | Yield |
|---|---|
| Multi-step reaction with 8 steps 1: 90 percent / PCl5 / xylene / 0.5 h / 90 °C 2: 95 percent / H2, sodium acetate / 5percent Pd/C / acetic acid / 0.5 h / 760 Torr / Ambient temperature 3: 90 percent / sodium azide / dimethylsulfoxide / 3 h / 80 °C 4: 96 percent / Bu4NBr, KOH / tetrahydrofuran / 1.5 h / Ambient temperature 5: 93 percent / H2 / 10percent Pd/C / ethanol / 1.5 h / 2280 Torr / Ambient temperature 7: 89 percent / aq. NaOH / methanol / 2 h / Ambient temperature 8: 12 percent / sodium cyanoborohydride / acetic acid; methanol / Ambient temperature View Scheme |
-
-
86499-22-1
3,3-dichloro-2,3,4,5-tetrahydro-1H-<1>benzazepin-2-one

-
-
86541-74-4
benazepril hydrochloride

| Conditions | Yield |
|---|---|
| Multi-step reaction with 7 steps 1: 95 percent / H2, sodium acetate / 5percent Pd/C / acetic acid / 0.5 h / 760 Torr / Ambient temperature 2: 90 percent / sodium azide / dimethylsulfoxide / 3 h / 80 °C 3: 96 percent / Bu4NBr, KOH / tetrahydrofuran / 1.5 h / Ambient temperature 4: 93 percent / H2 / 10percent Pd/C / ethanol / 1.5 h / 2280 Torr / Ambient temperature 6: 89 percent / aq. NaOH / methanol / 2 h / Ambient temperature 7: 12 percent / sodium cyanoborohydride / acetic acid; methanol / Ambient temperature View Scheme |
-
-
86499-23-2
3-chloro-2,3,4,5-tetrahydro-1H-<1>benzazepin-2-one

-
-
86541-74-4
benazepril hydrochloride

| Conditions | Yield |
|---|---|
| Multi-step reaction with 6 steps 1: 90 percent / sodium azide / dimethylsulfoxide / 3 h / 80 °C 2: 96 percent / Bu4NBr, KOH / tetrahydrofuran / 1.5 h / Ambient temperature 3: 93 percent / H2 / 10percent Pd/C / ethanol / 1.5 h / 2280 Torr / Ambient temperature 5: 89 percent / aq. NaOH / methanol / 2 h / Ambient temperature 6: 12 percent / sodium cyanoborohydride / acetic acid; methanol / Ambient temperature View Scheme |
-
-
97278-68-7
3-azido-2,3,4,5-tetrahydro-2-oxo-1 H-1-benzazepine

-
-
86541-74-4
benazepril hydrochloride

| Conditions | Yield |
|---|---|
| Multi-step reaction with 5 steps 1: 96 percent / Bu4NBr, KOH / tetrahydrofuran / 1.5 h / Ambient temperature 2: 93 percent / H2 / 10percent Pd/C / ethanol / 1.5 h / 2280 Torr / Ambient temperature 4: 89 percent / aq. NaOH / methanol / 2 h / Ambient temperature 5: 12 percent / sodium cyanoborohydride / acetic acid; methanol / Ambient temperature View Scheme |
-
-
92278-69-8
ethyl 3-azido-2,3,4,5-tetrahydro-1H-<1>benzazepin-2-one-1-acetate

-
-
86541-74-4
benazepril hydrochloride

| Conditions | Yield |
|---|---|
| Multi-step reaction with 4 steps 1: 93 percent / H2 / 10percent Pd/C / ethanol / 1.5 h / 2280 Torr / Ambient temperature 3: 89 percent / aq. NaOH / methanol / 2 h / Ambient temperature 4: 12 percent / sodium cyanoborohydride / acetic acid; methanol / Ambient temperature View Scheme |
-
-
94793-89-2
3-(S)-amino-1-ethoxycarbonylmethyl-2,3,4,5-tetrahydro-1H-[1]benzazepine-2-one

-
-
86541-74-4
benazepril hydrochloride

| Conditions | Yield |
|---|---|
| Multi-step reaction with 3 steps 2: 89 percent / aq. NaOH / methanol / 2 h / Ambient temperature 3: 12 percent / sodium cyanoborohydride / acetic acid; methanol / Ambient temperature View Scheme |
-
-
86499-52-7
(S)-3-amino-1-ethoxycarbonylmethyl-2,3,4,5-tetrahydro-1H-[1]benzazepin-2-one

-
-
86541-74-4
benazepril hydrochloride

| Conditions | Yield |
|---|---|
| Multi-step reaction with 2 steps 1: 89 percent / aq. NaOH / methanol / 2 h / Ambient temperature 2: 12 percent / sodium cyanoborohydride / acetic acid; methanol / Ambient temperature View Scheme |
-
-
859635-53-3
3-[[1-(t-butoxy-carbonyl)-3-phenyl-(1S)-propyl]amino]-2,3,4,5-tetrahydro-2-oxo-1H-1-(3S)-benzazepine-1-acetic acid ethyl ester

-
-
86541-74-4
benazepril hydrochloride

| Conditions | Yield |
|---|---|
| With hydrogenchloride In ethyl acetate at 0℃; for 2 - 3h; |
-
-
86541-75-5
benazepril

-
-
86541-74-4
benazepril hydrochloride

| Conditions | Yield |
|---|---|
| Stage #1: benazepril With Celite; pyrographite In acetone for 1h; Heating / reflux; Industry scale; Stage #2: With hydrogenchloride In water; acetone at 10 - 50℃; for 3h; |
-
-
64920-29-2
2-oxo-4-phenylbutanoic acid ethyl ester

-
-
86499-53-8
(3S)-3-amino-1-(carboxymethyl)-2,3,4,5-tetrahydro-1H-<1>benzazepin-2-one sodium salt

-
-
86541-74-4
benazepril hydrochloride

| Conditions | Yield |
|---|---|
| With hydrogenchloride; sodium cyanoborohydride In methanol; dichloromethane; acetic acid; butanone | |
| With hydrogenchloride; sodium cyanoborohydride In methanol; dichloromethane; acetic acid; butanone |
-
-
90315-82-5
ethyl (R)-2-hydroxy-4-phenylbutyrate

-
-
86541-74-4
benazepril hydrochloride

| Conditions | Yield |
|---|---|
| Multi-step reaction with 3 steps 1: triethylamine / ethyl acetate / 3 h / 0 - 25 °C 2: sodium carbonate / ethyl acetate / 20 h / 80 °C / 760.05 Torr 3: hydrogenchloride / ethyl acetate / 10 °C / 760.05 Torr / Reflux View Scheme |

-
-
86541-74-4
benazepril hydrochloride

| Conditions | Yield |
|---|---|
| Multi-step reaction with 3 steps 1.1: triethylamine / methanol / 24 h / 60 °C 2.1: 1,1'-carbonyldiimidazole / Isopropyl acetate / 4 h / 20 °C 2.2: 4 h 3.1: hydrogenchloride / Isopropyl acetate / 10 °C View Scheme | |
| Multi-step reaction with 3 steps 1.1: triethylamine / isopropyl alcohol / 24 h / 60 °C 2.1: 1,1'-carbonyldiimidazole / Isopropyl acetate / 4 h / 20 °C 2.2: 4 h 3.1: hydrogenchloride / Isopropyl acetate / 10 °C View Scheme |
-
-
943-73-7
D-homophenylalanine

-
-
86541-74-4
benazepril hydrochloride

| Conditions | Yield |
|---|---|
| Multi-step reaction with 3 steps 1.1: triethylamine / methanol / 24 h / 60 °C 2.1: 1,1'-carbonyldiimidazole / Isopropyl acetate / 4 h / 20 °C 2.2: 4 h 3.1: hydrogenchloride / Isopropyl acetate / 10 °C View Scheme | |
| Multi-step reaction with 3 steps 1.1: triethylamine / isopropyl alcohol / 24 h / 60 °C 2.1: 1,1'-carbonyldiimidazole / Isopropyl acetate / 4 h / 20 °C 2.2: 4 h 3.1: hydrogenchloride / Isopropyl acetate / 10 °C View Scheme |

-
-
86541-74-4
benazepril hydrochloride

| Conditions | Yield |
|---|---|
| Multi-step reaction with 2 steps 1.1: trifluoroacetic acid / 5 h / Reflux 2.1: palladium 10% on activated carbon; hydrogen / ethanol / 3 h / 20 °C 2.2: Reflux View Scheme |
-
-
104-53-0
3-phenyl-propionaldehyde

-
-
86541-74-4
benazepril hydrochloride

| Conditions | Yield |
|---|---|
| Multi-step reaction with 3 steps 1.1: titanium tetrachloride / 2,2,2-trifluoroethanol / 7 h / 25 °C 2.1: trifluoroacetic acid / 5 h / Reflux 3.1: palladium 10% on activated carbon; hydrogen / ethanol / 3 h / 20 °C 3.2: Reflux View Scheme |
-
-
183508-58-9
benzyl (3S)-3-amino-2,3,4,5-tetrahydro-2-oxo-1H-benzazepine-1-acetate

-
-
86541-74-4
benazepril hydrochloride

| Conditions | Yield |
|---|---|
| Multi-step reaction with 3 steps 1.1: titanium tetrachloride / 2,2,2-trifluoroethanol / 7 h / 25 °C 2.1: trifluoroacetic acid / 5 h / Reflux 3.1: palladium 10% on activated carbon; hydrogen / ethanol / 3 h / 20 °C 3.2: Reflux View Scheme |
-
-
86541-74-4
benazepril hydrochloride

-
-
86541-75-5
benazepril

| Conditions | Yield |
|---|---|
| With potassium carbonate In Isopropyl acetate; water at 20℃; pH=4 - 5; Industry scale; |
-
-
86541-74-4
benazepril hydrochloride

-
-
86541-78-8
benazeprilate

| Conditions | Yield |
|---|---|
| With hydrogenchloride; sodium hydroxide In methanol; water | |
| With sodium hydroxide for 0.5h; Reflux; |
Benazepril hydrochloride Specification
The Benazepril hydrochloride, with the CAS registry number 86541-74-4, is also known as (3S)-3-(((1S)-1-Carboxy-3-phenylpropyl)amino)-2,3,4,5-tetrahydro-2-oxo-1H-1-benzazepine-1-acetic acid, 3-ethyl ester, monohydrochloride; Benazepril HCl; Cibacen; Cibacen CHF; Labopol. It belongs to the product categories of Intermediates & Fine Chemicals; Pharmaceuticals; Amines; Aromatics; Heterocycles. This chemical's molecular formula is C24H29ClN2O5 and molecular weight is 460.96. What's more, its IUPAC name 2-[(3S)-3-[[(2S)-1-ethoxy-1-oxo-4-phenylbutan-2-yl]amino]-2-oxo-4,5-dihydro-3H-1-benzazepin-1-yl]acetic acid hydrochloride. In addition, Benazepril hydrochloride (CAS 86541-74-4) is crystalline solid which is soluble in DMSO. It is used in high blood pressure and congestive heart failure. When you are using this chemical, you should not breathe dust and avoid contact with skin and eyes.
Physical properties about Benazepril hydrochloride (CAS 86541-74-4) are: (1)ACD/LogP: 3.864; (2)# of Rule of 5 Violations: 0; (3)#H bond acceptors: 7; (4)#H bond donors: 2; (5)#Freely Rotating Bonds: 10; (6)Polar Surface Area: 76.15 Å2; (7)Flash Point: 371.8 °C; (8)Enthalpy of Vaporization: 106.37 kJ/mol; (9)Boiling Point: 691.2 °C at 760 mmHg; (10)Vapour Pressure: 4.69E-20 mmHg at 25°C.
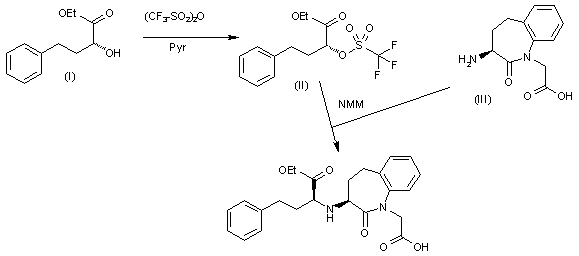
Preparation of Benazepril hydrochloride (CAS 86541-74-4): The reaction of 2(R)-hydroxy-4-phenyl butyric acid ethyl ester (I) with trifluoromethanesulfonic anhydride in dichloromethane gives the corresponding triflate (II), which is then condensed with the amino benzazepinone (III) by means of NMM in the same solvent to provide the target benazepril.
You can still convert the following datas of Benazepril hydrochloride (CAS 86541-74-4) into molecular structure:
(1) SMILES:Cl.O=C(OCC)[C@@H](N[C@@H]2C(=O)N(c1ccccc1CC2)CC(=O)O)CCc3ccccc3
(2) Std. InChI:InChI=1S/C24H28N2O5.ClH/c1-2-31-24(30)20(14-12-17-8-4-3-5-9-17)25-19-15-13-18-10-6-7-11-21(18)26(23(19)29)16-22(27)28;/h3-11,19-20,25H,2,12-16H2,1H3,(H,27,28);1H/t19-,20-;/m0./s1
(3) Std. InChIKey:VPSRQEHTHIMDQM-FKLPMGAJSA-N
The following are the toxicity data of Benazepril hydrochloride (CAS 86541-74-4) which has been tested.
| Organism | Test Type | Route | Reported Dose (Normalized Dose) | Effect | Source |
|---|---|---|---|---|---|
| dog | LDLo | oral | 1gm/kg (1000mg/kg) | SENSE ORGANS AND SPECIAL SENSES: OTHER CHANGES: OLFACTION BEHAVIORAL: FOOD INTAKE (ANIMAL) GASTROINTESTINAL: NAUSEA OR VOMITING | Cardiovascular Drug Reviews. Vol. 8, Pg. 89, 1990. |
| mouse | LD50 | oral | 4019mg/kg (4019mg/kg) | Cardiovascular Drug Reviews. Vol. 8, Pg. 89, 1990. | |
| rat | LD50 | oral | > 5gm/kg (5000mg/kg) | Cardiovascular Drug Reviews. Vol. 8, Pg. 89, 1990. |
Related Products
- Benazepril
- Benazepril hydrochloride
- Benazeprilat
- 86541-75-5
- 86541-78-8
- 86542-89-4
- 86543-84-2
- 86543-85-3
- 86-54-4
- 865-44-1
- 865443-41-0
- 865451-00-9
- 865466-24-6
Hot Products
About|Contact|Cas|Product Name|Molecular|Country|Encyclopedia
Message|New Cas|MSDS|Service|Advertisement|CAS DataBase|Article Data|Manufacturers | Chemical Catalog
©2008 LookChem.com,License: ICP
NO.:Zhejiang16009103
complaints:service@lookchem.com Desktop View