-
Name
Carbamazepine
- EINECS 206-062-7
- CAS No. 298-46-4
- Article Data34
- CAS DataBase
- Density 1.266 g/cm3
- Solubility insoluble in water
- Melting Point 189-192 °C
- Formula C15H12N2O
- Boiling Point 410.984 °C at 760 mmHg
- Molecular Weight 236.273
- Flash Point 202.356 °C
- Transport Information
- Appearance white solid
- Safety 37-24-22-36/37/39-36
- Risk Codes 42/43-22-20/21/22
-
Molecular Structure
-
Hazard Symbols
 Xn
Xn
- Synonyms Geigy 32883;Carbamazepine BP;Carbamazepine Bromine Free;5-Carbamyl-5H-dibenzo(b,f)azepine;Amizepin;Tegretal;Carbamazepine (JP14/USP);G 32883;Lexin;Karbamazepin;G-32883;Neurotol;Finlepsin;5-Carbamoyldibenzo(b,f)azepine;Carbamazepen;Tegretol (TN);5-Carbamoyl-5H-dibenzo(b,f)azepine;Carbazepine;Stazepine;Biston;5H-Dibenz[b,f]azepine-5-carboxamide;
- PSA 46.33000
- LogP 4.15250
Synthetic route

| Conditions | Yield |
|---|---|
| In water; acetic acid at 15 - 60℃; for 4h; Product distribution / selectivity; | 98.8% |
| In acetic acid at 18 - 60℃; for 5h; Product distribution / selectivity; | 95.9% |
| In ethanol; acetic acid at 60 - 80℃; for 1.5h; Product distribution / selectivity; | 93.7% |
-
-
59690-97-0
10-Brom-5-carbamoyl-5H-dibenzazepin

-
-
298-46-4
carbamazepin

| Conditions | Yield |
|---|---|
| With tetraethylammonium perchlorate In water; N,N-dimethyl-formamide cathode: Hg, working potential: -1.80 V, charge: 2.0-2.1 F/mol, 4-5 h; | 98% |
| With tetraethylammonium perchlorate In water; N,N-dimethyl-formamide Mechanism; cathode: Hg, working potential: -1.80 V, charge: 2.0-2.1 F, 4-5 h; other substituted azepines; | 98% |

| Conditions | Yield |
|---|---|
| In water; acetic acid at 20 - 80℃; for 2 - 5.5h; Product distribution / selectivity; | 93.2% |
-
-
59690-99-2, 141701-26-0, 143667-56-5
trans-10,11-dibromo-10,11-dihydro-5H-dibenzazepine-5-carboxamide

-
-
298-46-4
carbamazepin

| Conditions | Yield |
|---|---|
| With tetraethylammonium perchlorate In water; N,N-dimethyl-formamide cathode: Hg, working potential: -0.5 V, charge: 1.9-2.1 F/mol, 3-4 h; | 92% |
-
-
28721-07-5
oxcarbazepine

-
-
298-46-4
carbamazepin

| Conditions | Yield |
|---|---|
| With methanol; sodium tetrahydroborate at 45 - 50℃; for 3h; | 91.5% |

| Conditions | Yield |
|---|---|
| In dichloromethane; water at 5 - 30℃; | 78% |
-
-
143667-55-4
10,11-Dibrom-5-carbamoyl-5H-dibenzazepin

-
-
298-46-4
carbamazepin

| Conditions | Yield |
|---|---|
| With tetraethylammonium perchlorate In N,N-dimethyl-formamide for 12h; cathode: Hg, working potential: -1.35 V, charge: 2.6-3.0 F/mol, initial current density: 0.5 A/dm2; | 17% |
-
-
42787-75-7
5-cyanodibenzazepine

-
-
298-46-4
carbamazepin

| Conditions | Yield |
|---|---|
| With hydrogenchloride at 100℃; for 1h; Yield given; | |
| With sulfuric acid; acetic acid In ice-water |
-
-
33948-22-0
5H-dibenz[b,f]azepine-5-carbonylchloride

-
-
298-46-4
carbamazepin

| Conditions | Yield |
|---|---|
| With ammonia In ethanol for 3h; Heating; | 5 g |
| With ammonium hydroxide In methanol at 75 - 85℃; for 5h; Yield given; | |
| Multi-step reaction with 3 steps 1: 78 percent / Br2 / chlorobenzene / 170 °C 2: 90 percent / conc. aq. NH3 / various solvent(s) / 2 h / 100 °C 3: 17 percent / (C2H5)4NClO4 / dimethylformamide / 12 h / cathode: Hg, working potential: -1.35 V, charge: 2.6-3.0 F/mol, initial current density: 0.5 A/dm2 View Scheme |
-
-
52618-28-7, 94063-21-5
carbamazepine cyclobutyl dimer

-
-
298-46-4
carbamazepin

| Conditions | Yield |
|---|---|
| In ethanol Quantum yield; Irradiation; |

-
-
298-46-4
carbamazepin

| Conditions | Yield |
|---|---|
| With urea In acetic acid | |
| With urea In acetic acid |

| Conditions | Yield |
|---|---|
| With water at 25℃; Kinetics; Further Variations:; pH-values; |

-
-
298-46-4
carbamazepin

| Conditions | Yield |
|---|---|
| With water at 70℃; pH=4; Kinetics; Further Variations:; pH-values; Temperatures; |

| Conditions | Yield |
|---|---|
| Multi-step reaction with 4 steps 1: 90 percent / toluene / 3.5 h / 90 - 95 °C 2: Br2 / chlorobenzene / 1.5 h / 145 - 150 °C 3: chlorobenzene / 2 h / 150 - 155 °C 4: 25percent NH4OH / methanol / 5 h / 75 - 85 °C View Scheme | |
| Multi-step reaction with 3 steps 1: N-Bromosuccinimide 2: toluene 3: ammonia View Scheme |
-
-
33948-20-8
10-bromo-10,11-dihydro-dibenzo[b,f]azepine-5-carbonyl chloride

-
-
298-46-4
carbamazepin

| Conditions | Yield |
|---|---|
| Multi-step reaction with 2 steps 1: chlorobenzene / 2 h / 150 - 155 °C 2: 25percent NH4OH / methanol / 5 h / 75 - 85 °C View Scheme |
-
-
33948-19-5
10,11-dihydro-dibenzo[b,f]azepine-5-carbonyl chloride

-
-
298-46-4
carbamazepin

| Conditions | Yield |
|---|---|
| Multi-step reaction with 3 steps 1: Br2 / chlorobenzene / 1.5 h / 145 - 150 °C 2: chlorobenzene / 2 h / 150 - 155 °C 3: 25percent NH4OH / methanol / 5 h / 75 - 85 °C View Scheme |
-
-
143667-53-2
10-Brom-5-chlorcarbonyl-5H-dibenzazepin

-
-
298-46-4
carbamazepin

| Conditions | Yield |
|---|---|
| Multi-step reaction with 4 steps 1: 92 percent / (C2H5)4NClO4 / dimethylformamide; H2O / cathode: Hg, working potential: -1.60 V, charge: 2.0-2.1 F/mol, 4-5 h 2: 78 percent / Br2 / chlorobenzene / 170 °C 3: 90 percent / conc. aq. NH3 / various solvent(s) / 2 h / 100 °C 4: 17 percent / (C2H5)4NClO4 / dimethylformamide / 12 h / cathode: Hg, working potential: -1.35 V, charge: 2.6-3.0 F/mol, initial current density: 0.5 A/dm2 View Scheme |
-
-
143667-54-3
10,11-Dibrom-5-chlorcarbonyl-5H-dibenzazepin

-
-
298-46-4
carbamazepin

| Conditions | Yield |
|---|---|
| Multi-step reaction with 2 steps 1: 90 percent / conc. aq. NH3 / various solvent(s) / 2 h / 100 °C 2: 17 percent / (C2H5)4NClO4 / dimethylformamide / 12 h / cathode: Hg, working potential: -1.35 V, charge: 2.6-3.0 F/mol, initial current density: 0.5 A/dm2 View Scheme |

| Conditions | Yield |
|---|---|
| Multi-step reaction with 2 steps 1: CHCl3 / 0.5 h / cooling 2: 10 percent HCl / 1 h / 100 °C View Scheme | |
| Multi-step reaction with 2 steps 1: toluene / 0.5 h 2: 5 g / NH3 (gas) / ethanol / 3 h / Heating View Scheme | |
| With trichloroacetic acid In water; toluene |
-
-
32621-46-8
diphenylamine-2,2'-dicarbonyl chloride

-
-
298-46-4
carbamazepin

| Conditions | Yield |
|---|---|
| Multi-step reaction with 4 steps 1: H2 / Palladium-Barium sulphate; Quinolin sulphate / xylene / 2.5 h / Heating 2: 3.8 g / Hydrazine hydrate / acetic acid / 2 h / Heating 3: toluene / 0.5 h 4: 5 g / NH3 (gas) / ethanol / 3 h / Heating View Scheme |
-
-
49579-63-7
diphenylamine-2,2′-dicarboxaldehyde

-
-
298-46-4
carbamazepin

| Conditions | Yield |
|---|---|
| Multi-step reaction with 3 steps 1: 3.8 g / Hydrazine hydrate / acetic acid / 2 h / Heating 2: toluene / 0.5 h 3: 5 g / NH3 (gas) / ethanol / 3 h / Heating View Scheme |

| Conditions | Yield |
|---|---|
| Multi-step reaction with 5 steps 1: 11.8 g / Thionyl chloride; Pyridine / 5 h / Heating 2: H2 / Palladium-Barium sulphate; Quinolin sulphate / xylene / 2.5 h / Heating 3: 3.8 g / Hydrazine hydrate / acetic acid / 2 h / Heating 4: toluene / 0.5 h 5: 5 g / NH3 (gas) / ethanol / 3 h / Heating View Scheme |

| Conditions | Yield |
|---|---|
| With sodium bicarbonate In methanol; water |

| Conditions | Yield |
|---|---|
| With sulfuric acid In nitrogen; ethyl acetate | |
| With acetic acid In nitrogen | |
| In nitrogen; water; acetic acid |

| Conditions | Yield |
|---|---|
| In nitrogen; toluene | |
| In 5,5-dimethyl-1,3-cyclohexadiene; nitrogen |


| Conditions | Yield |
|---|---|
| With hydrogenchloride In nitrogen; ethyl acetate |

| Conditions | Yield |
|---|---|
| In acetic acid | |
| In acetic acid |

-
-
298-46-4
carbamazepin

| Conditions | Yield |
|---|---|
| Multi-step reaction with 3 steps 1.1: dicyclohexyl-carbodiimide / tetrahydrofuran; dichloromethane / 6 h / 0 °C / Inert atmosphere 2.1: methylhydrazine / tetrahydrofuran / -80 °C / Inert atmosphere 2.2: 24 h / 20 °C 3.1: 25 °C / pH 5 / aq. buffer View Scheme |

| Conditions | Yield |
|---|---|
| Multi-step reaction with 3 steps 1.1: dicyclohexyl-carbodiimide / tetrahydrofuran; dichloromethane / 6 h / 0 °C / Inert atmosphere 2.1: methylhydrazine / tetrahydrofuran / -80 °C / Inert atmosphere 2.2: 24 h / 20 °C 3.1: 25 °C / pH 10 / aq. buffer View Scheme |
-
-
298-46-4
carbamazepin

-
-
42787-75-7
5-cyanodibenzazepine

| Conditions | Yield |
|---|---|
| With bis(trichloromethyl) carbonate In toluene for 5h; Product distribution / selectivity; Reflux; | 96% |
| With sodium hydroxide; Aliquat In chloroform at 20℃; for 70h; | 62% |
| With oxalyl dichloride; dimethyl sulfoxide; triethylamine In dichloromethane at -78 - 20℃; | 4% |
| With sodium hydroxide; N-benzyl-N,N,N-triethylammonium chloride In dichloromethane; chloroform; water at 45 - 50℃; for 5 - 6h; |
-
-
298-46-4
carbamazepin

-
-
36507-30-9
carbamazepine 10,11-epoxide

| Conditions | Yield |
|---|---|
| With peracetic acid; potassium permanganate supported on alumina; sodium carbonate In dichloromethane; acetic acid at 20℃; for 1.66667h; Heating / reflux; | 91% |
| With peroxyacetic acid; potassium permanganate on alumina; sodium carbonate; acetic acid In dichloromethane for 1.66667h; Reflux; | 91% |
| With perpropionic acid; sodium carbonate; acetic acid In dichloromethane at 20℃; for 2h; Reflux; | 91% |
-
-
298-46-4
carbamazepin

-
-
59690-99-2, 141701-26-0, 143667-56-5
trans-10,11-dibromo-10,11-dihydro-5H-dibenzazepine-5-carboxamide

| Conditions | Yield |
|---|---|
| With bromine In 1,2-dichloro-ethane for 2h; Ambient temperature; | 90% |
| With bromine In 1,2-dichloro-ethane at 25℃; Rate constant; Mechanism; | 100 % Chromat. |

| Conditions | Yield |
|---|---|
| In ethanol for 3h; Reflux; | 88.1% |

| Conditions | Yield |
|---|---|
| With tetraethylammonium perchlorate; ammonium chloride In water; N,N-dimethyl-formamide cathodic (Hg, -2.0 V vs. SCE) reduction; | 85% |
| With tetraethylammonium perchlorate; ammonium chloride In water; N,N-dimethyl-formamide Mechanism; cathodic (Hg, -2.0 V vs. SCE) reduction; | 85% |
| With electrochemical reduction Mechanism; var. pH; |

| Conditions | Yield |
|---|---|
| With tetraethoxy tellurium(IV) In tetrachloromethane for 3h; Heating; | 85% |
| With hydrogenchloride In water for 12h; Reagent/catalyst; Darkness; | |
| With carbon dioxide at 160 - 200℃; |

| Conditions | Yield |
|---|---|
| In ethanol for 3h; Reflux; | 85% |

| Conditions | Yield |
|---|---|
| In ethanol for 3h; Reflux; | 84.15% |
-
-
298-46-4
carbamazepin

-
-
77287-34-4, 77287-35-5, 60100-09-6
formamide

-
-
139489-10-4
Carbamazepin*Formamid

| Conditions | Yield |
|---|---|
| In chloroform | 81.5% |
| In tetrahydrofuran | |
| In methanol |

| Conditions | Yield |
|---|---|
| In ethanol for 3h; Reflux; | 80.2% |

-
-
13520-92-8
ziconium(IV) oxychloride octahydrate

-
-
298-46-4
carbamazepin

| Conditions | Yield |
|---|---|
| In ethanol for 3h; Reflux; | 79.8% |
-
-
298-46-4
carbamazepin

-
-
109745-70-2, 19757-97-2
methyl glyoxylate methyl hemi-acetal

-
-
187866-36-0
[(Dibenzo[b,f]azepine-5-carbonyl)-amino]-hydroxy-acetic acid methyl ester

| Conditions | Yield |
|---|---|
| In chloroform for 2h; Heating; | 78% |
Carbamazepine Chemical Properties
IUPAC Name: Benzo[b][1]benzazepine-11-carboxamide
Molecular Formula: C15H12N2O
Molar mass: 236.27 g/mol
EINECS: 206-062-7
Density: 1.266 g/cm3
Flash Point: 202.4 °C
Index of Refraction: 1.669
Boiling Point: 411 °C at 760 mmHg
Vapour Pressure: 5.78E-07 mmHg at 25 °C
Melting point: 189-192 °C
Storage temp: 2-8 °C
Appearance: White Solid
Solubility: Soluble in Ethanol , Acetone , Propylene glycol , insoluble in water. Odorless, tasteless
Product categories: Acids and Derivatives;Active Pharmaceutical Ingredients;APIs;Intermediates & Fine Chemicals;Heterocycles
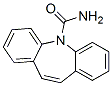
Structure of Carbamazepine (CAS NO.298-46-4):
XLogP3-AA: 2.5
H-Bond Donor: 1
H-Bond Acceptor: 1
Canonical SMILES: C1=CC=C2C(=C1)C=CC3=CC=CC=C3N2C(=O)N
InChI: InChI=1S/C15H12N2O/c16-15(18)17-13-7-3-1-5-11(13)9-10-12-6-2-4-8-14(12)
17/h1-10H,(H2,16,18)
Carbamazepine History
Carbamazepine (CAS NO.298-46-4) was discovered by chemist Walter Schindler at J.R. Geigy AG (now part of Novartis) in Basel, Switzerland, in 1953, and was first marketed as a drug to treat trigeminal neuralgia (formerly known as tic douloureux) in 1962. It has been approved in the U.S. since 1974. Carbamazepine would be studied for bipolar disorder throughout the 1970s.
Carbamazepine Uses
Carbamazepine (CAS NO.298-46-4) is an anticonvulsant and mood stabilizing drug used primarily in the treatment of epilepsy and bipolar disorder, as well as trigeminal neuralgia. It is also used off-label for a variety of indications, including schizophrenia, attention-deficit hyperactivity disorder (ADHD), paroxysmal extreme pain disorder, phantom limb syndrome, and post-traumatic stress disorder.
Carbamazepine Toxicity Data With Reference
| Organism | Test Type | Route | Reported Dose (Normalized Dose) | Effect | Source |
|---|---|---|---|---|---|
| child | TDLo | oral | 19mg/kg/4W-I (19mg/kg) | PERIPHERAL NERVE AND SENSATION: FASCICULATIONS | Pediatrics. Vol. 73, Pg. 841, 1984. |
| child | TDLo | oral | 25926ug/kg (25.926mg/kg) | BEHAVIORAL: SOMNOLENCE (GENERAL DEPRESSED ACTIVITY) BEHAVIORAL: CONVULSIONS OR EFFECT ON SEIZURE THRESHOLD | Gekkan Yakuji. Pharmaceuticals Monthly. Vol. 35, Pg. 320, 1993. |
| child | TDLo | oral | 55mg/kg (55mg/kg) | BEHAVIORAL: CONVULSIONS OR EFFECT ON SEIZURE THRESHOLD BEHAVIORAL: COMA | Journal of Toxicology, Clinical Toxicology. Vol. 38, Pg. 323, 2000. |
| child | TDLo | oral | 65mg/kg (65mg/kg) | BEHAVIORAL: CONVULSIONS OR EFFECT ON SEIZURE THRESHOLD BEHAVIORAL: COMA LUNGS, THORAX, OR RESPIRATION: OTHER CHANGES | Journal of Pediatrics. Vol. 121, Pg. 295, 1992. |
| child | TDLo | oral | 420mg/kg/3W-I (420mg/kg) | SENSE ORGANS AND SPECIAL SENSES: OTHER: EYE BEHAVIORAL: TREMOR BEHAVIORAL: GENERAL ANESTHETIC | Journal of Toxicology, Clinical Toxicology. Vol. 36, Pg. 109, 1998. |
| child | TDLo | oral | 1050mg/kg/6W- (1050mg/kg) | BEHAVIORAL: MUSCLE CONTRACTION OR SPASTICITY) | American Journal of Psychiatry. Vol. 143, Pg. 1176, 1985. |
| dog | LD50 | oral | 5620mg/kg (5620mg/kg) | "Psychotropic Drugs and Related Compounds," 2nd ed., Usdin, E., and D.H. Efron, Washington, DC, 1972Vol. -, Pg. 63, 1972. | |
| guinea pig | LD50 | oral | 920mg/kg (920mg/kg) | "Psychotropic Drugs and Related Compounds," 2nd ed., Usdin, E., and D.H. Efron, Washington, DC, 1972Vol. -, Pg. 63, 1972. | |
| human | TDLo | oral | 43mg/kg (43mg/kg) | BEHAVIORAL: SLEEP BEHAVIORAL: "HALLUCINATIONS, DISTORTED PERCEPTIONS" GASTROINTESTINAL: NAUSEA OR VOMITING | British Medical Journal. Vol. 1, Pg. 754, 1977. |
| man | LDLo | oral | 54mg/kg/9D-I (54mg/kg) | BEHAVIORAL: ATAXIA KIDNEY, URETER, AND BLADDER: URINE VOLUME INCREASED BLOOD: AGRANULOCYTOSIS | Canadian Medical Association Journal. Vol. 132, Pg. 1040, 1985. |
| man | TDLo | oral | 94mg/kg/11D-I (94mg/kg) | BLOOD: THROMBOCYTOPENIA | Journal of Clinical Pyschopharmacology. Vol. 10, Pg. 305, 1990. |
| man | TDLo | oral | 160mg/kg/3W-I (160mg/kg) | SKIN AND APPENDAGES (SKIN): "DERMATITIS, OTHER: AFTER SYSTEMIC EXPOSURE" | Journal of Clinical Pyschopharmacology. Vol. 5, Pg. 185, 1985. |
| man | TDLo | oral | 253mg/kg/6W-I (253mg/kg) | GASTROINTESTINAL: NAUSEA OR VOMITING LIVER: LIVER FUNCTION TESTS IMPAIRED | Journal of Clinical Pyschopharmacology. Vol. 6, Pg. 251, 1986. |
| mouse | LD50 | intraperitoneal | 114mg/kg (114mg/kg) | Farmakologiya i Toksikologiya Vol. 53(4), Pg. 19, 1990. | |
| mouse | LD50 | oral | 529mg/kg (529mg/kg) | Farmaco. Vol. 44, Pg. 595, 1989. | |
| mouse | LD50 | subcutaneous | > 1gm/kg (1000mg/kg) | Arzneimittel-Forschung. Drug Research. Vol. 30, Pg. 477, 1980. | |
| rabbit | LD50 | oral | 2680mg/kg (2680mg/kg) | "Psychotropic Drugs and Related Compounds," 2nd ed., Usdin, E., and D.H. Efron, Washington, DC, 1972Vol. -, Pg. 63, 1972. | |
| rat | LD50 | intraperitoneal | 158mg/kg (158mg/kg) | Archives Internationales de Pharmacodynamie et de Therapie. Vol. 202, Pg. 106, 1973. | |
| rat | LD50 | oral | 1957mg/kg (1957mg/kg) | BEHAVIORAL: ALTERED SLEEP TIME (INCLUDING CHANGE IN RIGHTING REFLEX) | Japanese Kokai Tokyo Koho Patents. Vol. #79-163823, |
| rat | LD50 | subcutaneous | > 1500mg/kg (1500mg/kg) | Arzneimittel-Forschung. Drug Research. Vol. 30, Pg. 477, 1980. | |
| women | LDLo | oral | 1920mg/kg/17W (1920mg/kg) | BLOOD: APLASTIC ANEMIA | American Journal of Psychiatry. Vol. 142, Pg. 974, 1985. |
| women | TDLo | oral | 28mg/kg/4D-I (28mg/kg) | BEHAVIORAL: SOMNOLENCE (GENERAL DEPRESSED ACTIVITY) GASTROINTESTINAL: NAUSEA OR VOMITING | American Journal of Psychiatry. Vol. 143, Pg. 1328, 1986. |
| women | TDLo | oral | 112mg/kg/2W-I (112mg/kg) | GASTROINTESTINAL: NAUSEA OR VOMITING BLOOD: THROMBOCYTOPENIA | American Journal of Psychiatry. Vol. 150, Pg. 1750, 1993. |
| women | TDLo | oral | 144mg/kg/2W-I (144mg/kg) | BLOOD: THROMBOCYTOPENIA SKIN AND APPENDAGES (SKIN): "DERMATITIS, ALLERGIC: AFTER SYSTEMIC EXPOSURE" | Journal of Clinical Psychiatry. Vol. 53, Pg. 378, 1992. |
| women | TDLo | oral | 560mg/kg/4W-I (560mg/kg) | BLOOD: CHANGES IN BONE MARROW NOT INCLUDED ABOVE | British Journal of Clinical Practice. Vol. 43, Pg. 302, 1989. |
| women | TDLo | oral | 7604mg/kg/69W (7604mg/kg) | BEHAVIORAL: GENERAL ANESTHETIC CARDIAC: EKG CHANGES NOT DIAGNOSTIC OF ABOVE | Japanese Heart Journal. Vol. 39, Pg. 469, 1998. |
Carbamazepine Safety Profile
Hazard Codes:  Xn
Xn
Risk Statements: 42/43-22-20/21/22
R20/21/22:Harmful by inhalation, in contact with skin and if swallowed.
R22:Harmful if swallowed.
R42/43:May cause sensitization by inhalation and skin contact.
Safety Statements: 37-24-22-36/37/39-36
S22:Do not breathe dust.
S24:Avoid contact with skin.
S36:Wear suitable protective clothing.
S37:Wear suitable gloves.
S36/37/39:Wear suitable protective clothing, gloves and eye/face protection.
Carbamazepine Specification
Carbamazepine ,its cas register number is 298-46-4. It can be called Finlepsin and Tegretol . And it has been sold under the names Biston, Tegretol, Calepsin, Equetro, Finlepsin, Sirtal, Carbatrol, Epitol, Stazepine, Telesmin, Teril, Trimonil, Timonil, Epimaz, Amizepin (Poland), Hermolepsin (Sweden), Carbama/Carbamaze (New Zealand), and Degranol (South Africa).
Related Products
- Carbamazepine
- Carbamazepine 10,11-epoxide
- Carbamazepine-D10
- 29847-23-2
- 29848-57-5
- 29848-59-7
- 29852-55-9
- 2985-59-3
- 298-57-7
- 29858-07-9
- 29858-53-5
- 298-59-9
- 2986-17-6
Hot Products
About|Contact|Cas|Product Name|Molecular|Country|Encyclopedia
Message|New Cas|MSDS|Service|Advertisement|CAS DataBase|Article Data|Manufacturers | Chemical Catalog
©2008 LookChem.com,License: ICP
NO.:Zhejiang16009103
complaints:service@lookchem.com Desktop View