-
Name
Canrenone
- EINECS 213-554-5
- CAS No. 976-71-6
- Article Data30
- CAS DataBase
- Density 1.19 g/cm3
- Solubility 272.4ug/L(25 oC)
- Melting Point 158-160 °C
- Formula C22H28O3
- Boiling Point 541.1 °C at 760 mmHg
- Molecular Weight 340.463
- Flash Point 237.6 °C
- Transport Information
- Appearance
- Safety 36/37-61
- Risk Codes 40-51/53
-
Molecular Structure
- Hazard Symbols Xn,N
- Synonyms 17a-Pregna-4,6-diene-21-carboxylicacid, 17-hydroxy-3-oxo-, g-lactone (6CI,7CI,8CI);17-Hydroxy-3-oxo-17a-pregna-4,6-diene-21-carboxylic acid g-lactone;17a-(2-Carboxyethyl)-17b-hydroxyandrosta-4,6-dien-3-one lactone;17b-Hydroxy-3-oxopregna-4,6-diene-21-carboxylicacid;20-Spiroxa-4,6-diene-3,21-dione;3-(17b-Hydroxy-3-oxoandrosta-4,6-dien-17a-yl)propionic acid g-lactone;3'-(3-Oxo-17b-hydroxyandrosta-4,6-dien-17a-yl)-propionic acid lactone;Aldadiene;Phanurane;
- PSA 43.37000
- LogP 4.37010
Synthetic route

| Conditions | Yield |
|---|---|
| With mercury dichloride In ethanol Ambient temperature; | 95% |
| With sodium methylate In tetrahydrofuran at 20℃; for 18h; Elimination; | 72% |
| With potassium dihydrogenphosphate In ethanol at 40℃; Rate constant; Kinetics; Thermodynamic data; var. buffers, pH, temp., ionic strengh, spironolactone and buffer conc.; energy of activation; |

-
-
976-71-6
canrenone

| Conditions | Yield |
|---|---|
| With NH2SO4 In methanol for 0.0833333h; Heating; | 92% |
| With hydrogenchloride In chloroform for 8h; Heating; | 86% |
| With acetic acid for 0.5h; Heating; |

-
-
976-71-6
canrenone

| Conditions | Yield |
|---|---|
| With chloranil In methanol; water; 1,2-dichloro-ethane at 20℃; for 1h; | 84% |

-
-
976-71-6
canrenone

| Conditions | Yield |
|---|---|
| Stage #1: 17β-hydroxy-3-ethoxy-17α-pregn-3,5-diene-21-carboxylic acid-γ-lactone With chloranil In methanol; dichloromethane; water at 20℃; for 1h; Stage #2: With sodium thiosulfate In methanol; water at 20℃; for 1h; | 83% |

| Conditions | Yield |
|---|---|
| With chloranil In tert-butyl alcohol Heating; | 70% |
| With chloranil; toluene-4-sulfonic acid; xylene |

| Conditions | Yield |
|---|---|
| With mercury dichloride In ethanol for 2h; Heating; | A 38% B 54% |
-
-
13934-61-7, 14009-58-6
C22H32O3

-
-
976-71-6
canrenone

| Conditions | Yield |
|---|---|
| Yield given. Multistep reaction; |

| Conditions | Yield |
|---|---|
| Multi-step reaction with 5 steps 1: 98 percent / trimethyl orthoformate, p-toluenesulfonic acid / methanol / 3 h / 50 °C 2: H2, triphenylphosphine, rhodium acetate dimer / ethyl acetate / 20 h / 80 °C / 9000.7 Torr 3: 100 percent / N-methylmorpholine N-oxide (NMO), RuCl2(Ph3P)3 / dimethylformamide / 0.17 h 4: 97 percent / 6 N HCl / tetrahydrofuran / 0.5 h 5: 70 percent / p-chloranil / 2-methyl-propan-2-ol / Heating View Scheme |
-
-
50407-76-6
17α-ethynyl-17β-hydroxy-5-androsten-3-one ethylene ketal

-
-
976-71-6
canrenone

| Conditions | Yield |
|---|---|
| Multi-step reaction with 4 steps 1: H2, triphenylphosphine, rhodium acetate dimer / ethyl acetate / 20 h / 80 °C / 9000.7 Torr 2: 100 percent / N-methylmorpholine N-oxide (NMO), RuCl2(Ph3P)3 / dimethylformamide / 0.17 h 3: 97 percent / 6 N HCl / tetrahydrofuran / 0.5 h 4: 70 percent / p-chloranil / 2-methyl-propan-2-ol / Heating View Scheme |
-
-
121936-43-4
5'-hydroxyspiro-5-en-3-one

-
-
976-71-6
canrenone

| Conditions | Yield |
|---|---|
| Multi-step reaction with 3 steps 1: 100 percent / N-methylmorpholine N-oxide (NMO), RuCl2(Ph3P)3 / dimethylformamide / 0.17 h 2: 97 percent / 6 N HCl / tetrahydrofuran / 0.5 h 3: 70 percent / p-chloranil / 2-methyl-propan-2-ol / Heating View Scheme |
-
-
75219-50-0
C24H34O4

-
-
976-71-6
canrenone

| Conditions | Yield |
|---|---|
| Multi-step reaction with 2 steps 1: 97 percent / 6 N HCl / tetrahydrofuran / 0.5 h 2: 70 percent / p-chloranil / 2-methyl-propan-2-ol / Heating View Scheme |
-
-
38753-76-3
3-(3-oxo-7α-thio-17β-hydroxy-4-androsten-17α-yl)propionic acid γ-lactone

-
-
74-88-4
methyl iodide

-
A
-
976-71-6
canrenone

-
B
-
38753-77-4
7-Thiomethylspirolactone

| Conditions | Yield |
|---|---|
| With sodium hydride In tetrahydrofuran at 20℃; for 2h; |

-
-
38753-76-3
3-(3-oxo-7α-thio-17β-hydroxy-4-androsten-17α-yl)propionic acid γ-lactone

-
-
976-71-6
canrenone

| Conditions | Yield |
|---|---|
| With sodium methylate; methyl iodide In methanol at 20℃; for 1h; |
-
-
52-01-7
SPIRONOLACTONE

-
A
-
38753-76-3
3-(3-oxo-7α-thio-17β-hydroxy-4-androsten-17α-yl)propionic acid γ-lactone

-
B
-
976-71-6
canrenone

| Conditions | Yield |
|---|---|
| With sodium hypochlorite; octabromotetrakis(2,6-dichlorophenyl)porphyrin Fe(III)Cl In water; acetonitrile at 80℃; for 0.833333h; Microwave irradiation; | |
| With curvularia lunata Microbiological reaction; |

| Conditions | Yield |
|---|---|
| Multi-step reaction with 5 steps 1: potassium hydroxide / tetrahydrofuran / 6.5 h / 15 - 20 °C / Inert atmosphere 2: 5%-palladium/activated carbon; hydrogen / ethanol / 5 h / 20 - 30 °C / Inert atmosphere 3: 2,2,6,6-Tetramethyl-1-piperidinyloxy free radical; potassium bromide; sodium hypochlorite; tetrabutyl-ammonium chloride / dichloromethane / 6 h / 10 - 15 °C 4: pyridine; sodium acetate; acetic acid; N-Bromosuccinimide / acetone; water / 2 h / -2 - 2 °C 5: lithium carbonate; lithium bromide / N,N-dimethyl-formamide / 3 h / 100 - 105 °C View Scheme |

-
-
976-71-6
canrenone

| Conditions | Yield |
|---|---|
| With lithium carbonate; lithium bromide In N,N-dimethyl-formamide at 100 - 105℃; for 3h; | 7.8 g |
-
-
55542-26-2
17,23-dihydroxy-21,24-dinor-17βH-chol-4-en-20-yn-3-one

-
-
976-71-6
canrenone

| Conditions | Yield |
|---|---|
| Multi-step reaction with 4 steps 1: 5%-palladium/activated carbon; hydrogen / ethanol / 5 h / 20 - 30 °C / Inert atmosphere 2: 2,2,6,6-Tetramethyl-1-piperidinyloxy free radical; potassium bromide; sodium hypochlorite; tetrabutyl-ammonium chloride / dichloromethane / 6 h / 10 - 15 °C 3: pyridine; sodium acetate; acetic acid; N-Bromosuccinimide / acetone; water / 2 h / -2 - 2 °C 4: lithium carbonate; lithium bromide / N,N-dimethyl-formamide / 3 h / 100 - 105 °C View Scheme |
-
-
55542-27-3
17α-(3-hydroxypropyl)-17β-hydroxy-4-androsten-3-one

-
-
976-71-6
canrenone

| Conditions | Yield |
|---|---|
| Multi-step reaction with 3 steps 1: 2,2,6,6-Tetramethyl-1-piperidinyloxy free radical; potassium bromide; sodium hypochlorite; tetrabutyl-ammonium chloride / dichloromethane / 6 h / 10 - 15 °C 2: pyridine; sodium acetate; acetic acid; N-Bromosuccinimide / acetone; water / 2 h / -2 - 2 °C 3: lithium carbonate; lithium bromide / N,N-dimethyl-formamide / 3 h / 100 - 105 °C View Scheme |
-
-
52-01-7
SPIRONOLACTONE

-
A
-
6785-74-6
3-(17β-hydroxy-3-oxo-1,4,6-androstadien-17α-yl)propionic acid γ-lactone

-
B
-
976-71-6
canrenone

| Conditions | Yield |
|---|---|
| With gibberella fujikuroi for 240h; Microbiological reaction; |
-
-
52-01-7
SPIRONOLACTONE

-
A
-
58551-67-0
15α-hydroxycanrenone

-
E
-
38753-76-3
3-(3-oxo-7α-thio-17β-hydroxy-4-androsten-17α-yl)propionic acid γ-lactone

-
F
-
6785-74-6
3-(17β-hydroxy-3-oxo-1,4,6-androstadien-17α-yl)propionic acid γ-lactone

-
G
-
976-71-6
canrenone

| Conditions | Yield |
|---|---|
| With aspergillus alliaceus Microbiological reaction; |

-
-
52-01-7
SPIRONOLACTONE

-
A
-
58551-67-0
15α-hydroxycanrenone

-
E
-
6785-74-6
3-(17β-hydroxy-3-oxo-1,4,6-androstadien-17α-yl)propionic acid γ-lactone

-
F
-
976-71-6
canrenone

| Conditions | Yield |
|---|---|
| With fusarium lini Microbiological reaction; |


| Conditions | Yield |
|---|---|
| Multi-step reaction with 2 steps 1: lithium bromide; C30H31Cl3N3(1+)*BF4(1-); triethylamine / 1,2-dichloro-ethane / 5 h / 20 °C / Inert atmosphere; Sealed tube 2: chloranil / 1,2-dichloro-ethane; methanol; water / 1 h / 20 °C View Scheme |

-
-
976-71-6
canrenone

| Conditions | Yield |
|---|---|
| With acetic acid In water; toluene at 0 - 100℃; for 24h; pH=7.1; Temperature; pH-value; Autoclave; Inert atmosphere; | 33.1 g |

-
-
976-71-6
canrenone

| Conditions | Yield |
|---|---|
| With methanesulfonyl chloride; triethylamine In dichloromethane at 0℃; for 4h; Temperature; Reagent/catalyst; Solvent; Inert atmosphere; | 8.6 g |

| Conditions | Yield |
|---|---|
| Multi-step reaction with 4 steps 1.1: iodine; mercury dichloride; magnesium / diethyl ether / 0.5 h / 40 °C 1.2: 2.5 h / 0 - 20 °C 2.1: lithium hexamethyldisilazane / tetrahydrofuran / 1 h / 0 °C 2.2: 2 h / 0 - 20 °C 2.3: 1 h / 0 - 20 °C 3.1: Dess-Martin periodane / dichloromethane / 4 h / 0 - 20 °C 4.1: chloranil / tert-butyl alcohol / 3 h / 80 °C View Scheme |
-
-
156894-83-6
21,24-dinor-3β,17β-dihydroxy-chol-5-en-22-yne

-
-
976-71-6
canrenone

| Conditions | Yield |
|---|---|
| Multi-step reaction with 3 steps 1.1: lithium hexamethyldisilazane / tetrahydrofuran / 1 h / 0 °C 1.2: 2 h / 0 - 20 °C 1.3: 1 h / 0 - 20 °C 2.1: Dess-Martin periodane / dichloromethane / 4 h / 0 - 20 °C 3.1: chloranil / tert-butyl alcohol / 3 h / 80 °C View Scheme |
-
-
13934-61-7
3β,17β-Dihydroxy-5-androsten-17α-propanoic Acid γ-Lactone

-
-
976-71-6
canrenone

| Conditions | Yield |
|---|---|
| Multi-step reaction with 2 steps 1: Dess-Martin periodane / dichloromethane / 4 h / 0 - 20 °C 2: chloranil / tert-butyl alcohol / 3 h / 80 °C View Scheme |

-
-
976-71-6
canrenone

| Conditions | Yield |
|---|---|
| With chloranil In tert-butyl alcohol at 80℃; for 3h; | 7.9 mg |
-
-
64-18-6
formic acid

-
-
976-71-6
canrenone

-
A
-
66175-65-3
C22H27ClO3

-
B
-
110078-67-6
7α-Chlor-6β-(formyloxy)-17β-hydroxy-3-oxo-4,6-pregnadien-21-carbonsaeure-γ-lacton

| Conditions | Yield |
|---|---|
| With tert-butylhypochlorite for 1h; Ambient temperature; Title compound not separated from byproducts; | A n/a B 91% |

-
-
976-71-6
canrenone

-
-
245076-35-1
4-(t-butyldimethylsilyloxy)-benzyl mercaptan

-
-
245076-30-6
3-oxo-17α-pregna-4-ene-7α-[4-(t-butyldimethylsilyloxy)-benzylthia]-21,17-carbolactone

| Conditions | Yield |
|---|---|
| With sodium at 45℃; for 0.75h; Addition; | 91% |
-
-
976-71-6
canrenone

-
-
192569-17-8
11α-hydroxyl canrenone

| Conditions | Yield |
|---|---|
| With D-glucose; peptone In water | 90% |
| With D-glucose In water for 72h; | 80% |
| With D-glucose In water for 72h; | 80% |
-
-
30354-18-8
N,N-dimethyl(methylene)ammonium chloride

-
-
976-71-6
canrenone

| Conditions | Yield |
|---|---|
| In acetonitrile for 24h; Ambient temperature; | 86% |
-
-
976-71-6
canrenone

-
-
245076-36-2
[4-(2-piperidin-1-yl-ethoxy)-phenyl]-methanethiol

| Conditions | Yield |
|---|---|
| With sodium at 45℃; for 1h; Addition; | 82% |
-
-
976-71-6
canrenone

| Conditions | Yield |
|---|---|
| With Nocardia farcinica NCAIM (P)-B 001342 9α-hydroxylase; 13-ethyl-10,11α-dihydroxy-4-gonene-3,17-dione In methanol; water; N,N-dimethyl-formamide at 30℃; for 16h; Enzymatic reaction; | 82% |
| With dipotassium hydrogenphosphate; D-glucose; sodium chloride In methanol; water at 26℃; for 5 - 48h; Conversion of starting material; | |
| With dipotassium hydrogenphosphate; D-glucose; sodium chloride In methanol; water at 26℃; for 5 - 48h; Conversion of starting material; |

| Conditions | Yield |
|---|---|
| Stage #1: tiolacetic acid With trifluoromethylsulfonic anhydride In tetrahydrofuran Stage #2: canrenone In tetrahydrofuran at 20℃; for 1h; | 76% |
| With trimethylsilyl trifluoromethanesulfonate In tetrahydrofuran at 20℃; for 3h; | 60% |
| In water at 20℃; for 2h; Michael addition; |

| Conditions | Yield |
|---|---|
| With sodium at 60℃; for 24h; Addition; | 74% |

| Conditions | Yield |
|---|---|
| titanium tetrachloride In dichloromethane at -70℃; | A 73% B 15% |


| Conditions | Yield |
|---|---|
| With sodium at 60℃; for 24h; Addition; | 72% |

| Conditions | Yield |
|---|---|
| With pyridine for 24h; Heating; | 70% |


| Conditions | Yield |
|---|---|
| With ethanol; boron trifluoride diethyl etherate In nitromethane at -19℃; for 17h; | A 69.5% B 19.8% |

-
-
976-71-6
canrenone

| Conditions | Yield |
|---|---|
| With pyridinium hydrobromide perbromide In acetic acid for 1.5h; Ambient temperature; | 69% |

| Conditions | Yield |
|---|---|
| With piperidine at 10℃; | 69% |
| With piperidine In methanol at 20℃; for 20h; |

| Conditions | Yield |
|---|---|
| With trimethylsilyl trifluoromethanesulfonate In tetrahydrofuran for 1h; Ambient temperature; Yields of byproduct given; | A n/a B 64% |
| With trimethylsilyl trifluoromethanesulfonate In tetrahydrofuran for 1h; Ambient temperature; Yield given; | A n/a B 64% |


| Conditions | Yield |
|---|---|
| With sodium at 60℃; for 24h; Addition; | 62% |

| Conditions | Yield |
|---|---|
| With sodium at 60℃; for 24h; Addition; | 59% |

| Conditions | Yield |
|---|---|
| With sodium at 60℃; for 24h; Addition; | 59% |
-
-
592-41-6
1-hexene

-
-
976-71-6
canrenone

-
A
-
1430412-93-3
(+)-7α-hexyl-17-hydroxy-3-oxo-17α-pregn-4-ene-21-carboxylic acid γ-lactone

-
B
-
1430412-96-6
(+)-7β-hexyl-17-hydroxy-3-oxo-17α-pregn-4-ene-21-carboxylic acid γ-lactone

| Conditions | Yield |
|---|---|
| Stage #1: 1-hexene With Schwartz's reagent In dichloromethane at 20℃; for 0.666667h; Inert atmosphere; Schlenk technique; Stage #2: canrenone With chloro-trimethyl-silane; copper(I) trifluoromethanesulfonate benzene In diethyl ether; dichloromethane at 20℃; for 15h; Inert atmosphere; Schlenk technique; diastereoselective reaction; | A 59% B 21% |
| Stage #1: 1-hexene With Schwartz's reagent In dichloromethane at 20℃; for 0.666667h; Inert atmosphere; Stage #2: With C36H30CuNO2P(1+)*CF3O3S(1-) In diethyl ether; dichloromethane at 20℃; for 0.166667h; Inert atmosphere; Stage #3: canrenone With chloro-trimethyl-silane In diethyl ether; dichloromethane at 20℃; for 15h; Inert atmosphere; | A 59% B 21% |

-
-
976-71-6
canrenone

-
-
67605-87-2
γ-lactone of 17α-pregna-4,6-diene-3β,17β-diol-20-carboxylic acid

| Conditions | Yield |
|---|---|
| With sodium tetrahydroborate In methanol | 56% |
-
-
976-71-6
canrenone

| Conditions | Yield |
|---|---|
| With 2,6-di-tert-butyl-4-methyl-phenol; 3-chloro-benzenecarboperoxoic acid In chloroform for 1.66667h; Heating; | 53% |
Canrenone Specification
The CAS registry number of Canrenone is 976-71-6. The IUPAC name is (8R,9S,10R,13S,14S,17R)-10,13-dimethylspiro[2,8,9,11,12,14,15,16-octahydro-1H-cyclopenta[a]phenanthrene-17,5'-oxolane]-2',3-dione. Its EINECS registry number is 213-554-5. In addition, the molecular formula is C22H28O3 and the molecular weight is 340.46. It is a kind of pale yellow to pale green solid and belongs to the classes of Spironolacrone; Intermediates & Fine Chemicals; Pharmaceuticals; Steroids. And it is a synthetic pregnadiene compound with anti-aldosterone activity.
Physical properties about this chemical are: (1)ACD/LogP: 2.99; (2)# of Rule of 5 Violations: 0; (3)ACD/LogD (pH 5.5): 2.99; (4)ACD/LogD (pH 7.4): 2.99; (5)ACD/BCF (pH 5.5): 110.05; (6)ACD/BCF (pH 7.4): 110.05; (7)ACD/KOC (pH 5.5): 1006.86; (8)ACD/KOC (pH 7.4): 1006.86; (9)#H bond acceptors: 3; (10)Polar Surface Area: 43.37 Å2; (11)Index of Refraction: 1.581; (12)Molar Refractivity: 95.22 cm3; (13)Molar Volume: 285.5 cm3; (14)Polarizability: 37.75 ×10-24cm3; (15)Surface Tension: 47.2 dyne/cm; (16)Density: 1.19 g/cm3; (17)Flash Point: 237.6 °C; (18)Enthalpy of Vaporization: 81.89 kJ/mol; (19)Boiling Point: 541.1 °C at 760 mmHg; (20)Vapour Pressure: 8.95E-12 mmHg at 25°C.
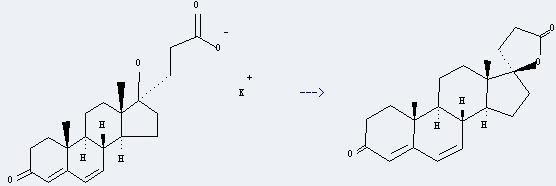
Preparation of Canrenone: it can be prepared by potassium canrenoate. This reaction will need reagent NH2SO4 and solvent methanol. The reaction time is 5 minutes by heating. The yield is about 92%.
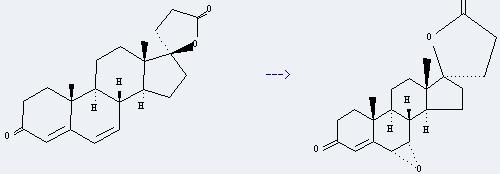
Uses of Canrenone: it can be used to get 6a,7a-Epoxycanrenone. This reaction will need reagents m-chloroperbenzoic acid and 2,6-di-t-butyl-4-methylphenol and solvent CHCl3. The reaction time is 100 minutes by heating. The yield is about 53%.
You can still convert the following datas into molecular structure:
(1)SMILES: O=C5\C=C4\C=C/[C@@H]1[C@H](CC[C@]3([C@H]1CC[C@]32OC(=O)CC2)C)[C@@]4(C)CC5
(2)InChI: InChI=1/C22H28O3/c1-20-9-5-15(23)13-14(20)3-4-16-17(20)6-10-21(2)18(16)7-11-22(21)12-8-19(24)25-22/h3-4,13,16-18H,5-12H2,1-2H3/t16-,17+,18+,20+,21+,22-/m1/s1
(3)InChIKey: UJVLDDZCTMKXJK-WNHSNXHDBD
The toxicity data is as follows:
| Organism | Test Type | Route | Reported Dose (Normalized Dose) | Effect | Source |
|---|---|---|---|---|---|
| rat | LD50 | oral | > 5gm/kg (5000mg/kg) | Acute Toxicity Data. Journal of the American College of Toxicology, Part B. Vol. 1, Pg. 36, 1990. |
About|Contact|Cas|Product Name|Molecular|Country|Encyclopedia
Message|New Cas|MSDS|Service|Advertisement|CAS DataBase|Article Data|Manufacturers | Chemical Catalog
©2008 LookChem.com,License: ICP
NO.:Zhejiang16009103
complaints:service@lookchem.com Desktop View