-
Name
Cedrol
- EINECS 201-035-6
- CAS No. 77-53-2
- Article Data12
- CAS DataBase
- Density 1 g/cm3
- Solubility
- Melting Point 55-59 °C(lit.)
- Formula C15H26O
- Boiling Point 277.2 °C at 760 mmHg
- Molecular Weight 222.371
- Flash Point 115.5 °C
- Transport Information
- Appearance
- Safety 22-24/25
- Risk Codes
-
Molecular Structure
- Hazard Symbols
- Synonyms 1H-3a,7-Methanoazulen-6-ol,octahydro-3,6,8,8-tetramethyl-, [3R-(3a,3ab,6a,7b,8aa)]-;8bH-Cedran-8-ol (8CI);Cedrol(6CI,7CI);(+)-Cedrol;a-Cedrol;1H-3a,7-Methanoazulen-6-ol,octahydro-3,6,8,8-tetramethyl-, (3R,3aS,6R,7R,8aS)-;
- PSA 20.23000
- LogP 3.60980
Synthetic route

| Conditions | Yield |
|---|---|
| In diethyl ether Heating; |

-
-
77-53-2
(+)-cedrol

| Conditions | Yield |
|---|---|
| (i) KOH, N2H4, HOCH2CH2OH, (ii) (heating); Multistep reaction; |

-
-
77-53-2
(+)-cedrol

| Conditions | Yield |
|---|---|
| Multi-step reaction with 2 steps 1: CrO3, Py 2: (i) KOH, N2H4, HOCH2CH2OH, (ii) (heating) View Scheme |
-
-
109507-91-7
3-Nor-cedrol

-
-
77-53-2
(+)-cedrol

| Conditions | Yield |
|---|---|
| Multi-step reaction with 2 steps 1: CrO3, Py 2: diethyl ether / Heating View Scheme |
-
-
40768-85-2, 109312-44-9
3-Norcedr-2-en-3-on

-
-
77-53-2
(+)-cedrol

| Conditions | Yield |
|---|---|
| Multi-step reaction with 2 steps 1: H2 / Pd-C / ethanol 2: diethyl ether / Heating View Scheme |
-
-
76679-86-2, 101262-59-3
(3aR)-2c-acetyl-1,1,4c-trimethyl-(6ac)-hexahydro-pentalene-3ar-carboxylic acid methyl ester

-
-
77-53-2
(+)-cedrol

| Conditions | Yield |
|---|---|
| Multi-step reaction with 3 steps 1: (i) LiAlH4, (ii) CrO3, Py, (iii) aq. KOH 2: H2 / Pd-C / ethanol 3: diethyl ether / Heating View Scheme |
-
-
70639-04-2
(3aR)-1.1.4c-trimethyl-(6acH)-hexahydro-3H-pentalene-dicarboxylic acid-(2c.3ar)

-
-
77-53-2
(+)-cedrol

| Conditions | Yield |
|---|---|
| Multi-step reaction with 6 steps 2: KOH, EtOH / Heating 4: (i) LiAlH4, (ii) CrO3, Py, (iii) aq. KOH 5: H2 / Pd-C / ethanol 6: diethyl ether / Heating View Scheme |
-
-
70639-05-3
(-)-Norcedren-dicarbonsaeuredimethylester

-
-
77-53-2
(+)-cedrol

| Conditions | Yield |
|---|---|
| Multi-step reaction with 5 steps 1: KOH, EtOH / Heating 3: (i) LiAlH4, (ii) CrO3, Py, (iii) aq. KOH 4: H2 / Pd-C / ethanol 5: diethyl ether / Heating View Scheme |

-
-
77-53-2
(+)-cedrol

| Conditions | Yield |
|---|---|
| Multi-step reaction with 4 steps 2: (i) LiAlH4, (ii) CrO3, Py, (iii) aq. KOH 3: H2 / Pd-C / ethanol 4: diethyl ether / Heating View Scheme |

-
-
77-53-2
(+)-cedrol

| Conditions | Yield |
|---|---|
| Multi-step reaction with 7 steps 1: (i) Raney-Ni, (ii) aq. KOH, EtOH, (iii) (racemate resolution using quinine) 3: KOH, EtOH / Heating 5: (i) LiAlH4, (ii) CrO3, Py, (iii) aq. KOH 6: H2 / Pd-C / ethanol 7: diethyl ether / Heating View Scheme |
-
-
372-97-4, 13058-04-3, 25744-33-6, 27248-37-9, 27248-38-0, 40716-68-5
farnesyl pyrophosphate

-
A
-
546-28-1
β-(+)-cedrene

-
B
-
77-53-2
(+)-cedrol

-
C
-
469-61-4
alpha-cedrene

-
D
-
19903-73-2
(-)-(3R,3aS,6S,7R,8aS)-isocedrol

| Conditions | Yield |
|---|---|
| With epicedrol synthase In aq. buffer at 30℃; for 1h; pH=8.5; Enzymatic reaction; |

-
-
372-97-4, 13058-04-3, 25744-33-6, 27248-37-9, 27248-38-0, 40716-68-5
farnesyl pyrophosphate

-
-
77-53-2
(+)-cedrol

| Conditions | Yield |
|---|---|
| With cedrol synthase gene from the transcriptome of the glandular trichomes of a woody Lamiaceae plant Leucosceptrum canum In aq. buffer at 30℃; for 3h; pH=7.4; Enzymatic reaction; |
-
-
77-53-2
(+)-cedrol

-
-
16000-39-8
1-cyano-2-methoxynaphthalene

| Conditions | Yield |
|---|---|
| With potassium hexamethylsilazane In 1,4-dioxane at 20℃; for 16h; Inert atmosphere; Sealed tube; Glovebox; | 98% |

| Conditions | Yield |
|---|---|
| Stage #1: (+)-cedrol With potassium tert-butylate In toluene; tert-butyl alcohol at 85 - 110℃; Green chemistry; Stage #2: methylene chloride In tert-butyl methyl ether at 60℃; under 1875.19 Torr; Reagent/catalyst; Temperature; Pressure; Autoclave; Green chemistry; | 96.3% |

| Conditions | Yield |
|---|---|
| With p-toluenesulfonic acid monohydrate In toluene for 42h; Reflux; | 91% |

| Conditions | Yield |
|---|---|
| Stage #1: (+)-cedrol With sodium hydroxide In tetrahydrofuran at 0℃; for 0.333333h; Inert atmosphere; Stage #2: ethyl bromoacetate In tetrahydrofuran at 25℃; for 6.33333h; | 88% |

| Conditions | Yield |
|---|---|
| With pyridine In dichloromethane at 0 - 20℃; for 2h; Inert atmosphere; | 83% |
-
-
77-53-2
(+)-cedrol

-
-
18319-31-8
(1R,3aS,3bR,6R)-1,3a,6-trimethyl-octahydro-1H-1,6a-cedarane[1,2-c]furan

| Conditions | Yield |
|---|---|
| With [bis(acetoxy)iodo]benzene; iodine In cyclohexane at 23℃; for 1.5h; Irradiation; Inert atmosphere; | 73% |
| With diphenylselenium hydroxyacetate; iodine In cyclohexane at 50℃; for 12h; Irradiation; | 67% |
| With [bis(acetoxy)iodo]benzene; iodine In cyclohexane at 20℃; for 1.5h; Irradiation; Inert atmosphere; regioselective reaction; |
-
-
77-53-2
(+)-cedrol

-
-
67152-03-8
(3S,3aR,6R,7R,8aS) -3,6,8,8-tetramethyloctahydro-1H-3a,7-methanoazulene-3,6-diol

| Conditions | Yield |
|---|---|
| Stage #1: (+)-cedrol With cis-RuCl2(4,4'-di-tert-butyl-2,2'-bipyridine)2; acetic acid In water for 0.0333333h; Darkness; Stage #2: With periodic acid In water for 4h; Darkness; chemoselective reaction; | 55% |
| With iodosylbenzene; C40H39ClN4OPRu(1+)*Cl(1-); trifluoroacetic acid In 1,1,2,2-tetrachloroethane at 35℃; for 18h; regioselective reaction; | 53% |
| With 1,3,5-trichlorobenzene; iodosylbenzene; C38H35ClN4OPRu(1+)*Cl(1-); trifluoroacetic acid In 1,1,2,2-tetrachloroethane at 30℃; for 48h; | 36.5% |
| With ammonium cerium (IV) nitrate; 2C7H7O3S(1-)*C20H30N4O2Ru(2+) In water; tert-butyl alcohol at 20℃; for 0.666667h; |
-
-
63527-43-5
2,3,5-p-Nitrobenzoyl-D-ribofuranosylbromid

-
-
77-53-2
(+)-cedrol

-
-
99049-93-1
(Cedran-8-yl)-2,3,5-tri-O-(p-nitrobenzoyl)-β-D-ribofuranosid

| Conditions | Yield |
|---|---|
| With molecular sieve; silver silicate In dichloromethane for 24h; | 43% |
-
-
77-53-2
(+)-cedrol

-
-
572-09-8
2,3,4,6-tetra-O-acetyl-α-D-glucopyranosyl bromide

-
-
99049-91-9
(Cedran-8-yl)-2,3,4,6-tetra-O-acetyl-β-D-glucopyranosid

| Conditions | Yield |
|---|---|
| With calcium sulfate; silver carbonate In dichloromethane for 1.5h; Ambient temperature; | 40% |

| Conditions | Yield |
|---|---|
| With n-butyllithium In hexane for 24h; Ambient temperature; | 38% |
-
-
77-53-2
(+)-cedrol

-
A
-
142864-21-9
((3aR)-1.1.4c-trimethyl-2c-acetyl-(6acH)-hexahydro-3H-pentalenyl-(3ar))-acetic acid

-
B
-
67152-03-8
(3S,3aR,6R,7R,8aS) -3,6,8,8-tetramethyloctahydro-1H-3a,7-methanoazulene-3,6-diol

| Conditions | Yield |
|---|---|
| With ruthenium trichloride; sodium periodate In tetrachloromethane; water; acetonitrile at 70℃; for 24h; | A n/a B 29% |

| Conditions | Yield |
|---|---|
| With 4-Fluorobenzoic acid In octane at 85℃; for 144h; | 28% |

| Conditions | Yield |
|---|---|
| With cyclopentadienyl titanium(IV) trichloride; triethylsilyl chloride; zinc In tetrahydrofuran at 60℃; for 12h; Molecular sieve; Sealed tube; Inert atmosphere; enantioselective reaction; | 27% |
-
-
77-53-2
(+)-cedrol

-
-
22860-91-9
2,3,5-tri-O-benzoyl-D-ribofuranosyl bromide

-
-
99049-94-2
(Cedran-8-yl)-2,3,5-tri-O-benzoyl-β-D-ribofuranosid

| Conditions | Yield |
|---|---|
| With molecular sieve; silver silicate In dichloromethane for 1h; | 14% |

| Conditions | Yield |
|---|---|
| With n-butyllithium In tetrahydrofuran; hexane for 24h; Ambient temperature; | 13% |
-
-
77-53-2
(+)-cedrol

-
A
-
50539-24-7
3α,8β-dihydroxycedrane

-
B
-
67152-03-8
(3S,3aR,6R,7R,8aS) -3,6,8,8-tetramethyloctahydro-1H-3a,7-methanoazulene-3,6-diol

| Conditions | Yield |
|---|---|
| With Mucor plumbeus In ethanol for 144h; Further byproducts given; | A 5 mg B 3% C 5 mg D 2% |

-
-
77-53-2
(+)-cedrol

-
A
-
50539-24-7
3α,8β-dihydroxycedrane

-
B
-
67152-03-8
(3S,3aR,6R,7R,8aS) -3,6,8,8-tetramethyloctahydro-1H-3a,7-methanoazulene-3,6-diol

| Conditions | Yield |
|---|---|
| With Mucor plumbeus In ethanol for 144h; Product distribution; microbiological hydroxylation of cedrol and related compounds by Mucor plumbeus; | A 5 mg B 3% C 6 mg D 2% E n/a |

-
-
77-53-2
(+)-cedrol

| Conditions | Yield |
|---|---|
| With pyridine; zinc; Fe3O(OAc)6Pyr3.5 In water; acetic acid for 6h; Ambient temperature; | 1.3% |

| Conditions | Yield |
|---|---|
| With chloroform; N,N-dimethyl-aniline |
-
-
77-53-2
(+)-cedrol
Cedrol Chemical Properties
The Molecular Structure of Cedrol (CAS NO.77-53-2):
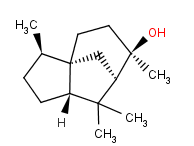
Molecular Formula C15H26O
Molecular Weight 222.37
Melting Point: 55-59°C
Boiling Point: 273°C
Flash Point: 200 ºF
Product Categories: Industrial/Fine Chemicals
Synonyms: (+)-Cedrol ; AI3-02178 ; Cedarwood oil alcohols ; Cedrol (natural) ; EINECS 201-035-6 ; EUDESMOL ; UNII-63ZM9703BO ; a-Cedrol ; (3R-(3alpha,3Abeta,6alpha,7beta,8aalpha))-octahydro-3,6,8,8-tetramethyl-1H-3a,7-methanoazulen-6-ol ; 1H-3a,7-Methanoazulen-6-ol, octahydro-3,6,8,8-tetramethyl- ; (3R,3aS,6R,7R,8aS)- 8-betaH-Cedran-8-ol (8CI) 8betaH-Cedran-8-ol (8CI) Cedrol (6CI,7CI)
Cedrol Uses
Cedrol (CAS NO.77-53-2) can be used as a perfume fixative for variety of cosmetic compunds and the materials of Cedryl Acetate and Methyl Cedryl Ether etc.
Cedrol Toxicity Data With Reference
| Organism | Test Type | Route | Reported Dose (Normalized Dose) | Effect | Source |
|---|---|---|---|---|---|
| mouse | LD | intraperitoneal | > 500mg/kg (500mg/kg) | "Summary Tables of Biological Tests," National Research Council Chemical-Biological Coordination Center. Vol. 8, Pg. 102, 1956. | |
| rabbit | LD50 | skin | > 5gm/kg (5000mg/kg) | Food and Cosmetics Toxicology. Vol. 13, Pg. 745, 1975. |
Cedrol Safety Profile
Safety Statements: 22-24/25
S22: Do not breathe dust
S24/25: Avoid contact with skin and eyes
WGK Germany: 2
RTECS: PB7728666
Human experience: 8 % solution: no irritation or sensitization.
Maximised Survey-derived Daily Intakes (MSDI-EU) : 13.00 (μg/capita/day)
modified Theoretical Added Maximum Daily Intake (mTAMDI) : 3900 (μg/person/day)
Threshold of concern : 1800 (μg/person/day)
R 36/37/38: Irritating to eyes, respiratory system, and skin.
S 02: Keep out of the reach of children.
S 24/25: Avoid contact with skin and eyes.
S 26: In case of contact with eyes, rinse immediately with plenty of water and seek medical advice.
S 36: Wear suitable protective clothing.
Dermal Toxicity(LD50): Skin-Rabbit >5.00 gm/kg
Related Products
- Cedrol
- Cedrol formate
- Cedrol methyl ether
- 77532-79-7
- 77532-86-6
- 77532-89-9
- 77532-90-2
- 775-33-7
- 77534-70-4
- 7753-60-8
- 7753-72-2
- 77538-14-8
- 77538-19-3
Hot Products
About|Contact|Cas|Product Name|Molecular|Country|Encyclopedia
Message|New Cas|MSDS|Service|Advertisement|CAS DataBase|Article Data|Manufacturers | Chemical Catalog
©2008 LookChem.com,License: ICP
NO.:Zhejiang16009103
complaints:service@lookchem.com Desktop View