-
Name
Benzarone
- EINECS 216-026-2
- CAS No. 1477-19-6
- Article Data10
- CAS DataBase
- Density 1.234g/cm3
- Solubility
- Melting Point 124.3°
- Formula C17H14 O3
- Boiling Point 473.6°Cat760mmHg
- Molecular Weight 266.296
- Flash Point 240.2°C
- Transport Information
- Appearance
- Safety Poison by intraperitoneal route. An experimental teratogen. Other experimental reproductive effects. A flammable liquid. When heated to decomposition it emits acrid and irritating smoke and fumes. See also KETONES.
- Risk Codes
-
Molecular Structure
- Hazard Symbols
- Synonyms Ketone,2-ethyl-3-benzofuranyl p-hydroxyphenyl (6CI,7CI,8CI);2-Ethyl-3-(4-hydroxybenzoyl)benzofuran; 2-Ethyl-3-(p-hydroxybenzoyl)benzofuran;2-Ethyl-3-benzofuranyl p-hydroxyphenyl ketone;2-Ethyl-4'-hydroxy-3-benzoylbenzofuran; Benzaron; Benzarone; Fagivil; Fragivil;Fragivix; L 2179-Labaz; L 2197; NSC 82134
- PSA 50.44000
- LogP 3.93180
Synthetic route

| Conditions | Yield |
|---|---|
| With aluminum (III) chloride In chloroform at 80℃; for 0.5h; Temperature; Reagent/catalyst; Large scale; | 95% |
| Stage #1: (2-ethylbenzofuran-3-yl)(4-methoxyphenyl)methanone With sodium thioethylate In N,N-dimethyl-formamide at 125 - 130℃; for 1h; Stage #2: With water; ammonium chloride In N,N-dimethyl-formamide | 90% |
| With sodium thioethylate In N,N-dimethyl-formamide at 125 - 130℃; for 1h; | 90% |

| Conditions | Yield |
|---|---|
| Stage #1: benzbromarone With dichloro[1,1′-bis[bis(1,1-dimethylethyl)phosphino]ferrocene-P,P′]palladium; triethylamine In water at 20℃; for 0.0333333h; Stage #2: With 1,1,3,3-Tetramethyldisiloxane for 11.9667h; | 86% |

| Conditions | Yield |
|---|---|
| With lewis acid In chloroform at 10 - 30℃; for 4h; Solvent; Temperature; | 68.2% |
-
-
3131-63-3
2-ethylbenzofuran

-
-
1486-50-6
4-(benzyloxy)benzoic acid chloride

-
A
-
101596-43-4
3-benzyl-2-ethylbenzofuran

-
B
-
1477-19-6
benzarone

| Conditions | Yield |
|---|---|
| With tin(IV) chloride; benzene |

-
-
90908-77-3
(4-acetoxy-phenyl)-(2-ethyl-benzofuran-3-yl)-ketone

-
-
1477-19-6
benzarone

| Conditions | Yield |
|---|---|
| With sodium hydroxide |

| Conditions | Yield |
|---|---|
| Multi-step reaction with 2 steps 1: tin (IV)-chloride; benzene 2: aq.-ethanolic NaOH-solution View Scheme | |
| Multi-step reaction with 2 steps 1: tin (IV)-chloride; carbon disulfide 2: pyridine hydrochloride View Scheme | |
| Multi-step reaction with 2 steps 1: tin(IV) chloride / carbon disulfide / 5 - 20 °C / Inert atmosphere; Cooling with ice 2: sodium thioethylate / N,N-dimethyl-formamide / 1 h / 125 - 130 °C View Scheme |
-
-
27914-73-4
4-acetoxybenzoyl chloride

-
-
1477-19-6
benzarone

| Conditions | Yield |
|---|---|
| Multi-step reaction with 2 steps 1: tin (IV)-chloride; benzene 2: aq.-ethanolic NaOH-solution View Scheme |

| Conditions | Yield |
|---|---|
| Multi-step reaction with 2 steps 1: tin (IV)-chloride; carbon disulfide 2: pyridine hydrochloride View Scheme | |
| Multi-step reaction with 2 steps 1: tin(IV) chloride / carbon disulfide / 5 - 20 °C / Inert atmosphere; Cooling with ice 2: sodium thioethylate / N,N-dimethyl-formamide / 1 h / 125 - 130 °C View Scheme | |
| Multi-step reaction with 2 steps 1: polystyrene-supported aluminum trichloride / dichloromethane / 1 h / 5 - 20 °C / Large scale 2: aluminum (III) chloride / chloroform / 0.5 h / 80 °C / Large scale View Scheme |

| Conditions | Yield |
|---|---|
| Multi-step reaction with 3 steps 1.1: hydrazine / water; diethylene glycol / 120 - 190 °C 1.2: 6 h / 120 - 130 °C 2.1: tin(IV) chloride / carbon disulfide / 5 - 20 °C / Inert atmosphere; Cooling with ice 3.1: sodium thioethylate / N,N-dimethyl-formamide / 1 h / 125 - 130 °C View Scheme | |
| Multi-step reaction with 3 steps 1.1: hydrazine / water; diethylene glycol / 120 - 190 °C 1.2: 6 h / 120 - 130 °C 2.1: carbon disulfide / 0.5 h / Inert atmosphere; Cooling with ice 2.2: 6 h / 20 °C / Cooling with ice 3.1: sodium thioethylate / N,N-dimethyl-formamide / 1 h / 125 - 130 °C View Scheme |

| Conditions | Yield |
|---|---|
| Multi-step reaction with 4 steps 1.1: potassium carbonate / acetone / 6 h / Reflux 2.1: hydrazine / water; diethylene glycol / 120 - 190 °C 2.2: 6 h / 120 - 130 °C 3.1: tin(IV) chloride / carbon disulfide / 5 - 20 °C / Inert atmosphere; Cooling with ice 4.1: sodium thioethylate / N,N-dimethyl-formamide / 1 h / 125 - 130 °C View Scheme | |
| Multi-step reaction with 3 steps 1.1: potassium hydroxide / ethanol / 2 h / Reflux 1.2: 2 h / Reflux 1.3: 0.17 h / Reflux 2.1: tin(IV) chloride / chloroform / 20 °C / Cooling with ice 3.1: ethanethiol; sodium hydride / N,N-dimethyl-formamide / 3 h / 110 °C View Scheme |

| Conditions | Yield |
|---|---|
| Multi-step reaction with 4 steps 1.1: potassium carbonate / acetone / 6 h / Reflux 2.1: hydrazine / water; diethylene glycol / 120 - 190 °C 2.2: 6 h / 120 - 130 °C 3.1: carbon disulfide / 0.5 h / Inert atmosphere; Cooling with ice 3.2: 6 h / 20 °C / Cooling with ice 4.1: sodium thioethylate / N,N-dimethyl-formamide / 1 h / 125 - 130 °C View Scheme |
-
-
54103-36-5
1-(4-methoxyphenyl)-1,3-pentanedione

-
-
1477-19-6
benzarone

| Conditions | Yield |
|---|---|
| Multi-step reaction with 2 steps 1: N-Bromosuccinimide; aluminum (III) chloride / nitromethane / 6 h / 80 °C 2: boron tribromide / dichloromethane / 10 h View Scheme |

| Conditions | Yield |
|---|---|
| Multi-step reaction with 3 steps 1: sodium hydride / mineral oil; tetrahydrofuran / Reflux 2: N-Bromosuccinimide; aluminum (III) chloride / nitromethane / 6 h / 80 °C 3: boron tribromide / dichloromethane / 10 h View Scheme |

| Conditions | Yield |
|---|---|
| With bromine; acetic acid In water at 20℃; for 2h; | 98% |
| With N-Bromosuccinimide In dichloromethane; N,N-dimethyl-formamide at -10 - 20℃; for 17h; | 82% |
| With bromine; acetic acid for 0.25h; Product distribution / selectivity; | 35% |

| Conditions | Yield |
|---|---|
| With sodium hydroxide; potassium carbonate In N,N-dimethyl-formamide; Petroleum ether | 97.4% |
-
-
1477-19-6
benzarone

-
-
82699-09-0
Natriumbenzaronsulfat

| Conditions | Yield |
|---|---|
| With aminosulfonic acid; sodium carbonate 1.) pyridine, 90 deg C, 2 h, 2.) H2O; | 74% |

| Conditions | Yield |
|---|---|
| With iodine; silver nitrate In methanol at 20℃; for 3h; | 46.2% |
-
-
1477-19-6
benzarone

-
-
51073-13-3
(3,5-dibromo-4-methoxy-phenyl)-(2-ethyl-benzofuran-3-yl)-methanone

| Conditions | Yield |
|---|---|
| Multi-step reaction with 2 steps 1: aqueous acetic acid; bromine 2: ethanolic KOH-solution View Scheme | |
| Multi-step reaction with 2 steps 1.1: bromine; acetic acid / 0.25 h 2.1: potassium carbonate / tetrahydrofuran / 0.17 h 2.2: 16 h / 40 °C View Scheme |

-
-
1477-19-6
benzarone

-
-
107-14-2
chloroacetonitrile

-
-
91627-69-9
5-[4-(2-ethyl-3-benzofuroyl)phenoxymethyl]tetrazole

| Conditions | Yield |
|---|---|
| With hydrogenchloride; NaH In tetrahydrofuran; (2S)-N-methyl-1-phenylpropan-2-amine hydrate; N,N-dimethyl-formamide |

-
-
1477-19-6
benzarone

-
-
106-89-8
epichlorohydrin


-
B
-
61085-19-6
2-ethyl-3-[4'-(2,3-epoxy)propoxybenzoyl]benzofuran

-
-
1477-19-6
benzarone

| Conditions | Yield |
|---|---|
| Multi-step reaction with 2 steps 1: silver nitrate; iodine / methanol / 3 h / 20 °C 2: N,N-dimethyl-formamide / 5 h / 100 °C View Scheme |
-
-
1477-19-6
benzarone

| Conditions | Yield |
|---|---|
| Multi-step reaction with 3 steps 1: silver nitrate; iodine / methanol / 3 h / 20 °C 2: N,N-dimethyl-formamide / 5 h / 100 °C 3: 5%-palladium/activated carbon; hydrogen / ethyl acetate / 20 °C / 760.05 Torr View Scheme |
-
-
1477-19-6
benzarone

| Conditions | Yield |
|---|---|
| Multi-step reaction with 4 steps 1: silver nitrate; iodine / methanol / 3 h / 20 °C 2: N,N-dimethyl-formamide / 5 h / 100 °C 3: 5%-palladium/activated carbon; hydrogen / ethyl acetate / 20 °C / 760.05 Torr 4: N-Bromosuccinimide / N,N-dimethyl-formamide / 20 °C View Scheme |
-
-
1477-19-6
benzarone

| Conditions | Yield |
|---|---|
| Multi-step reaction with 2 steps 1: silver nitrate; iodine / methanol / 3 h / 20 °C 2: dmap; pyridine / 2 h / 20 °C View Scheme |
-
-
1477-19-6
benzarone

| Conditions | Yield |
|---|---|
| Multi-step reaction with 3 steps 1: silver nitrate; iodine / methanol / 3 h / 20 °C 2: dmap; pyridine / 2 h / 20 °C 3: copper(l) iodide / N,N-dimethyl-formamide / 1 h / 90 °C / Inert atmosphere View Scheme |
-
-
1477-19-6
benzarone

| Conditions | Yield |
|---|---|
| Multi-step reaction with 4 steps 1: silver nitrate; iodine / methanol / 3 h / 20 °C 2: dmap; pyridine / 2 h / 20 °C 3: copper(l) iodide / N,N-dimethyl-formamide / 1 h / 90 °C / Inert atmosphere 4: potassium carbonate / methanol / 2 h / 20 °C View Scheme |
-
-
1477-19-6
benzarone

| Conditions | Yield |
|---|---|
| Multi-step reaction with 5 steps 1: silver nitrate; iodine / methanol / 3 h / 20 °C 2: dmap; pyridine / 2 h / 20 °C 3: copper(l) iodide / N,N-dimethyl-formamide / 1 h / 90 °C / Inert atmosphere 4: potassium carbonate / methanol / 2 h / 20 °C 5: N,N-dimethyl-formamide / 100 °C View Scheme |
Benzarone Chemical Properties
Molecular Structure of Benzarone (CAS NO. 1477-19-6):
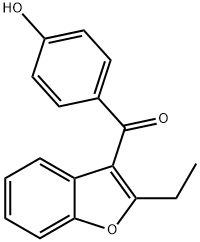
EINECS: 216-026-2
IUPAC Name: (2-Ethyl-1-benzofuran-3-yl)-(4-hydroxyphenyl)methanone
Molecular Formula: C17H14O3
Molecular Weight: 266.291260 g/mol
XLogP3: 4.3
H-Bond Donor: 1
H-Bond Acceptor: 3
Canonical SMILES: CCC1=C(C2=CC=CC=C2O1)C(=O)C3=CC=C(C=C3)O
InChI: InChI=1S/C17H14O3/c1-2-14-16(13-5-3-4-6-15(13)20-14)17(19)11-7-9-12(18)10-8-11/h3-10,18H,2H2,1H3
InChIKey: RFRXIWQYSOIBDI-UHFFFAOYSA-N
Index of Refraction: 1.638
Molar Refractivity: 77.61 cm3
Molar Volume: 215.7 cm3
Surface Tension: 50.9 dyne/cm
Density: 1.234 g/cm3
Flash Point: 240.2 °C
Enthalpy of Vaporization: 76.49 kJ/mol
Boiling Point: 473.6 °C at 760 mmHg
Vapour Pressure: 1.36E-09 mmHg at 25 °C
Water Solubility of Benzarone (CAS NO. 1477-19-6): 17.07 mg/L at 25 °C
Benzarone Toxicity Data With Reference
| 1. | ipr-mus LD50:200 mg/kg | AIPTAK Archives Internationales de Pharmacodynamie et de Therapie, 154 (1965),94. |
Benzarone Consensus Reports
Reported in EPA TSCA Inventory.
Benzarone Safety Profile
Poison by intraperitoneal route. An experimental teratogen. Other experimental reproductive effects. A flammable liquid. When heated to decomposition it emits acrid and irritating smoke and fumes. See also KETONES.
Benzarone Standards and Recommendations
DOT Classification: 3; Label: Flammable Liquid
Benzarone Specification
Benzarone with cas registry number of 1477-19-6 is also known as 2-Ethyl-3-(4-hydroxybenzoyl)benzofuran ; 2-Ethyl-3-(p-hydroxybenzoyl)benzofuran ; 2-Ethyl-3-benzofuranyl p-hydroxyphenyl ketone ; 2-Ethyl-3-(4'-hydroxybenzoyl)benzofuran ; 2-Ethyl-4'-hydroxy-3-benzoylbenzofuran ; 5-18-02-00334 (Beilstein Handbook Reference) ; Benzarona ; Benzarona [INN-Spanish] ; Benzarone [INN:DCF] ; Benzarone ; Benzaronum ; Benzaronum [INN-Latin] ; Benzofuran, (2-ethyl-3-(4'-hydroxybenzoyl)) ; Ethyl-2 (hydroxy-4 benzoyl)-3 benzofuranne ; Ethyl-2 (hydroxy-4 benzoyl)-3 benzofuranne [French] ; Fragivix ; L 2197 ; L 2197-Labaz ; Methanone, (2-ethyl-3-benzofuranyl)(4-hydroxyphenyl)- ; NSC 82134 ; UNII-23ZW4BG89C ; Vasoc ; Venagil ; Ketone, 2-ethyl-3-benzofuranyl p-hydroxyphenyl .
About|Contact|Cas|Product Name|Molecular|Country|Encyclopedia
Message|New Cas|MSDS|Service|Advertisement|CAS DataBase|Article Data|Manufacturers | Chemical Catalog
©2008 LookChem.com,License: ICP
NO.:Zhejiang16009103
complaints:service@lookchem.com Desktop View