-
Name
Berberine
- EINECS 218-229-1
- CAS No. 2086-83-1
- Article Data11
- CAS DataBase
- Density 1.2976 (rough estimate)
- Solubility SOLUBLE IN COLD WATER
- Melting Point 204-206 °C (dec.)
- Formula C20H18NO4
- Boiling Point 486.8°C (rough estimate)
- Molecular Weight 336.367
- Flash Point
- Transport Information UN 1544
- Appearance Yellow
- Safety
- Risk Codes
-
Molecular Structure
- Hazard Symbols
- Synonyms Berbinium,7,8,13,13a-tetradehydro-9,10-dimethoxy-2,3-(methylenedioxy)- (8CI);Umbellatine(6CI);Berbericine;Berberin;Majarine;Thalsine;Umbellatin;5,6-Dihydro-9,10-dimethoxybenzo[g]-1,3-benzodioxolo[5,6-a]quinolizinium;
- PSA 40.80000
- LogP 3.09630
Synthetic route
-
-
94272-86-3
C21H19NO6

-
-
2086-83-1
berberine

| Conditions | Yield |
|---|---|
| With sodium cyanoborohydride In methanol | 96% |
-
-
29074-38-2
canadine

-
-
2086-83-1
berberine

| Conditions | Yield |
|---|---|
| With iodine In ethanol at 20℃; | 90% |
| Multi-step reaction with 2 steps 1.1: 81 percent / mCPBA / CHCl3 / 20 °C 2.1: TFAA / CH2Cl2 / -30 - 20 °C 2.2: NaOAc / CH2Cl2; H2O / 0.5 h / 20 °C View Scheme | |
| With (S)-tetrahydroprotoberberine oxidase; tris hydrochloride at 37℃; for 2h; pH=8.8; Enzymatic reaction; |

-
-
2086-83-1
berberine

| Conditions | Yield |
|---|---|
| With methanesulfonyl chloride; triethylamine In dichloromethane at 20℃; for 6h; | 88% |

| Conditions | Yield |
|---|---|
| Stage #1: (+/-)-trans-canadine N-oxide With trifluoroacetic anhydride In dichloromethane at -30 - 20℃; Elimination; Polonovski-Potier reaction; Stage #2: potassium cyanide With sodium acetate In dichloromethane; water at 20℃; for 0.5h; Addition; tautomerization; aromatization; | A n/a B 29% |

-
-
29074-38-2
canadine

-
A
-
549-21-3
oxyberberine

-
B
-
2086-83-1
berberine

-
C
-
64939-64-6, 66408-44-4
3-<3,4-dihydro-1-oxo-6,7-(methylenedioxy)-2H-isoquinolin-2-yl>-4,5-dimethoxy-1(3H)-isobenzofuranone

| Conditions | Yield |
|---|---|
| With iodosylbenzene; tetra-(n-butyl)ammonium iodide In water; acetonitrile at 50℃; for 18h; | A 6% B 15% C 25% |


| Conditions | Yield |
|---|---|
| With (S)-protoberberine oxidase; oxygen Equilibrium constant; enzyme activity for the substrate; |
-
-
113975-46-5
9,10-dimethoxy-5,6,7,8-tetrahydro-2H-1,3-dioxolano[4,5-g]isoquinolino[3,2-a]isoquinoline-8-carbonitrile

-
-
2086-83-1
berberine

| Conditions | Yield |
|---|---|
| Multi-step reaction with 3 steps 1.1: NaBH4 2.1: 81 percent / mCPBA / CHCl3 / 20 °C 3.1: TFAA / CH2Cl2 / -30 - 20 °C 3.2: NaOAc / CH2Cl2; H2O / 0.5 h / 20 °C View Scheme |

| Conditions | Yield |
|---|---|
| Multi-step reaction with 3 steps 1.1: NaBH4 / methanol 2.1: 81 percent / mCPBA / CHCl3 / 20 °C 3.1: TFAA / CH2Cl2 / -30 - 20 °C 3.2: NaOAc / CH2Cl2; H2O / 0.5 h / 20 °C View Scheme | |
| Multi-step reaction with 4 steps 1.1: 95 percent / methanol; H2O 2.1: NaBH4 3.1: 81 percent / mCPBA / CHCl3 / 20 °C 4.1: TFAA / CH2Cl2 / -30 - 20 °C 4.2: NaOAc / CH2Cl2; H2O / 0.5 h / 20 °C View Scheme | |
| With sodium tetrahydroborate In pyridine at 20℃; |
-
-
94272-85-2
C21H21NO6

-
-
2086-83-1
berberine

| Conditions | Yield |
|---|---|
| Multi-step reaction with 2 steps 1: 93 percent / silver carbonate / benzene / Heating 2: 96 percent / sodium cyanoborohydride / methanol View Scheme |
-
-
84229-90-3
spirobenzylisoquinoline

-
-
2086-83-1
berberine

| Conditions | Yield |
|---|---|
| Multi-step reaction with 3 steps 1: 94 percent / methanol / Ambient temperature 2: 93 percent / silver carbonate / benzene / Heating 3: 96 percent / sodium cyanoborohydride / methanol View Scheme |

| Conditions | Yield |
|---|---|
| Multi-step reaction with 2 steps 1: oxalyl dichloride / chloroform / 1 h / Reflux 2: dimethyl sulfoxide / 25.5 h / 115 - 120 °C View Scheme |
-
-
47474-50-0
N-methyl-7,8-dihydroberberine

-
-
2086-83-1
berberine

| Conditions | Yield |
|---|---|
| In dimethyl sulfoxide at 115 - 120℃; for 25.5h; |

| Conditions | Yield |
|---|---|
| With iodine; potassium acetate In ethanol at 20℃; Inert atmosphere; | 6.7 mg |
| Multi-step reaction with 2 steps 1: sodium tetrahydroborate / methanol / 2 h / 20 °C / Inert atmosphere 2: iodine / ethanol / 20 °C View Scheme | |
| Multi-step reaction with 2 steps 1: diborane 2: iodine / ethanol / 20 °C View Scheme |

-
-
2086-83-1
berberine

| Conditions | Yield |
|---|---|
| Multi-step reaction with 2 steps 1: Wilkinson's catalyst 2: iodine / ethanol / 20 °C View Scheme |

-
-
2086-83-1
berberine

| Conditions | Yield |
|---|---|
| Multi-step reaction with 4 steps 1: sodium formate; tetrakis(triphenylphosphine) palladium(0) / N,N-dimethyl-formamide; water / 1 h / 100 °C / Inert atmosphere 2: 9-borabicyclo[3.3.1]nonane dimer; sodium hydroperoxide 3: Wilkinson's catalyst 4: iodine / ethanol / 20 °C View Scheme | |
| Multi-step reaction with 5 steps 1: sodium formate; tetrakis(triphenylphosphine) palladium(0) / N,N-dimethyl-formamide; water / 1 h / 100 °C / Inert atmosphere 2: tetrapropylammonium perruthennate; 4-methylmorpholine N-oxide / acetonitrile / 2 h / 50 °C / Inert atmosphere; Molecular sieve 3: Wilkinson's catalyst; diphenyl phosphoryl azide / diethylene glycol dimethyl ether / 12 h / 160 °C / Inert atmosphere 4: lithium aluminium tetrahydride; aluminum (III) chloride / diethyl ether / 2 h / Reflux; Inert atmosphere 5: potassium acetate; iodine / ethanol / 20 °C / Inert atmosphere View Scheme | |
| Multi-step reaction with 6 steps 1: sodium formate; tetrakis(triphenylphosphine) palladium(0) / N,N-dimethyl-formamide; water / 1 h / 100 °C / Inert atmosphere 2: tetrapropylammonium perruthennate; 4-methylmorpholine N-oxide / acetonitrile / 2 h / 50 °C / Inert atmosphere; Molecular sieve 3: Wilkinson's catalyst; diphenyl phosphoryl azide / diethylene glycol dimethyl ether / 12 h / 160 °C / Inert atmosphere 4: lithium aluminium tetrahydride; aluminum (III) chloride / diethyl ether / 2 h / Reflux; Inert atmosphere 5: sodium tetrahydroborate / methanol / 2 h / 20 °C / Inert atmosphere 6: iodine / ethanol / 20 °C View Scheme | |
| Multi-step reaction with 6 steps 1: sodium formate; tetrakis(triphenylphosphine) palladium(0) / N,N-dimethyl-formamide; water / 1 h / 100 °C / Inert atmosphere 2: tetrapropylammonium perruthennate; 4-methylmorpholine N-oxide / acetonitrile / 2 h / 50 °C / Inert atmosphere; Molecular sieve 3: Wilkinson's catalyst; diphenyl phosphoryl azide / diethylene glycol dimethyl ether / 12 h / 160 °C / Inert atmosphere 4: lithium aluminium tetrahydride; aluminum (III) chloride / diethyl ether / 2 h / Reflux; Inert atmosphere 5: diborane 6: iodine / ethanol / 20 °C View Scheme |
-
-
94143-83-6
5,6,7,8-tetrahydro-[1,3]dioxolo[4,5-g]isoquinoline

-
-
2086-83-1
berberine

| Conditions | Yield |
|---|---|
| Multi-step reaction with 6 steps 1: copper(l) iodide; benzoic acid / toluene / 12 h / 80 °C / Inert atmosphere; Molecular sieve 2: potassium carbonate / methanol / 0.5 h / 20 °C / Inert atmosphere 3: sodium formate; tetrakis(triphenylphosphine) palladium(0) / N,N-dimethyl-formamide; water / 1 h / 100 °C / Inert atmosphere 4: 9-borabicyclo[3.3.1]nonane dimer; sodium hydroperoxide 5: Wilkinson's catalyst 6: iodine / ethanol / 20 °C View Scheme | |
| Multi-step reaction with 7 steps 1: copper(l) iodide; benzoic acid / toluene / 12 h / 80 °C / Inert atmosphere; Molecular sieve 2: potassium carbonate / methanol / 0.5 h / 20 °C / Inert atmosphere 3: sodium formate; tetrakis(triphenylphosphine) palladium(0) / N,N-dimethyl-formamide; water / 1 h / 100 °C / Inert atmosphere 4: tetrapropylammonium perruthennate; 4-methylmorpholine N-oxide / acetonitrile / 2 h / 50 °C / Inert atmosphere; Molecular sieve 5: Wilkinson's catalyst; diphenyl phosphoryl azide / diethylene glycol dimethyl ether / 12 h / 160 °C / Inert atmosphere 6: lithium aluminium tetrahydride; aluminum (III) chloride / diethyl ether / 2 h / Reflux; Inert atmosphere 7: potassium acetate; iodine / ethanol / 20 °C / Inert atmosphere View Scheme | |
| Multi-step reaction with 8 steps 1: copper(l) iodide; benzoic acid / toluene / 12 h / 80 °C / Inert atmosphere; Molecular sieve 2: potassium carbonate / methanol / 0.5 h / 20 °C / Inert atmosphere 3: sodium formate; tetrakis(triphenylphosphine) palladium(0) / N,N-dimethyl-formamide; water / 1 h / 100 °C / Inert atmosphere 4: tetrapropylammonium perruthennate; 4-methylmorpholine N-oxide / acetonitrile / 2 h / 50 °C / Inert atmosphere; Molecular sieve 5: Wilkinson's catalyst; diphenyl phosphoryl azide / diethylene glycol dimethyl ether / 12 h / 160 °C / Inert atmosphere 6: lithium aluminium tetrahydride; aluminum (III) chloride / diethyl ether / 2 h / Reflux; Inert atmosphere 7: sodium tetrahydroborate / methanol / 2 h / 20 °C / Inert atmosphere 8: iodine / ethanol / 20 °C View Scheme | |
| Multi-step reaction with 8 steps 1: copper(l) iodide; benzoic acid / toluene / 12 h / 80 °C / Inert atmosphere; Molecular sieve 2: potassium carbonate / methanol / 0.5 h / 20 °C / Inert atmosphere 3: sodium formate; tetrakis(triphenylphosphine) palladium(0) / N,N-dimethyl-formamide; water / 1 h / 100 °C / Inert atmosphere 4: tetrapropylammonium perruthennate; 4-methylmorpholine N-oxide / acetonitrile / 2 h / 50 °C / Inert atmosphere; Molecular sieve 5: Wilkinson's catalyst; diphenyl phosphoryl azide / diethylene glycol dimethyl ether / 12 h / 160 °C / Inert atmosphere 6: lithium aluminium tetrahydride; aluminum (III) chloride / diethyl ether / 2 h / Reflux; Inert atmosphere 7: diborane 8: iodine / ethanol / 20 °C View Scheme |
-
-
53811-50-0
6-bromo-2,3-dimethoxybenzaldehyde

-
-
2086-83-1
berberine

| Conditions | Yield |
|---|---|
| Multi-step reaction with 6 steps 1: copper(l) iodide; benzoic acid / toluene / 12 h / 80 °C / Inert atmosphere; Molecular sieve 2: potassium carbonate / methanol / 0.5 h / 20 °C / Inert atmosphere 3: sodium formate; tetrakis(triphenylphosphine) palladium(0) / N,N-dimethyl-formamide; water / 1 h / 100 °C / Inert atmosphere 4: 9-borabicyclo[3.3.1]nonane dimer; sodium hydroperoxide 5: Wilkinson's catalyst 6: iodine / ethanol / 20 °C View Scheme | |
| Multi-step reaction with 7 steps 1: copper(l) iodide; benzoic acid / toluene / 12 h / 80 °C / Inert atmosphere; Molecular sieve 2: potassium carbonate / methanol / 0.5 h / 20 °C / Inert atmosphere 3: sodium formate; tetrakis(triphenylphosphine) palladium(0) / N,N-dimethyl-formamide; water / 1 h / 100 °C / Inert atmosphere 4: tetrapropylammonium perruthennate; 4-methylmorpholine N-oxide / acetonitrile / 2 h / 50 °C / Inert atmosphere; Molecular sieve 5: Wilkinson's catalyst; diphenyl phosphoryl azide / diethylene glycol dimethyl ether / 12 h / 160 °C / Inert atmosphere 6: lithium aluminium tetrahydride; aluminum (III) chloride / diethyl ether / 2 h / Reflux; Inert atmosphere 7: potassium acetate; iodine / ethanol / 20 °C / Inert atmosphere View Scheme | |
| Multi-step reaction with 8 steps 1: copper(l) iodide; benzoic acid / toluene / 12 h / 80 °C / Inert atmosphere; Molecular sieve 2: potassium carbonate / methanol / 0.5 h / 20 °C / Inert atmosphere 3: sodium formate; tetrakis(triphenylphosphine) palladium(0) / N,N-dimethyl-formamide; water / 1 h / 100 °C / Inert atmosphere 4: tetrapropylammonium perruthennate; 4-methylmorpholine N-oxide / acetonitrile / 2 h / 50 °C / Inert atmosphere; Molecular sieve 5: Wilkinson's catalyst; diphenyl phosphoryl azide / diethylene glycol dimethyl ether / 12 h / 160 °C / Inert atmosphere 6: lithium aluminium tetrahydride; aluminum (III) chloride / diethyl ether / 2 h / Reflux; Inert atmosphere 7: sodium tetrahydroborate / methanol / 2 h / 20 °C / Inert atmosphere 8: iodine / ethanol / 20 °C View Scheme | |
| Multi-step reaction with 8 steps 1: copper(l) iodide; benzoic acid / toluene / 12 h / 80 °C / Inert atmosphere; Molecular sieve 2: potassium carbonate / methanol / 0.5 h / 20 °C / Inert atmosphere 3: sodium formate; tetrakis(triphenylphosphine) palladium(0) / N,N-dimethyl-formamide; water / 1 h / 100 °C / Inert atmosphere 4: tetrapropylammonium perruthennate; 4-methylmorpholine N-oxide / acetonitrile / 2 h / 50 °C / Inert atmosphere; Molecular sieve 5: Wilkinson's catalyst; diphenyl phosphoryl azide / diethylene glycol dimethyl ether / 12 h / 160 °C / Inert atmosphere 6: lithium aluminium tetrahydride; aluminum (III) chloride / diethyl ether / 2 h / Reflux; Inert atmosphere 7: diborane 8: iodine / ethanol / 20 °C View Scheme |

-
-
2086-83-1
berberine

| Conditions | Yield |
|---|---|
| Multi-step reaction with 3 steps 1: 9-borabicyclo[3.3.1]nonane dimer; sodium hydroperoxide 2: Wilkinson's catalyst 3: iodine / ethanol / 20 °C View Scheme | |
| Multi-step reaction with 4 steps 1: tetrapropylammonium perruthennate; 4-methylmorpholine N-oxide / acetonitrile / 2 h / 50 °C / Inert atmosphere; Molecular sieve 2: Wilkinson's catalyst; diphenyl phosphoryl azide / diethylene glycol dimethyl ether / 12 h / 160 °C / Inert atmosphere 3: lithium aluminium tetrahydride; aluminum (III) chloride / diethyl ether / 2 h / Reflux; Inert atmosphere 4: potassium acetate; iodine / ethanol / 20 °C / Inert atmosphere View Scheme | |
| Multi-step reaction with 5 steps 1: tetrapropylammonium perruthennate; 4-methylmorpholine N-oxide / acetonitrile / 2 h / 50 °C / Inert atmosphere; Molecular sieve 2: Wilkinson's catalyst; diphenyl phosphoryl azide / diethylene glycol dimethyl ether / 12 h / 160 °C / Inert atmosphere 3: lithium aluminium tetrahydride; aluminum (III) chloride / diethyl ether / 2 h / Reflux; Inert atmosphere 4: sodium tetrahydroborate / methanol / 2 h / 20 °C / Inert atmosphere 5: iodine / ethanol / 20 °C View Scheme | |
| Multi-step reaction with 5 steps 1: tetrapropylammonium perruthennate; 4-methylmorpholine N-oxide / acetonitrile / 2 h / 50 °C / Inert atmosphere; Molecular sieve 2: Wilkinson's catalyst; diphenyl phosphoryl azide / diethylene glycol dimethyl ether / 12 h / 160 °C / Inert atmosphere 3: lithium aluminium tetrahydride; aluminum (III) chloride / diethyl ether / 2 h / Reflux; Inert atmosphere 4: diborane 5: iodine / ethanol / 20 °C View Scheme |

-
-
2086-83-1
berberine

| Conditions | Yield |
|---|---|
| Multi-step reaction with 5 steps 1: potassium carbonate / methanol / 0.5 h / 20 °C / Inert atmosphere 2: sodium formate; tetrakis(triphenylphosphine) palladium(0) / N,N-dimethyl-formamide; water / 1 h / 100 °C / Inert atmosphere 3: 9-borabicyclo[3.3.1]nonane dimer; sodium hydroperoxide 4: Wilkinson's catalyst 5: iodine / ethanol / 20 °C View Scheme | |
| Multi-step reaction with 6 steps 1: potassium carbonate / methanol / 0.5 h / 20 °C / Inert atmosphere 2: sodium formate; tetrakis(triphenylphosphine) palladium(0) / N,N-dimethyl-formamide; water / 1 h / 100 °C / Inert atmosphere 3: tetrapropylammonium perruthennate; 4-methylmorpholine N-oxide / acetonitrile / 2 h / 50 °C / Inert atmosphere; Molecular sieve 4: Wilkinson's catalyst; diphenyl phosphoryl azide / diethylene glycol dimethyl ether / 12 h / 160 °C / Inert atmosphere 5: lithium aluminium tetrahydride; aluminum (III) chloride / diethyl ether / 2 h / Reflux; Inert atmosphere 6: potassium acetate; iodine / ethanol / 20 °C / Inert atmosphere View Scheme | |
| Multi-step reaction with 7 steps 1: potassium carbonate / methanol / 0.5 h / 20 °C / Inert atmosphere 2: sodium formate; tetrakis(triphenylphosphine) palladium(0) / N,N-dimethyl-formamide; water / 1 h / 100 °C / Inert atmosphere 3: tetrapropylammonium perruthennate; 4-methylmorpholine N-oxide / acetonitrile / 2 h / 50 °C / Inert atmosphere; Molecular sieve 4: Wilkinson's catalyst; diphenyl phosphoryl azide / diethylene glycol dimethyl ether / 12 h / 160 °C / Inert atmosphere 5: lithium aluminium tetrahydride; aluminum (III) chloride / diethyl ether / 2 h / Reflux; Inert atmosphere 6: sodium tetrahydroborate / methanol / 2 h / 20 °C / Inert atmosphere 7: iodine / ethanol / 20 °C View Scheme | |
| Multi-step reaction with 7 steps 1: potassium carbonate / methanol / 0.5 h / 20 °C / Inert atmosphere 2: sodium formate; tetrakis(triphenylphosphine) palladium(0) / N,N-dimethyl-formamide; water / 1 h / 100 °C / Inert atmosphere 3: tetrapropylammonium perruthennate; 4-methylmorpholine N-oxide / acetonitrile / 2 h / 50 °C / Inert atmosphere; Molecular sieve 4: Wilkinson's catalyst; diphenyl phosphoryl azide / diethylene glycol dimethyl ether / 12 h / 160 °C / Inert atmosphere 5: lithium aluminium tetrahydride; aluminum (III) chloride / diethyl ether / 2 h / Reflux; Inert atmosphere 6: diborane 7: iodine / ethanol / 20 °C View Scheme |
-
-
75767-29-2
Oxyberberine-13-carboxaldehyde

-
-
2086-83-1
berberine

| Conditions | Yield |
|---|---|
| Multi-step reaction with 3 steps 1: Wilkinson's catalyst; diphenyl phosphoryl azide / diethylene glycol dimethyl ether / 12 h / 160 °C / Inert atmosphere 2: lithium aluminium tetrahydride; aluminum (III) chloride / diethyl ether / 2 h / Reflux; Inert atmosphere 3: potassium acetate; iodine / ethanol / 20 °C / Inert atmosphere View Scheme | |
| Multi-step reaction with 4 steps 1: Wilkinson's catalyst; diphenyl phosphoryl azide / diethylene glycol dimethyl ether / 12 h / 160 °C / Inert atmosphere 2: lithium aluminium tetrahydride; aluminum (III) chloride / diethyl ether / 2 h / Reflux; Inert atmosphere 3: sodium tetrahydroborate / methanol / 2 h / 20 °C / Inert atmosphere 4: iodine / ethanol / 20 °C View Scheme | |
| Multi-step reaction with 4 steps 1: Wilkinson's catalyst; diphenyl phosphoryl azide / diethylene glycol dimethyl ether / 12 h / 160 °C / Inert atmosphere 2: lithium aluminium tetrahydride; aluminum (III) chloride / diethyl ether / 2 h / Reflux; Inert atmosphere 3: diborane 4: iodine / ethanol / 20 °C View Scheme |

| Conditions | Yield |
|---|---|
| Multi-step reaction with 2 steps 1: lithium aluminium tetrahydride; aluminum (III) chloride / diethyl ether / 2 h / Reflux; Inert atmosphere 2: potassium acetate; iodine / ethanol / 20 °C / Inert atmosphere View Scheme | |
| Multi-step reaction with 3 steps 1: lithium aluminium tetrahydride; aluminum (III) chloride / diethyl ether / 2 h / Reflux; Inert atmosphere 2: sodium tetrahydroborate / methanol / 2 h / 20 °C / Inert atmosphere 3: iodine / ethanol / 20 °C View Scheme | |
| Multi-step reaction with 3 steps 1: lithium aluminium tetrahydride; aluminum (III) chloride / diethyl ether / 2 h / Reflux; Inert atmosphere 2: diborane 3: iodine / ethanol / 20 °C View Scheme |

-
-
2086-83-1
berberine

| Conditions | Yield |
|---|---|
| Multi-step reaction with 3 steps 1: potassium carbonate / tetrahydrofuran / 65 °C 2: sodium carbonate; tris-(dibenzylideneacetone)dipalladium(0); XPhos / toluene; ethanol / 12 h / 110 °C / Inert atmosphere 3: triethylamine; methanesulfonyl chloride / dichloromethane / 6 h / 20 °C View Scheme |

-
-
2086-83-1
berberine

| Conditions | Yield |
|---|---|
| Multi-step reaction with 2 steps 1: sodium carbonate; tris-(dibenzylideneacetone)dipalladium(0); XPhos / toluene; ethanol / 12 h / 110 °C / Inert atmosphere 2: triethylamine; methanesulfonyl chloride / dichloromethane / 6 h / 20 °C View Scheme |
-
-
2086-83-1
berberine

-
-
108275-17-8, 6847-93-4
berberrubine

| Conditions | Yield |
|---|---|
| In chloroform for 0.0833333h; microwave irradiation; | 98% |
| In neat (no solvent) at 190℃; for 0.75h; | 79% |
| at 180℃; for 1h; |

| Conditions | Yield |
|---|---|
| With sodium hydroxide In ethanol at 60 - 70℃; pH=7 - 8; | 92% |

| Conditions | Yield |
|---|---|
| In ethanol at 60 - 70℃; pH=7 - 8; | 86% |
-
-
2086-83-1
berberine

-
-
29074-38-2
canadine

| Conditions | Yield |
|---|---|
| With indium; ammonium chloride In ethanol for 10h; Heating; | 83% |
| With hydrogenchloride; zinc for 3h; Heating; | 65% |
| With sodium tetrahydroborate | |
| With sodium tetrahydroborate In water for 1h; Cooling with ice; | |
| With sodium tetrahydroborate In methanol for 24.25h; |

| Conditions | Yield |
|---|---|
| With sodium tetrahydroborate; potassium carbonate; sodium hydroxide In methanol at 20℃; for 3h; | 81% |
| With pyridine; sodium tetrahydroborate at 20℃; for 1h; | |
| With sodium tetrahydroborate | |
| With pyridine; sodium tetrahydroborate at 25℃; |
-
-
2086-83-1
berberine

-
-
17388-19-1
berberrubine

| Conditions | Yield |
|---|---|
| With C60H54N2O10(2+) In N,N-dimethyl-formamide at 50 - 400℃; Solvent; | 75% |
| at 190 - 200℃; under 20 - 30 Torr; for 0.5h; | 75% |
| at 190 - 200℃; under 20 - 30 Torr; for 0.5h; | 75% |
-
-
2086-83-1
berberine

-
-
54313-01-8
Dehydro-berberubinium

| Conditions | Yield |
|---|---|
| at 190 - 200℃; under 20 - 30 Torr; for 0.5h; | 75% |

| Conditions | Yield |
|---|---|
| Stage #1: Mangiferin With sodium hydrogencarbonate In ethanol; water Stage #2: berberine In ethanol; water for 12h; | 70.5% |

-
-
2086-83-1
berberine

| Conditions | Yield |
|---|---|
| With sodium hydride In dimethyl sulfoxide; mineral oil at 50℃; for 5h; | 70% |

-
-
2086-83-1
berberine

| Conditions | Yield |
|---|---|
| With sodium hydride In N,N-dimethyl-formamide at 50℃; for 8h; | 69% |

-
-
2086-83-1
berberine

| Conditions | Yield |
|---|---|
| With sodium hydride In dimethyl sulfoxide; mineral oil at 60℃; for 6h; | 66% |

-
-
2086-83-1
berberine

| Conditions | Yield |
|---|---|
| With sodium hydride In dimethyl sulfoxide; mineral oil at 55℃; for 7h; | 65% |

-
-
2086-83-1
berberine

| Conditions | Yield |
|---|---|
| With sodium hydride In dimethyl sulfoxide; mineral oil at 60℃; for 5h; | 61.7% |
-
-
124-13-0
Octanal

-
-
2086-83-1
berberine

-
-
947599-36-2
9,10-dimethoxy-13-octyl-5,6-dihydro-[1,3]dioxolo[4,5-g]isoquinolino[3,2-a]isoquinolin-7-ium chloride

| Conditions | Yield |
|---|---|
| Stage #1: berberine With sodium tetrahydroborate; potassium carbonate; sodium hydroxide In methanol at 20℃; for 2h; Stage #2: Octanal With acetic acid In ethanol at 90℃; for 6h; | 51% |
-
-
2086-83-1
berberine

-
A
-
549-21-3
oxyberberine

-
B
-
64939-64-6, 66408-44-4
3-<3,4-dihydro-1-oxo-6,7-(methylenedioxy)-2H-isoquinolin-2-yl>-4,5-dimethoxy-1(3H)-isobenzofuranone

-
C
-
66408-27-3
13-hydroxy-8-oxo-berberine

| Conditions | Yield |
|---|---|
| With iodosylbenzene; tetra-(n-butyl)ammonium iodide In water; acetonitrile at 50℃; for 18h; | A 5% B 37% C 14% |


| Conditions | Yield |
|---|---|
| (i) K3Fe(CN)6, (ii) /BRN= 1098229/, HCl; Multistep reaction; |
-
-
67-56-1
methanol

-
-
2086-83-1
berberine

-
-
71733-98-7
1-[(3',4'-dimethoxy-2'-methylcarboxy)benzoyl]-6,7-methylenedioxyisoquinoline

| Conditions | Yield |
|---|---|
| (i) K3Fe(CN)6, (ii) Py*HCl, (iii) HCl, /BRN= 1098229/; Multistep reaction; |
-
-
67-56-1
methanol

-
-
2086-83-1
berberine

-
-
71733-96-5, 95585-80-1
9,10,13a-trimethoxy-2,3-(methylenedioxy)-8,13-dioxo-5,6,13,13a-tetrahydro-8H-dibenzoquinolizine

| Conditions | Yield |
|---|---|
| (i) K3Fe(CN)6, (ii) Py*HCl, (iii) /BRN= 1098229/; Multistep reaction; |
-
-
2086-83-1
berberine

-
-
71766-69-3
13a-hydroxy-9,10-dimethoxy-2,3-(methylenedioxy)-8,13-dioxo-5,6,13,13a-tetrahydro-8H-dibenzoquinolizine

| Conditions | Yield |
|---|---|
| (i) K3Fe(CN)6, (ii) Py*HCl; Multistep reaction; |

| Conditions | Yield |
|---|---|
| In methanol Ambient temperature; |

| Conditions | Yield |
|---|---|
| Multistep reaction; |
-
-
2086-83-1
berberine

-
-
80665-65-2
9,10-dimethoxy-2,3-methylenedioxy-8,14-cycloberbin-13-one

| Conditions | Yield |
|---|---|
| With alkali |
-
-
2086-83-1
berberine

-
-
77-78-1
dimethyl sulfate

-
-
32245-50-4
9,10-dimethoxy-7-methyl-5,8-dihydro-6H-[1,3]dioxolo[4,5-g]isoquino[3,2-a]isoquinolinium; methyl sulfate
Berberine Specification
The Berberine, with the CAS registry number 2086-83-1, is also known as 5,6-Dihydro-9,10-dimethoxybenzo[g]-1,3-benzodioxolo[5,6-a]quinolizinium. Its EINECS number is 211-195-9. This chemical's molecular formula is C20H18NO4 and molecular weight is 336.37. What's more, its systematic name is 9,10-Dimethoxy-5,6-dihydro[1,3]dioxolo[4,5-g]isoquinolino[3,2-a]isoquinolin-7-ium. Its classification code is Mutation data. This chemical is used in histology for staining heparin in mast cells because of a strong yellow fluorescence. As a traditional medicine or dietary supplement, it has showed some activity against fungal infections, Candida albicans, yeast, parasites and bacterial/viral infections. Berberine is considered as an ineffective antibiotic and is a component of some eye drop formulations.
Physical properties of Berberine are: (1)ACD/LogP: 0.053; (2)# of Rule of 5 Violations: 0; (3)ACD/LogD (pH 5.5): 0.05; (4)ACD/LogD (pH 7.4): 0.05; (5)ACD/BCF (pH 5.5): 1.00; (6)ACD/BCF (pH 7.4): 1.00; (7)ACD/KOC (pH 5.5): 25.46; (8)ACD/KOC (pH 7.4): 25.46; (9)#H bond acceptors: 5; (10)#H bond donors: 0; (11)#Freely Rotating Bonds: 2; (12)Polar Surface Area: 40.8 Å2.
Preparation: this chemical can be prepared by C21H19NO6. This reaction will need reagent sodium cyanoborohydride and solvent methanol. The yield is about 96%.
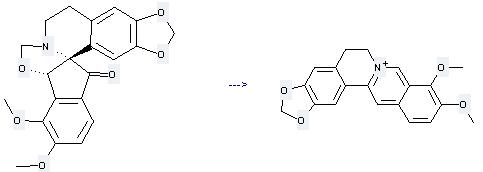
Uses of Berberine: it can be used to produce 9,10-dimethoxy-5,8,13,13a-tetrahydro-6H-[1,3]dioxolo[4,5-g]isoquino[3,2-a]isoquinoline by heating. It will need reagents In, aq. NH4Cl and solvent ethanol with the reaction time of 10 hours. The yield is about 83%.
![Berberine can be used to produce 9,10-dimethoxy-5,8,13,13a-tetrahydro-6H-[1,3]dioxolo[4,5-g]isoquino[3,2-a]isoquinoline by heating](/UserFilesUpload/Uses of Berberine.jpeg)
You can still convert the following datas into molecular structure:
(1)SMILES: O1c2c(OC1)cc5c(c2)c4cc3ccc(OC)c(OC)c3c[n+]4CC5
(2)Std. InChI: InChI=1S/C20H18NO4/c1-22-17-4-3-12-7-16-14-9-19-18(24-11-25-19)8-13(14)5-6-21(16)10-15(12)20(17)23-2/h3-4,7-10H,5-6,11H2,1-2H3/q+1
(3)Std. InChIKey: YBHILYKTIRIUTE-UHFFFAOYSA-N
The toxicity data is as follows:
| Organism | Test Type | Route | Reported Dose (Normalized Dose) | Effect | Source |
|---|---|---|---|---|---|
| mouse | LD50 | oral | 329mg/kg (329mg/kg) | Yakugaku Zasshi. Journal of Pharmacy. Vol. 82, Pg. 726, 1962. | |
| mouse | LD50 | subcutaneous | 18mg/kg (18mg/kg) | Russian Pharmacology and Toxicology Vol. 31, Pg. 129, 1968. | |
| rabbit | LDLo | subcutaneous | 100mg/kg (100mg/kg) | "Abdernalden's Handbuch der Biologischen Arbeitsmethoden." Vol. 4, Pg. 1289, 1935. | |
| rat | LD | intraperitoneal | > 500mg/kg (500mg/kg) | National Academy of Sciences, National Research Council, Chemical-Biological Coordination Center, Review. Vol. 5, Pg. 17, 1953. |
Related Products
- Berberine
- Berberine chloride dihydrate
- Berberine Deriv JCI 2223
- Berberine hydrochloride
- Berberine hydrogen sulphate
- Berberine sulfate
- Berberine sulfate trihydrate
- 20869-95-8
- 20870-77-3
- 20870-78-4
- 20870-79-5
- 20870-90-0
- 20870-91-1
- 208709-55-1
- 20872-29-1
- 20872-93-9
- 20873-58-9
Hot Products
About|Contact|Cas|Product Name|Molecular|Country|Encyclopedia
Message|New Cas|MSDS|Service|Advertisement|CAS DataBase|Article Data|Manufacturers | Chemical Catalog
©2008 LookChem.com,License: ICP
NO.:Zhejiang16009103
complaints:service@lookchem.com Desktop View