-
Name
Ciclopirox
- EINECS 249-577-2
- CAS No. 29342-05-0
- Article Data12
- CAS DataBase
- Density 1.193 g/cm3
- Solubility soluble in methanol, ethanol or chloroform
- Melting Point 144 °C
- Formula C12H17NO2
- Boiling Point 350 °C at 760 mmHg
- Molecular Weight 207.272
- Flash Point 165.5 °C
- Transport Information
- Appearance white or light-yellow powder
- Safety
- Risk Codes R8; R35; R34; R20
-
Molecular Structure
-
Hazard Symbols
 C,
C, O
O
- Synonyms 2(1H)-Pyridone,6-cyclohexyl-1-hydroxy-4-methyl- (8CI);2(1H)-Pyridinone,6-cyclohexyl-1-hydroxy-4-methyl-;HOE 296b;Penlac;
- PSA 42.23000
- LogP 2.44170
Synthetic route

-
-
29342-05-0
CICLOPIROX

| Conditions | Yield |
|---|---|
| Stage #1: 2-cyclohexyl-6-methoxy-4-methylpyridine N-oxide With acetyl chloride for 1h; Reflux; Stage #2: With methanol at 20℃; | 95% |
-
-
41621-49-2
cyclopirox olamine

-
-
29342-05-0
CICLOPIROX

| Conditions | Yield |
|---|---|
| With hydrogenchloride In water | 84% |
| With hydrogenchloride In water; ethyl acetate | 84% |

| Conditions | Yield |
|---|---|
| Stage #1: Methyl 3,3-dimethylacrylate; cyclohexanylcarbonyl chloride With aluminum (III) chloride In dichloromethane for 3h; Reflux; Stage #2: With hydroxylamine hydrochloride; sodium acetate In methanol; water at 20 - 30℃; for 20h; Stage #3: With sodium hydroxide In methanol; water at 20℃; for 1h; | 34% |
| With hydroxylamine hydrochloride; sodium acetate 2.) water, methanol, 25 deg C, 20 h; Yield given. Multistep reaction; |
-
-
14818-35-0
4-methyl-6-cyclohexyl02-pyrone

-
-
29342-05-0
CICLOPIROX

| Conditions | Yield |
|---|---|
| With 1H-imidazole; hydroxyammonium sulfate at 90℃; for 5h; | 11.8 g |

| Conditions | Yield |
|---|---|
| With hydroxylamine sulfate | |
| With hydroxylamine sulfate |

| Conditions | Yield |
|---|---|
| With hydroxylamine hydrochloride In dichloromethane |
-
-
504-29-0
2-aminopyridine

-
-
1824-81-3
2-Amino-6-methylpyridine

-
-
14818-35-0
4-methyl-6-cyclohexyl02-pyrone

-
-
29342-05-0
CICLOPIROX

| Conditions | Yield |
|---|---|
| With hydroxylamine |


-
-
29342-05-0
CICLOPIROX

| Conditions | Yield |
|---|---|
| Multi-step reaction with 2 steps 1.1: tris(2,2-bipyridine)ruthenium(II) hexafluorophosphate; 1-acetoxy-1,2-benziodoxol-3-one; trifluoroacetic acid / water; dichloromethane / 24 h / 20 °C / Schlenk technique; Inert atmosphere; Irradiation 2.1: acetyl chloride / 1 h / Reflux 2.2: 20 °C View Scheme |
-
-
100848-70-2
2-methoxy-4-methyl pyridine

-
-
29342-05-0
CICLOPIROX

| Conditions | Yield |
|---|---|
| Multi-step reaction with 3 steps 1.1: 3-chloro-benzenecarboperoxoic acid / chloroform / 12 h / 0 - 20 °C 2.1: tris(2,2-bipyridine)ruthenium(II) hexafluorophosphate; 1-acetoxy-1,2-benziodoxol-3-one; trifluoroacetic acid / water; dichloromethane / 24 h / 20 °C / Schlenk technique; Inert atmosphere; Irradiation 3.1: acetyl chloride / 1 h / Reflux 3.2: 20 °C View Scheme |
-
-
541-47-9
3-Methylbutenoic acid

-
-
29342-05-0
CICLOPIROX

| Conditions | Yield |
|---|---|
| Multi-step reaction with 2 steps 1.1: sulfuric acid / 5 h / Reflux 2.1: aluminum (III) chloride / dichloromethane / 3 h / Reflux 2.2: 20 h / 20 - 30 °C 2.3: 1 h / 20 °C View Scheme |
-
-
98-89-5
Cyclohexanecarboxylic acid

-
-
29342-05-0
CICLOPIROX

| Conditions | Yield |
|---|---|
| Multi-step reaction with 2 steps 1.1: pyridine; thionyl chloride / 3 h / Reflux 2.1: aluminum (III) chloride / dichloromethane / 3 h / Reflux 2.2: 20 h / 20 - 30 °C 2.3: 1 h / 20 °C View Scheme |
-
-
29342-05-0
CICLOPIROX

| Conditions | Yield |
|---|---|
| With N-Bromosuccinimide; 2,2'-azobis(isobutyronitrile) In tetrachloromethane at 0℃; for 6h; Reflux; | A 90% B 5% |
-
-
29342-05-0
CICLOPIROX

-
-
96-32-2
bromoacetic acid methyl ester

| Conditions | Yield |
|---|---|
| With potassium carbonate In acetonitrile Reflux; | 80% |

| Conditions | Yield |
|---|---|
| With potassium carbonate In N,N-dimethyl-formamide at 60℃; | 74% |
-
-
29342-05-0
CICLOPIROX

| Conditions | Yield |
|---|---|
| With N-chloro-succinimide; 2,2'-azobis(isobutyronitrile) In tetrachloromethane at 0℃; for 6h; Reflux; | 70% |

| Conditions | Yield |
|---|---|
| In aq. phosphate buffer; water for 0.75h; pH=7.4; Sonication; | 67.3% |
-
-
29342-05-0
CICLOPIROX

-
-
258516-84-6
dibenzyloxy-chloromethylphosphoric acid

| Conditions | Yield |
|---|---|
| With sodium hydride In N,N-dimethyl-formamide at 0 - 20℃; for 1.5h; | 67% |
| With sodium hydride In N,N-dimethyl-formamide; mineral oil at 0 - 20℃; for 1.5h; Concentration; | 67% |
-
-
29342-05-0
CICLOPIROX

-
-
19715-49-2
4-(dimethylamino)benzenesulfonyl chloride

| Conditions | Yield |
|---|---|
| With pyridine at 20℃; for 24h; | 67% |
-
-
29342-05-0
CICLOPIROX

| Conditions | Yield |
|---|---|
| With N-iodo-succinimide; 2,2'-azobis(isobutyronitrile) In tetrachloromethane at 0℃; for 6h; Reflux; | 63% |
-
-
29342-05-0
CICLOPIROX

-
-
98-09-9
benzenesulfonyl chloride

| Conditions | Yield |
|---|---|
| With pyridine at 20℃; for 24h; | 19% |
-
-
29342-05-0
CICLOPIROX

-
-
98-88-4
benzoyl chloride

-
-
1351572-55-8
1-benzoyloxy 6-cyclohexyl-4-methylpyridine-2(1H)-one

| Conditions | Yield |
|---|---|
| With dmap In dichloromethane at 7℃; for 1.5h; |
-
-
29342-05-0
CICLOPIROX

-
-
229625-50-7
di-tert-butyl chloromethyl phosphate

| Conditions | Yield |
|---|---|
| With sodium hydride at 0 - 20℃; for 2.5h; | |
| With sodium hydride In mineral oil at 0 - 20℃; for 2.5h; |
-
-
29342-05-0
CICLOPIROX

| Conditions | Yield |
|---|---|
| Multi-step reaction with 2 steps 1: sodium hydride / N,N-dimethyl-formamide / 1.5 h / 0 - 20 °C 2: palladium on activated charcoal; hydrogen / tetrahydrofuran View Scheme | |
| Multi-step reaction with 2 steps 1: sodium hydride / 2.5 h / 0 - 20 °C 2: sodium carbonate / water; acetonitrile View Scheme | |
| Multi-step reaction with 3 steps 1: sodium hydride / N,N-dimethyl-formamide / 1.5 h / 0 - 20 °C 2: palladium on activated charcoal; hydrogen / tetrahydrofuran 3: sodium cation View Scheme | |
| Multi-step reaction with 3 steps 1: sodium hydride / 2.5 h / 0 - 20 °C 2: sodium carbonate / water; acetonitrile 3: sodium cation View Scheme | |
| Multi-step reaction with 4 steps 1: sodium hydride / 2.5 h / 0 - 20 °C 2: sodium carbonate / water; acetonitrile 3: sodium carbonate / water; acetonitrile 4: sodium cation View Scheme |
-
-
29342-05-0
CICLOPIROX

| Conditions | Yield |
|---|---|
| Multi-step reaction with 2 steps 1: sodium hydride / N,N-dimethyl-formamide / 1.5 h / 0 - 20 °C 2: palladium on activated charcoal; hydrogen / tetrahydrofuran View Scheme | |
| Multi-step reaction with 2 steps 1: sodium hydride / 2.5 h / 0 - 20 °C 2: sodium carbonate / water; acetonitrile View Scheme | |
| Multi-step reaction with 3 steps 1: sodium hydride / 2.5 h / 0 - 20 °C 2: sodium carbonate / water; acetonitrile 3: sodium carbonate / water; acetonitrile View Scheme |
-
-
29342-05-0
CICLOPIROX

| Conditions | Yield |
|---|---|
| Multi-step reaction with 2 steps 1: sodium hydride / 2.5 h / 0 - 20 °C 2: sodium carbonate / water; acetonitrile View Scheme |
-
-
29342-05-0
CICLOPIROX

| Conditions | Yield |
|---|---|
| With tetraphosphorus decasulfide In toluene at 80℃; for 5h; Inert atmosphere; | 700 mg |

| Conditions | Yield |
|---|---|
| With potassium carbonate In N,N-dimethyl-formamide at 20℃; Inert atmosphere; | 0.15 g |
-
-
29342-05-0
CICLOPIROX

| Conditions | Yield |
|---|---|
| Multi-step reaction with 2 steps 1: sodium hydride / mineral oil / 2.5 h / 0 - 20 °C 2: tetrahydrofuran; dichloromethane / 2 h / 20 °C View Scheme | |
| Multi-step reaction with 2 steps 1: sodium hydride / mineral oil; N,N-dimethyl-formamide / 1.5 h / 0 - 20 °C 2: palladium on activated charcoal; hydrogen / tetrahydrofuran / 3 h / 20 °C View Scheme |
-
-
29342-05-0
CICLOPIROX

| Conditions | Yield |
|---|---|
| Multi-step reaction with 3 steps 1: sodium hydride / mineral oil / 2.5 h / 0 - 20 °C 2: tetrahydrofuran; dichloromethane / 2 h / 20 °C 3: sodium View Scheme | |
| Multi-step reaction with 3 steps 1: sodium hydride / mineral oil; N,N-dimethyl-formamide / 1.5 h / 0 - 20 °C 2: palladium on activated charcoal; hydrogen / tetrahydrofuran / 3 h / 20 °C 3: sodium View Scheme |

| Conditions | Yield |
|---|---|
| In ethyl acetate at 50℃; for 0.5h; |
Ciclopirox Specification
1. Introduction of Ciclopirox
Ciclopirox is one kind of white or light-yellow powder. It is soluble in methanol, ethanol or chloroform, slightly soluble in DMF or water, slightly soluble in ethyl ether. The IUPAC Name of it is 6-Cyclohexyl-1-hydroxy-4-methylpyridin-2-one. It belongs to Intermediates & Fine Chemicals;Pharmaceuticals;Heterocycles. In addition, the Classification Code of it is Anti-Infective Agents; Antifungal; Antifungal Agents.
2. Properties of Ciclopirox
Physical properties about Ciclopirox are:
(1)Melting Point: 144 °C; (2)Density: 1.193 g/cm3; (3)Boiling Point: 350 °C at 760 mmHg; (4)Flash Point: 165.5 °C; (5)Molar Volume: 173.6 cm3; (6)Polarizability: 22.98×10-24 cm3; (7)Surface Tension: 51.7 dyne/cm; (8)Enthalpy of Vaporization: 68.85 kJ/mol; (9)Vapour Pressure: 2.71E-06 mmHg at 25 °C; (10)log P (octanol-water): 2.730 (none); (11)XLogP3-AA: 2; (12)H-Bond Donor: 1; (13)H-Bond Acceptor: 2; (14)Rotatable Bond Count: 1; (15)Exact Mass: 207.125929; (16)MonoIsotopic Mass: 207.125929; (17)Topological Polar Surface Area: 40.5; (18)Heavy Atom Count: 15; (19)Complexity: 325.
3. Structure Descriptors of Ciclopirox
(1)Canonical SMILES: CC1=CC(=O)N(C(=C1)C2CCCCC2)O
(2)InChI: InChI=1S/C12H17NO2/c1-9-7-11(13(15)12(14)8-9)10-5-3-2-4-6-10/h7-8,10,15H,2-6H2,1H3
(3)InChIKey: SCKYRAXSEDYPSA-UHFFFAOYSA-N
(4)Smiles: c1(cc(cc(n1O)C1CCCCC1)C)=O
4. Physical Properties of Ciclopirox
| Physical Property | Value | Units | Temp (deg C) | Source |
|---|---|---|---|---|
| Melting Point | 144 | deg C | EXP | |
| log P (octanol-water) | 2.730 | (none) | EST | |
| Atmospheric OH Rate Constant | 7.97E-11 | cm3/molecule-sec | 25 | EST |
5. Uses of Ciclopirox
Ciclopirox olamine (also called Batrafen, Loprox, Penlac and Stieprox) is a synthetic antifungal agent for topical dermatologic treatment of superficial mycoses. It is most useful against Tinea versicolor. It can be used as broad spectrum antimycotic agent with some antibacterial activity.
6. Production of Ciclopirox
Cyclohexane carboxylic acid and thionyl chloride reacted to get cyclohexane chloride. It reacts with 3-methyl-2-butenoate (I) in dichloromethane solvent under the action of the three aluminum chloride, stirring 4h, after post-processing, the vacuum collection of 140-145 °C (0.4kPa), may get 5-oxo-3-methyl-5- Ring Hexyl-3-ene acid methyl ester (ciclopirox Division, II), yield 75%. Again, with hydroxylamine hydrochloride, sodium acetate, methanol and water at room temperature, stirring 20h, adding 50% sodium hydroxide and then stirred 1h. Extracted with benzene after cooling, the water phase acidified to Ph = 6. Then ethanol precipitation of crystals recrystallized, you can get Ciclopirox (CAS NO.29342-05-0) , melting point 140 ~ 142 °C, the yield of 48.3%. Finally with the hydroxyl amine salt in dichloromethane, get almost quantitative ciclopirox olamine, melting point 97 ~ 99 °C.
Or MESITYL OXIDE can be used to manufacture Ciclopirox which is showed as follows:
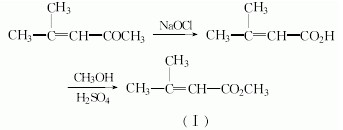
Related Products
- Ciclopirox
- Ciclopirox ethanolamine
- 29349-22-2
- 2934-97-6
- 2935-32-2
- 2935-35-5
- 2935-44-6
- 2935-63-9
- 29358-99-4
- 2935-90-2
- 2936-08-5
- 29361-78-2
Hot Products
About|Contact|Cas|Product Name|Molecular|Country|Encyclopedia
Message|New Cas|MSDS|Service|Advertisement|CAS DataBase|Article Data|Manufacturers | Chemical Catalog
©2008 LookChem.com,License: ICP
NO.:Zhejiang16009103
complaints:service@lookchem.com Desktop View