-
Name
Cefotaxime
- EINECS 264-299-1
- CAS No. 63527-52-6
- Article Data47
- CAS DataBase
- Density 1.8 g/cm3
- Solubility soluble in water
- Melting Point
- Formula C16H17N5O7S2
- Boiling Point
- Molecular Weight 455.472
- Flash Point
- Transport Information
- Appearance white power
- Safety
- Risk Codes
-
Molecular Structure
- Hazard Symbols
- Synonyms Zeefotax;5-Thia-1-azabicyclo[4.2.0]oct-2-ene-2-carboxylicacid,3-[(acetyloxy)methyl]-7-[[(2-amino-4-thiazolyl)(methoxyimino)acetyl]amino]-8-oxo-,[6R-[6a,7b(Z)]]-;5-Thia-1-azabicyclo[4.2.0]oct-2-ene-2-carboxylic acid,3-[(acetyloxy)methyl]-7-[[(2Z)-(2-amino-4-thiazolyl)(methoxyimino)acetyl]amino]-8-oxo-,(6R,7R)- (9CI);Cefabol;Cefotaxime acid;Ceftax;Claforan;Omnatax;Taxim;
- PSA 227.05000
- LogP 0.28750
Synthetic route
-
-
80756-85-0
(Z)-S-benzo[d]thiazol-2-yl 2-(2-aminothiazol-4-yl)-2-(methoxyimino)ethanethioate

-
-
957-68-6
7-Aminocephalosporanic acid

-
-
63527-52-6
cefotaxime

| Conditions | Yield |
|---|---|
| With TEA In dichloromethane at 20℃; for 1h; Substitution; | 95% |
| With triethylamine In dichloromethane at 20℃; for 1h; | 95% |
| Stage #1: (Z)-S-benzo[d]thiazol-2-yl 2-(2-aminothiazol-4-yl)-2-(methoxyimino)ethanethioate; 7-Aminocephalosporanic acid With triethylamine In methanol at 0 - 5℃; Stage #2: With hydrogenchloride In methanol; water at 0 - 5℃; for 1h; pH=2.3 - 2.5; | |
| Stage #1: (Z)-S-benzo[d]thiazol-2-yl 2-(2-aminothiazol-4-yl)-2-(methoxyimino)ethanethioate; 7-Aminocephalosporanic acid With triethylamine In 1,2-dimethoxyethane at -5 - 0℃; Stage #2: With hydrogenchloride In 1,2-dimethoxyethane; water for 1h; pH=2.6 - 2.8; Product distribution / selectivity; |

-
-
957-68-6
7-Aminocephalosporanic acid

-
-
63527-52-6
cefotaxime

| Conditions | Yield |
|---|---|
| Stage #1: 7-Aminocephalosporanic acid With N,O-bis-(trimethylsilyl)-acetamide In dichloromethane at 20℃; for 1h; Stage #2: diethylthiophosphoryl-(Z)-(2-aminothiazol-4-yl)methoxyimino acetate In dichloromethane at 20℃; for 8h; Stage #3: With sodium hydroxide In dichloromethane; water at 15 - 20℃; pH=7.5 - 7.8; | 94.4% |
| Stage #1: 7-Aminocephalosporanic acid With N,O-bis-(trimethylsilyl)-acetamide In DMF (N,N-dimethyl-formamide) at 20 - 25℃; Stage #2: diethylthiophosphoryl-(Z)-(2-aminothiazol-4-yl)methoxyimino acetate In DMF (N,N-dimethyl-formamide) at 20℃; for 18h; | 93.5% |

| Conditions | Yield |
|---|---|
| With N,O-bis-(trimethylsilyl)-acetamide In acetonitrile at -5 - 0℃; for 1h; Temperature; | 93.1% |
-
-
957-68-6
7-Aminocephalosporanic acid

-
-
207725-19-7
(2-Amino-thiazol-4-yl)-[(Z)-methoxyimino]-acetic acid 4,6-dimethoxy-[1,3,5]triazin-2-yl ester

-
-
63527-52-6
cefotaxime

| Conditions | Yield |
|---|---|
| With N,O-bis-dimethylsilyl acetamide In acetonitrile at 0 - 5℃; for 1.5h; | 75.4% |

-
-
17356-08-0
thiourea

-
-
63527-52-6
cefotaxime

| Conditions | Yield |
|---|---|
| Stage #1: 7-[4-bromo-2(Z)-methoxyimino-3-oxobutyramido]-cephalosporanic acid; thiourea With sodium acetate In dichloromethane; water for 2.5 - 3.5h; Stage #2: With hydrogenchloride In tetrahydrofuran; water pH=2.8; | 40.3% |
-
-
957-68-6
7-Aminocephalosporanic acid

-
-
64485-90-1
2-(2-aminothiazol-4-yl)-2-(methoxy)iminoacetic acid

-
-
63527-52-6
cefotaxime

| Conditions | Yield |
|---|---|
| (i) DCC, CH2Cl2, (ii) /BRN= 622638/, Et3N, (iii) aq. HCO2H; Multistep reaction; |
-
-
65243-53-0
pyvaloyloxymethyl 7β-<2-(2-aminothiazol-4-yl)-(Z)-methoxyiminoacetamido>-3-acetoxymethyl-3-cephem-4-carboxylate

-
A
-
63527-52-6
cefotaxime

-
B
-
126747-48-6
(2R,6R,7R)-3-Acetoxymethyl-7-{2-(2-amino-thiazol-4-yl)-2-[(Z)-methoxyimino]-acetylamino}-8-oxo-5-thia-1-aza-bicyclo[4.2.0]oct-3-ene-2-carboxylic acid

| Conditions | Yield |
|---|---|
| With water In N,N-dimethyl-formamide at 37℃; Rate constant; phosphate buffer, var. pH; |

-
-
64485-88-7
ethyl 2-(2-aminothiazol-4-yl)-2-(anti)-methoxyiminoacetate

-
-
63527-52-6
cefotaxime

| Conditions | Yield |
|---|---|
| Multi-step reaction with 3 steps 1: Et3N / dimethylformamide / 3 h 2: aq. NaOH / dioxane / 0.5 h / Heating 3: (i) DCC, CH2Cl2, (ii) /BRN= 622638/, Et3N, (iii) aq. HCO2H View Scheme |
-
-
65872-39-1
(Z)-γ-bromo-β-oxo-α-methoxyiminobutyric acid ethyl ester

-
-
63527-52-6
cefotaxime

| Conditions | Yield |
|---|---|
| Multi-step reaction with 4 steps 1: ethanol; H2O / 1 h / 20 °C 2: Et3N / dimethylformamide / 3 h 3: aq. NaOH / dioxane / 0.5 h / Heating 4: (i) DCC, CH2Cl2, (ii) /BRN= 622638/, Et3N, (iii) aq. HCO2H View Scheme |
-
-
64485-89-8
ethyl (Z)-2-(methoxyimino)-2-[2-(triphenylmethyl)-aminothiazol-4-yl]acetate

-
-
63527-52-6
cefotaxime

| Conditions | Yield |
|---|---|
| Multi-step reaction with 2 steps 1: aq. NaOH / dioxane / 0.5 h / Heating 2: (i) DCC, CH2Cl2, (ii) /BRN= 622638/, Et3N, (iii) aq. HCO2H View Scheme |

| Conditions | Yield |
|---|---|
| Stage #1: C15H16ClN3O8S; thiourea With triethylamine In tetrahydrofuran; water at 20 - 25℃; for 4h; pH=7.5; Stage #2: With hydrogenchloride In water at 15 - 20℃; for 0.5h; pH=3 - 4.2; |

| Conditions | Yield |
|---|---|
| Stage #1: C15H16ClN3O8S; thiourea With sodium acetate In tetrahydrofuran; water at 20℃; for 1h; Stage #2: With hydrogenchloride In tetrahydrofuran; water at 10℃; for 1h; pH=2.5; |
-
-
64486-19-7
7β-[2-(2-chloroacetamidothiazol-4-yl)-(Z)-2-methoxyiminoacetamido]-3-acetoxymethyl-3-cephem-4-carboxylic acid

-
-
63527-52-6
cefotaxime

| Conditions | Yield |
|---|---|
| Stage #1: 7β-[2-(2-chloroacetamidothiazol-4-yl)-(Z)-2-methoxyiminoacetamido]-3-acetoxymethyl-3-cephem-4-carboxylic acid With water; sodium carbonate; thiourea; isopropyl alcohol at 20 - 30℃; Stage #2: With hydrogenchloride; water In isopropyl alcohol at 20 - 30℃; for 2h; pH=2.7 - 3.0; |
-
-
80756-85-0
(Z)-S-benzo[d]thiazol-2-yl 2-(2-aminothiazol-4-yl)-2-(methoxyimino)ethanethioate

-
-
957-68-6
7-Aminocephalosporanic acid

-
A
-
63527-52-6
cefotaxime

-
B
-
149-30-4
2-Mercaptobenzothiazole

| Conditions | Yield |
|---|---|
| Stage #1: (Z)-S-benzo[d]thiazol-2-yl 2-(2-aminothiazol-4-yl)-2-(methoxyimino)ethanethioate; 7-Aminocephalosporanic acid With sodium 2-ethylhexanoate In water; acetonitrile at 6 - 8℃; for 12h; Stage #2: In water; acetonitrile pH=2.5 - 3.0; |

-
-
111230-59-2
4-chloro-2-methoxyimino-3-oxo-butyric acid

-
-
17356-08-0
thiourea

-
-
957-68-6
7-Aminocephalosporanic acid

-
-
63527-52-6
cefotaxime

| Conditions | Yield |
|---|---|
| Stage #1: 4-chloro-2-methoxyimino-3-oxo-butyric acid With phosphorus pentachloride In dichloromethane at -5 - 0℃; for 1h; Stage #2: 7-Aminocephalosporanic acid With ammonia In water; acetone at -5 - 0℃; for 0.5 - 0.666667h; pH=6.5 - 7; Stage #3: thiourea With sodium acetate In water; acetone at 18 - 20℃; for 1h; |


| Conditions | Yield |
|---|---|
| Stage #1: C13H11N5O2S2; 7-Aminocephalosporanic acid With sodium hydrogensulfite; triethylamine In ethanol; dichloromethane; isopropyl alcohol at 5℃; pH=7 - 8; Stage #2: With hydrogenchloride In water; acetone at 10℃; for 1.5h; pH=2.5; Temperature; |
-
-
10416-59-8
N,O-bis-(trimethylsilyl)-acetamide

-
-
57079-45-5
D-(-)-2-formyloxy-2-phenylacetyl chloride

-
-
141-78-6
ethyl acetate

-
-
63527-52-6
cefotaxime

| Conditions | Yield |
|---|---|
| at 20℃; |

-
-
91868-79-0
2-(2-amino-1,3-thiazol-4-yl)-2-(methoxyimino)acetic acid

-
-
63527-52-6
cefotaxime

| Conditions | Yield |
|---|---|
| Multi-step reaction with 2 steps 1: 1,8-diazabicyclo[5.4.0]undec-7-ene / N,N-dimethyl-formamide / 2.5 h / 2 - 25 °C 2: N,O-bis-(trimethylsilyl)-acetamide / acetonitrile / 1 h / -5 - 0 °C View Scheme |
-
-
63527-52-6
cefotaxime

-
-
64485-93-4
cefotaxime sodium salt

| Conditions | Yield |
|---|---|
| With sodium hydrogencarbonate In ethanol Substitution; | 95.4% |
| With sodium isooctanoate; sodium sulfite In water; acetone at 5 - 10℃; for 0.5h; | 95% |
| With triethylamine; sodium 2-ethylhexanoic acid In methanol; ethyl acetate | 88.7% |
-
-
63527-52-6
cefotaxime

-
-
79-04-9
chloroacetyl chloride

-
-
64486-19-7
7β-[2-(2-chloroacetamidothiazol-4-yl)-(Z)-2-methoxyiminoacetamido]-3-acetoxymethyl-3-cephem-4-carboxylic acid

| Conditions | Yield |
|---|---|
| In N,N-dimethyl acetamide at 20℃; for 1h; | 90.2% |
-
-
63527-52-6
cefotaxime

-
-
908093-98-1
diazodiphenylmethane

| Conditions | Yield |
|---|---|
| With toluene-4-sulfonic acid In water; acetonitrile at 20℃; for 0.75h; pH=3; Inert atmosphere; | 80% |
-
-
63527-52-6
cefotaxime

-
-
239468-67-8
5-[(4-methylquinolin-2-yloxy)methyl]-1,3,4-thiadiazole-2-thiol

| Conditions | Yield |
|---|---|
| With boron trifluoride diethyl etherate In acetic acid for 0.0333333h; Condensation; microwave irradiation; | 58% |
-
-
63527-52-6
cefotaxime

-
-
239468-65-6
5-[(quinolin-8-yloxy)methyl]-1,3,4-thiadiazole-2-thiol

| Conditions | Yield |
|---|---|
| With boron trifluoride diethyl etherate In acetic acid for 0.0166667h; Condensation; microwave irradiation; | 54% |

| Conditions | Yield |
|---|---|
| With methanesulfonic acid; sulfuric acid In acetonitrile at 15 - 20℃; | 54% |
| With methanesulfonic acid In dichloromethane at 23 - 27℃; | 47% |
| With methanesulfonic acid In ethyl acetate at 30℃; | 47% |
-
-
63527-52-6
cefotaxime

-
-
202116-95-8
5-[(5-methyl-1,3,4-thiadiazol-2-ylthio)methyl]-1,3,4-thiadiazole-2-thiol

| Conditions | Yield |
|---|---|
| With boron trifluoride diethyl etherate In acetic acid for 0.025h; Condensation; microwave irradiation; | 52% |
-
-
63527-52-6
cefotaxime

-
-
202116-96-9
5-[(tetrazol-1-yl)methyl]-1,3,4-thiadiazole-2-thiol

| Conditions | Yield |
|---|---|
| With boron trifluoride diethyl etherate In acetic acid for 0.025h; Condensation; microwave irradiation; | 48% |

| Conditions | Yield |
|---|---|
| at 35℃; for 2.5h; | 35.8% |
-
-
63527-52-6
cefotaxime

-
-
24589-78-4
N-methyl-N-trimethylsilyl-2,2,2-trifluoroacetamide

-
-
104498-86-4
C19H25N5O7S2Si

| Conditions | Yield |
|---|---|
| In dichloromethane at 40℃; |

| Conditions | Yield |
|---|---|
| In dichloromethane for 1h; |
-
-
63527-52-6
cefotaxime

| Conditions | Yield |
|---|---|
| In dichloromethane |
-
-
63527-52-6
cefotaxime

-
-
86070-92-0
(6R,7R)-7-{2-(2-Amino-thiazol-4-yl)-2-[(Z)-methoxyimino]-acetylamino}-3-iodomethyl-8-oxo-5-thia-1-aza-bicyclo[4.2.0]oct-2-ene-2-carboxylic acid

| Conditions | Yield |
|---|---|
| In dichloromethane | |
| With trimethylsilyl iodide; N-methyl-N-trimethylsilyl-2,2,2-trifluoroacetamide In dichloromethane at 20℃; for 0.5h; Substitution; |
-
-
63527-52-6
cefotaxime

-
-
533-37-9
2,3-cyclopentenopyridine

-
-
84957-29-9, 97164-55-1, 130431-30-0
cefpirome

| Conditions | Yield |
|---|---|
| With potassium iodide In water at 70℃; |
-
-
63527-52-6
cefotaxime

-
-
24589-78-4
N-methyl-N-trimethylsilyl-2,2,2-trifluoroacetamide

-
-
83421-24-3
7β-<2-(2-aminothiazol-4yl)-2-(Z)-methoximinoacetamide>-3-iodomethyl-2-<(trimethylsilyloxy)carbonyl>cephalosporin

| Conditions | Yield |
|---|---|
| Stage #1: cefotaxime; N-methyl-N-trimethylsilyl-2,2,2-trifluoroacetamide In dichloromethane for 1h; Iodination; Stage #2: With trimethylsilyl iodide In dichloromethane for 0.5h; Condensation; |
-
-
63527-52-6
cefotaxime

-
-
87239-81-4
cefpodoxime proxetil

| Conditions | Yield |
|---|---|
| Multi-step reaction with 4 steps 1: 90.2 percent / N,N-dimethyl-acetamide / 1 h / 20 °C 2: 65 percent / aq. NaHCO3; CaCl2*2H2O / 1.25 h / 70 °C 3: 2.4 g / dicyclohexylamine / N,N-dimethyl-acetamide / 0.75 h / 0 - 5 °C 4: 1.44 g / thiourea / N,N-dimethyl-acetamide / 3 h / 20 °C View Scheme |
-
-
63527-52-6
cefotaxime

-
-
82618-67-5
7β-[2-(2-chloroacetamidothiazol-4-yl)-(Z)-2-methoxyiminoacetamido]-3-methoxymethyl-3-cephem-4-carboxylic acid

| Conditions | Yield |
|---|---|
| Multi-step reaction with 2 steps 1: 90.2 percent / N,N-dimethyl-acetamide / 1 h / 20 °C 2: 65 percent / aq. NaHCO3; CaCl2*2H2O / 1.25 h / 70 °C View Scheme |
-
-
63527-52-6
cefotaxime

-
-
620962-96-1
(6R,7R)-7-{2-[2-(2-Chloro-acetylamino)-thiazol-4-yl]-2-[(Z)-methoxyimino]-acetylamino}-3-methoxymethyl-8-oxo-5-thia-1-aza-bicyclo[4.2.0]oct-2-ene-2-carboxylic acid 1-isopropoxycarbonyloxy-ethyl ester

| Conditions | Yield |
|---|---|
| Multi-step reaction with 3 steps 1: 90.2 percent / N,N-dimethyl-acetamide / 1 h / 20 °C 2: 65 percent / aq. NaHCO3; CaCl2*2H2O / 1.25 h / 70 °C 3: 2.4 g / dicyclohexylamine / N,N-dimethyl-acetamide / 0.75 h / 0 - 5 °C View Scheme |
-
-
63527-52-6
cefotaxime

| Conditions | Yield |
|---|---|
| Multi-step reaction with 2 steps 1: MSTFA; TMSI / CH2Cl2 / 0.5 h / 20 °C 2: 0.10 g / MSTFA / acetonitrile; tetrahydrofuran / 20 h / 20 °C View Scheme |
-
-
63527-52-6
cefotaxime

| Conditions | Yield |
|---|---|
| Multi-step reaction with 2 steps 1: MSTFA; TMSI / CH2Cl2 / 0.5 h / 20 °C 2: MSTFA / acetonitrile; tetrahydrofuran / 20 h / 20 °C View Scheme |
-
-
63527-52-6
cefotaxime

| Conditions | Yield |
|---|---|
| Multi-step reaction with 2 steps 1.1: CH2Cl2 / 1 h 1.2: Me3SiI / CH2Cl2 / 0.5 h 2.1: acetonitrile; tetrahydrofuran / 16 h / 20 °C View Scheme |
Cefotaxime Specification
The Cefotaxime with CAS registry number of 63527-52-6 is also known as Cefotaximum. The IUPAC name is (6R,7R)-3-(Acetyloxymethyl)-7-[[(2Z)-2-(2-amino-1,3-thiazol-4-yl)-2-methoxyiminoacetyl]amino]-8-oxo-5-thia-1-azabicyclo[4.2.0]oct-2-ene-2-carboxylic acid. Its EINECS registry number is 264-299-1. In addition, the formula is C16H17N5O7S2 and the molecular weight is 455.47. This chemical is a white power.
Physical properties about Cefotaxime are: (1)ACD/LogP: 1.20; (2)# of Rule of 5 Violations: 1; (3)ACD/BCF (pH 5.5): 1; (4)ACD/BCF (pH 7.4): 1; (5)ACD/KOC (pH 5.5): 1; (6)ACD/KOC (pH 7.4): 1; (7)#H bond acceptors: 12; (8)#H bond donors: 4; (9)#Freely Rotating Bonds: 8; (10)Index of Refraction: 1.778; (11)Molar Refractivity: 105.95 cm3; (12)Molar Volume: 252.8 cm3; (13)Surface Tension: 81.2 dyne/cm; (14)Density: 1.8 g/cm3.
Preparation of Cefotaxime: it is prepared by reaction of cephalosporin C. Firstly, cefotaxime is crystallizated after cracking, silicon esterification, condensation and hydrolysis. Secondly, add sodium acetate to get sodium. Product is obtained after bleaching, sterile filtration and crystallization.
Uses of Cefotaxime: it is used as antibacterial drugs and is a semi-synthetic oxime-type cephalosporins. It is used for infections of the respiratory tract, skin, bones, joints, urogenital system, meningitis, and septicemia. This chemical is used to produce 7b-(2-(2-Aminothiazol-4-yl)-(Z)-2-methoxyiminoacetamido)-3-acetoxymethyl-3-cephem-4-carboxylic acid sodium salt. The reaction occurs with reagent aq. NaHCO3 and solvent ethanol. The yield is about 95.4%.
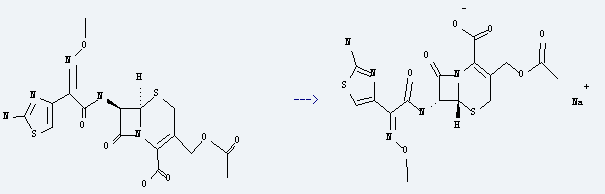
You can still convert the following datas into molecular structure:
1. SMILES: O=C2N1/C(=C(\CS[C@@H]1[C@@H]2NC(=O)C(=NOC)c3nc(sc3)N)COC(=O)C)C(=O)O
2. InChI: InChI=1/C16H17N5O7S2/c1-6(22)28-3-7-4-29-14-10(13(24)21(14)11(7)15(25)26)19-12(23)9(20-27-2)8-5-30-16(17)18-8/h5,10,14H,3-4H2,1-2H3,(H2,17,18)(H,19,23)(H,25,26)/t10-,14-/m1/s1
3. InChIKey: GPRBEKHLDVQUJE-QMTHXVAHBP
Related Products
- Cefotaxime
- Cefotaxime sodium
- 635-27-8
- 635287-26-2
- 63528-78-9
- 635302-32-8
- 635303-41-2
- 635305-47-4
- 635318-11-5
- 63534-56-5
- 63535-27-3
- 6353-54-4
Hot Products
About|Contact|Cas|Product Name|Molecular|Country|Encyclopedia
Message|New Cas|MSDS|Service|Advertisement|CAS DataBase|Article Data|Manufacturers | Chemical Catalog
©2008 LookChem.com,License: ICP
NO.:Zhejiang16009103
complaints:service@lookchem.com Desktop View