-
Name
Cyclobutyl bromide
- EINECS 224-530-9
- CAS No. 4399-47-7
- Article Data30
- CAS DataBase
- Density 1.581 g/cm3
- Solubility insoluble in water
- Melting Point
- Formula C4H7Br
- Boiling Point 107.1 °C at 760 mmHg
- Molecular Weight 135.004
- Flash Point 22.2 °C
- Transport Information UN 1993 3/PG 2
- Appearance Clear colourless liquid
- Safety 26-36-36/37/39-16
- Risk Codes 10-36/37/38-20/21/22
-
Molecular Structure
-
Hazard Symbols
 Xi,
Xi,  Xn
Xn
- Synonyms Cyclobutyl bromide;AC1L2UIA;SBB070682;
- PSA 0.00000
- LogP 1.93380
Synthetic route
-
-
5162-44-7
1-bromo-4-butene

-
-
1229666-20-9
4-methoxy-3-pyridinesulfonamide

-
B
-
4399-47-7
Bromocyclobutane

| Conditions | Yield |
|---|---|
| In acetonitrile at 20℃; for 84h; Reflux; regioselective reaction; | A 75% B 37.5% |


| Conditions | Yield |
|---|---|
| With hydrogen bromide In 1,4-dioxane; chloroform at 10 - 20℃; for 2h; | A 9% B 74% |
| With phosphorus tribromide In diethyl ether at -80℃; Product distribution; variation of temperatures; | |
| With hydrogen bromide; 1-octyl-3-methyl-imidazolium bromide at 25℃; for 0.333333h; Reagent/catalyst; Temperature; |
-
-
42392-28-9
Cyclobutancarbonsaeure - Silbersalz

-
-
4399-47-7
Bromocyclobutane

| Conditions | Yield |
|---|---|
| With bromine In tetrachloromethane at -25℃; for 12h; | 60% |
| With bromine In tetrachloromethane at 26.9℃; for 1h; | 23% |

| Conditions | Yield |
|---|---|
| With NbCl3(N,N′-bis-(2,6-diisopropylphenyl)-1,4-diaza-2,3-dimethyl-1,3-butadiene) In benzene-d6 at 120℃; for 14h; Catalytic behavior; Inert atmosphere; Schlenk technique; Glovebox; | A 59% B 33% |
-
-
503-17-3
dimethylacetylene

-
-
33742-81-3
1,1-dibromocyclobutane

-
A
-
4399-47-7
Bromocyclobutane

-
B
-
80204-24-6
1-Brom-1-methylcyclobutan

-
C
-
108909-90-6
1,2-dimethylspiro(2.3)hex-1-ene

-
D
-
6142-73-0
methylene cyclopropane

| Conditions | Yield |
|---|---|
| With methyllithium In diethyl ether at -35℃; Further byproducts given; | A n/a B 12% C 21% D 30% |

-
-
33742-81-3
1,1-dibromocyclobutane

-
A
-
4399-47-7
Bromocyclobutane

-
B
-
80204-24-6
1-Brom-1-methylcyclobutan

-
C
-
108909-90-6
1,2-dimethylspiro(2.3)hex-1-ene

-
D
-
6142-73-0
methylene cyclopropane

| Conditions | Yield |
|---|---|
| With dimethylacetylene; methyllithium In diethyl ether at -35℃; Further byproducts given; | A n/a B 12% C 21% D 30% |


| Conditions | Yield |
|---|---|
| With carbon tetrabromide; triphenylphosphine In diethyl ether |

| Conditions | Yield |
|---|---|
| Multistep reaction; |
-
-
498-66-8
norborn-2-ene

-
A
-
4399-47-7
Bromocyclobutane

-
-
38537-30-3, 38537-32-5, 38537-61-0, 38537-64-3, 98431-04-0
7-anti-Chlor-8,9,10-trinorbornan-2-exo-ol

| Conditions | Yield |
|---|---|
| With hypochloric acid |

| Conditions | Yield |
|---|---|
| With hydroxide; bromine; silver nitrate 2.) CCl4; Multistep reaction; | |
| With [bis(acetoxy)iodo]benzene; potassium bromide In dichloromethane at 25℃; Irradiation; | 86 %Chromat. |
-
-
1506-77-0
1,1‐dichlorocyclobutane

-
A
-
4399-47-7
Bromocyclobutane

-
B
-
33954-15-3
1-bromocyclobut-1-ene

-
C
-
822-35-5
cyclobutene

-
D
-
6142-73-0
methylene cyclopropane

| Conditions | Yield |
|---|---|
| With n-butyllithium at 20℃; under 0.001 Torr; for 0.5h; Yield given. Yields of byproduct given; |

-
-
157-33-5
bicyclo[1.1.0]butane

-
A
-
5162-44-7
1-bromo-4-butene

-
B
-
4399-47-7
Bromocyclobutane

-
C
-
7051-34-5
cyclopropylcarbinyl bromide

| Conditions | Yield |
|---|---|
| With sodium hydroxide; sodium tetrahydroborate; mercury dibromide 1.) CH2Cl2; Yield given. Multistep reaction. Yields of byproduct given. Title compound not separated from byproducts; | |
| With hydrogen bromide Product distribution; influence of reaction time; |

-
-
42392-28-9
Cyclobutancarbonsaeure - Silbersalz

-
A
-
4399-47-7
Bromocyclobutane

-
B
-
7051-34-5
cyclopropylcarbinyl bromide

| Conditions | Yield |
|---|---|
| With bromine In tetrachloromethane at -20℃; for 1h; Yield given. Yields of byproduct given; |

-
A
-
4399-47-7
Bromocyclobutane

-
B
-
33954-15-3
1-bromocyclobut-1-ene

-
C
-
822-35-5
cyclobutene

-
D
-
6142-73-0
methylene cyclopropane

| Conditions | Yield |
|---|---|
| With methyllithium at 20℃; under 0.001 Torr; for 0.5h; Yield given. Yields of byproduct given; |


-
-
4399-47-7
Bromocyclobutane

| Conditions | Yield |
|---|---|
| With carbon disulfide; bromine at -25℃; |

-
-
4399-47-7
Bromocyclobutane

| Conditions | Yield |
|---|---|
| With tetrachloromethane; bromine at -25℃; |

-
-
4399-47-7
Bromocyclobutane

| Conditions | Yield |
|---|---|
| With tetrachloromethane; bromine at -20℃; |
-
-
2516-33-8
Cyclopropylmethanol

-
-
10035-10-6, 12258-64-9
hydrogen bromide

-
A
-
5162-44-7
1-bromo-4-butene

-
B
-
4399-47-7
Bromocyclobutane

-
C
-
7051-34-5
cyclopropylcarbinyl bromide

-
-
75-09-2
dichloromethane

-
-
2516-33-8
Cyclopropylmethanol

-
-
7789-60-8
phosphorus tribromide

-
A
-
5162-44-7
1-bromo-4-butene

-
B
-
4399-47-7
Bromocyclobutane

-
C
-
7051-34-5
cyclopropylcarbinyl bromide

-
-
5911-08-0
chloro(cyclopropyl)methane

-
A
-
5162-44-7
1-bromo-4-butene

-
B
-
4399-47-7
Bromocyclobutane

-
C
-
7051-34-5
cyclopropylcarbinyl bromide

| Conditions | Yield |
|---|---|
| With sodium bromide; NaY zeolite In pentane at 25℃; Kinetics; | A 58 % Chromat. B 32 % Chromat. C 10 % Chromat. |

-
-
2516-33-8
Cyclopropylmethanol

-
A
-
5162-44-7
1-bromo-4-butene

-
B
-
4399-47-7
Bromocyclobutane

-
C
-
7051-34-5
cyclopropylcarbinyl bromide

| Conditions | Yield |
|---|---|
| With hydrogen bromide; 3-ethyl-1-methyl-1H-imidazol-3-ium bromide at 80℃; for 0.333333h; Reagent/catalyst; Temperature; |

-
-
526214-00-6
5-methyloxazol-2-yl(phenyl)methanone

-
-
4399-47-7
Bromocyclobutane

-
-
1046818-32-9
cyclobutyl-(5-methyl-oxazol-2-yl)-phenyl-methanol

| Conditions | Yield |
|---|---|
| Stage #1: Bromocyclobutane With magnesium; iodine In tetrahydrofuran at 65℃; Inert atmosphere; Stage #2: 5-methyloxazol-2-yl(phenyl)methanone In tetrahydrofuran at 20℃; Cooling with ice; | 100% |
| Stage #1: Bromocyclobutane With magnesium In diethyl ether at 20℃; for 1h; Heating / reflux; Stage #2: 5-methyloxazol-2-yl(phenyl)methanone In tetrahydrofuran; diethyl ether at -10 - 20℃; for 16h; Stage #3: With water; ammonium chloride In tetrahydrofuran; diethyl ether at -20℃; | 38% |
-
-
1092448-19-5
2-(2-hydroxy-3-isopropyl-benzoylamino)-indan-2-carboxylic acid ethyl ester

-
-
4399-47-7
Bromocyclobutane

-
-
1092448-20-8
2-(2-cyclobutyloxy-3-isopropyl-benzoylamino)-indan-2-carboxylic acid ethyl ester

| Conditions | Yield |
|---|---|
| With caesium carbonate; potassium iodide In N,N-dimethyl-formamide at 130℃; for 2h; Microwave irradiation; | 98% |

| Conditions | Yield |
|---|---|
| With potassium carbonate at 80℃; for 16h; | 98% |

| Conditions | Yield |
|---|---|
| With potassium carbonate In N,N-dimethyl-formamide at 80℃; for 16h; | 98% |

| Conditions | Yield |
|---|---|
| With caesium carbonate In N,N-dimethyl-formamide at 95℃; for 16h; | 97% |
-
-
942473-86-1
4-bromo-3-fluorobenzenethiol

-
-
4399-47-7
Bromocyclobutane

-
-
1614246-39-7
(4-bromo-3-fluorophenyl)(cyclobutyl)sulfane

| Conditions | Yield |
|---|---|
| With caesium carbonate In dimethyl sulfoxide at 70℃; for 19h; | 95% |
-
-
4399-47-7
Bromocyclobutane

| Conditions | Yield |
|---|---|
| With caesium carbonate In acetonitrile at 80℃; for 6h; | 95% |
-
-
4399-47-7
Bromocyclobutane

-
-
175968-15-7
4-((tert-butyldimethylsilyl)oxy)-2-fluorobenzonitrile

-
-
1221235-53-5
cyclobutyl(2-fluoro-4-hydroxyphenyl)methanone

| Conditions | Yield |
|---|---|
| Stage #1: Bromocyclobutane With iodine; magnesium; ethylene dibromide In diethyl ether at 20℃; Stage #2: 4-((tert-butyldimethylsilyl)oxy)-2-fluorobenzonitrile With copper(I) bromide In tetrahydrofuran; diethyl ether at 20 - 60℃; for 0.75h; Inert atmosphere; Stage #3: With hydrogenchloride; water In tetrahydrofuran; diethyl ether at 0 - 60℃; for 1h; Inert atmosphere; | 95% |

| Conditions | Yield |
|---|---|
| Stage #1: benzo[1,3,2]dioxaborole With bis(cyclopentadienyl)titanium dichloride; potassium carbonate In tert-butyl methyl ether for 0.5h; Stage #2: Bromocyclobutane In tert-butyl methyl ether at 80℃; under 760.051 Torr; for 24h; Inert atmosphere; Stage #3: 2,3-dimethyl-2,3-butane diol With triethylamine In tert-butyl methyl ether at 20℃; for 1h; Inert atmosphere; | 95% |
| Stage #1: benzo[1,3,2]dioxaborole With bis(cyclopentadienyl)titanium dichloride; potassium carbonate In tert-butyl methyl ether for 0.5h; Inert atmosphere; Glovebox; Stage #2: Bromocyclobutane In tert-butyl methyl ether at 80℃; for 24h; Sealed tube; Stage #3: 2,3-dimethyl-2,3-butane diol With triethylamine In tert-butyl methyl ether at 20℃; for 1h; Sealed tube; | 95% |

-
-
1092448-55-9
5-formyl-2-hydroxy-3-methyl-benzoic acid methyl ester

-
-
4399-47-7
Bromocyclobutane

-
-
1092448-56-0
2-cyclobutyloxy-5-formyl-3-methyl-benzoic acid methyl ester

| Conditions | Yield |
|---|---|
| With caesium carbonate; potassium iodide In N,N-dimethyl-formamide at 110℃; for 6h; | 94% |

| Conditions | Yield |
|---|---|
| With caesium carbonate In N,N-dimethyl-formamide at 60℃; for 48h; Inert atmosphere; | 94% |

| Conditions | Yield |
|---|---|
| Stage #1: Bromocyclobutane With magnesium In tetrahydrofuran at 60℃; for 3h; Stage #2: With zinc(II) chloride In tetrahydrofuran at -78 - 20℃; for 1h; Stage #3: 2,3-dibromopyridine With tetrakis(triphenylphosphine) palladium(0) In tetrahydrofuran at 60℃; for 1h; Inert atmosphere; | 94% |

| Conditions | Yield |
|---|---|
| In N,N-dimethyl-formamide for 1.5h; Heating; | 93% |
| In N,N-dimethyl-formamide at 25℃; for 2h; | 63% |
| In N,N-dimethyl-formamide at 0℃; Kinetics; |

| Conditions | Yield |
|---|---|
| With potassium carbonate In N,N-dimethyl-formamide at 120℃; Sealed tube; Inert atmosphere; | 93% |
-
-
4399-47-7
Bromocyclobutane

-
-
1603018-85-4
6-mercaptopyridine-2-carboxylic acid ethyl ester

-
-
1609289-69-1
6-cyclobutylsulfanylpyridine-2-carboxylic acid ethyl ester

| Conditions | Yield |
|---|---|
| With caesium carbonate In acetonitrile at 50℃; for 18h; | 92% |
-
-
4399-47-7
Bromocyclobutane

-
-
942473-85-0
3-bromo-4-fluorobenzenethiol

| Conditions | Yield |
|---|---|
| With potassium carbonate In dimethyl sulfoxide at 60℃; Inert atmosphere; | 92% |

| Conditions | Yield |
|---|---|
| With potassium carbonate In N,N-dimethyl-formamide at 120℃; for 6h; | 91% |
| With potassium carbonate In N,N-dimethyl-formamide at 65℃; for 71h; Inert atmosphere; | 70% |

| Conditions | Yield |
|---|---|
| Stage #1: C8H11LiSi*LiCl With magnesium bromide In tetrahydrofuran at 66℃; for 0.166667h; Schlenk technique; Inert atmosphere; Stage #2: Bromocyclobutane With ferric(III) bromide; N,N,N,N,-tetramethylethylenediamine In tetrahydrofuran at 0℃; for 1.5h; Schlenk technique; Inert atmosphere; | 91% |
-
-
4399-47-7
Bromocyclobutane

-
-
40530-18-5
5-bromo-2-hydroxybenzonitrile

-
-
1594671-86-9
5-bromo-2-cyclobutoxybenzonitrile

| Conditions | Yield |
|---|---|
| With potassium carbonate In N,N-dimethyl-formamide at 90℃; for 6h; Inert atmosphere; | 90% |
| With potassium carbonate In N,N-dimethyl-formamide at 80℃; for 16h; Sealed tube; | 77% |

| Conditions | Yield |
|---|---|
| Stage #1: Bromocyclobutane With tert.-butyl lithium at -78℃; for 2h; Inert atmosphere; Stage #2: phenyl isothiocyanate at -78 - 20℃; Inert atmosphere; | 90% |
-
-
33543-78-1
ethyl 1H-imidazole-2-carboxylate

-
-
4399-47-7
Bromocyclobutane

| Conditions | Yield |
|---|---|
| Stage #1: ethyl 1H-imidazole-2-carboxylate With potassium carbonate In N,N-dimethyl-formamide for 0.5h; Stage #2: Bromocyclobutane In N,N-dimethyl-formamide at 100℃; for 5h; | 89.7% |
| Stage #1: ethyl 1H-imidazole-2-carboxylate With potassium carbonate In N,N-dimethyl-formamide for 0.5h; Stage #2: Bromocyclobutane In N,N-dimethyl-formamide at 100℃; for 5h; |
-
-
4399-47-7
Bromocyclobutane

-
-
138851-61-3
N-(2-fluorophenyl)pyrimidin-2-amine

| Conditions | Yield |
|---|---|
| With (1,2-dimethoxyethane)dichloronickel(II); lithium tert-butoxide In 1,4-dioxane at 100℃; for 16h; Schlenk technique; Inert atmosphere; | 89% |
| With N,N'-di-tert-butylethylenediamine; C40H32N12Ni2; lithium tert-butoxide In 1,4-dioxane at 120℃; for 16h; | 57% |

| Conditions | Yield |
|---|---|
| With caesium carbonate In N,N-dimethyl-formamide at 90℃; for 20h; | 89% |

| Conditions | Yield |
|---|---|
| With potassium carbonate In N,N-dimethyl-formamide at 90℃; for 14h; Inert atmosphere; | 89% |

| Conditions | Yield |
|---|---|
| With sodium azide; sodium L-ascorbate In water at 50℃; for 1h; Green chemistry; | 88% |
-
-
4399-47-7
Bromocyclobutane

| Conditions | Yield |
|---|---|
| Stage #1: Bromocyclobutane With sodium azide In N,N-dimethyl-formamide at 80℃; for 2h; Microwave irradiation; Stage #2: benzyl 3-(4-chloro-3-(((4-methoxybenzyl)oxy)methyl)phenyl)-2,2-dimethylhept-6-ynoate With copper(l) iodide; N-ethyl-N,N-diisopropylamine In dichloromethane; water; N,N-dimethyl-formamide; tert-butyl alcohol at 70℃; for 1h; Microwave irradiation; | 88% |

| Conditions | Yield |
|---|---|
| With tetrakis(triphenylphosphine) palladium(0); 1,3,4,6,7,8-hexahydro-2H-pyrimido[1,2-a]pyrimidine In dimethyl sulfoxide at 20℃; under 760.051 Torr; for 24h; Inert atmosphere; Schlenk technique; Sealed tube; Irradiation; | 88% |

-
-
4399-47-7
Bromocyclobutane

-
-
73183-34-3
bis(pinacol)diborane

| Conditions | Yield |
|---|---|
| With lithium tert-butoxide In N,N-dimethyl-formamide at 30℃; under 760.051 Torr; for 3h; Irradiation; | 87% |
| With copper(I) oxide; lithium methanolate; pyrographite In ethanol at 20℃; for 12h; Inert atmosphere; | 50% |
| With potassium methanolate; [1,3-bis(2,4,6-trimethylphenyl)imidazol]-2-ylidene; copper dichloride In tetrahydrofuran at 20℃; |

-
-
4399-47-7
Bromocyclobutane

| Conditions | Yield |
|---|---|
| With potassium carbonate In N,N-dimethyl-formamide at 90 - 95℃; for 16h; | 87% |
-
-
4399-47-7
Bromocyclobutane

-
-
2315-86-8
3-bromo-4-hydroxybenzonitrile

-
-
1065640-60-9
3-bromo-4-cyclobutoxybenzonitrile

| Conditions | Yield |
|---|---|
| With potassium carbonate In N,N-dimethyl-formamide at 60℃; for 72h; | 86% |
| With potassium carbonate In N,N-dimethyl-formamide at 60℃; for 72h; |
Cyclobutyl bromide Specification
The Cyclobutane, bromo- with CAS registry number of 4399-47-7 is also known as Cyclobutyl bromide. The IUPAC name is Cyclobutyl bromide. It belongs to product categories of Cyclobutanes & Cyclobutenes; Simple 4-Membered Ring Compounds; Cycloalkanes. Its EINECS registry number is 224-530-9. In addition, the formula is C4H7Br and the molecular weight is 135.00. This chemical is a clear colourless liquid that miscible with water. It may cause inflammation to the skin or other mucous membranes and may cause damage to health. Therefore this chemical should be sealed in ventilated, dry place without light at the temperature of 0-6 °C.
Physical properties about Cyclobutane, bromo- are: (1)ACD/LogP: 2.08; (2)# of Rule of 5 Violations: 0; (3)ACD/LogD (pH 5.5): 2.08; (4)ACD/LogD (pH 7.4): 2.08; (5)ACD/BCF (pH 5.5): 22.39; (6)ACD/BCF (pH 7.4): 22.39; (7)ACD/KOC (pH 5.5): 322.13; (8)ACD/KOC (pH 7.4): 322.13; (9)Index of Refraction: 1.526; (10)Molar Refractivity: 26.23 cm3; (11)Molar Volume: 85.3 cm3; (12)Surface Tension: 37.6 dyne/cm; (13)Density: 1.581 g/cm3; (14)Flash Point: 22.2 °C; (15)Enthalpy of Vaporization: 33.17 kJ/mol; (16)Boiling Point: 107.1 °C at 760 mmHg; (17)Vapour Pressure: 32 mmHg at 25 °C.
Preparation of Cyclobutane, bromo-: it is prepared by reaction of cyclobutanecarboxylic acid; silver (I)-compound. The reaction needs reagent Br2 and solvent CCl4 at the temperature of -25 °C for 12 hours. The yield is about 60%.
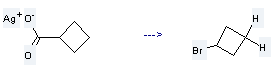
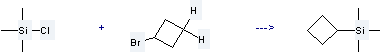
Uses of Cyclobutane, bromo-: it is used to produce cyclobutyltrimethylsilane by reaction with chloro-trimethyl-silane. The reaction occurs with reagent Li and solvent tetrahydrofuran at the temperature of -20 °C for 20 hours. The yield is about 50%.
When you are using this chemical, please be cautious about it. As a chemical, it is irritating to eyes, respiratory system and skin. Furthermore, it is harmful by inhalation, in contact with skin and if swallowed. During using it, wear suitable protective clothing, gloves and eye/face protection. Keep away from sources of ignition as this chemical is flammable. If contact with eyes accidently, rinse immediately with plenty of water and seek medical advice.
You can still convert the following datas into molecular structure:
1. Canonical SMILES: C1CC(C1)Br
2. InChI: InChI=1S/C4H7Br/c5-4-2-1-3-4/h4H,1-3H2
3. InChIKey: KXVUSQIDCZRUKF-UHFFFAOYSA-N
About|Contact|Cas|Product Name|Molecular|Country|Encyclopedia
Message|New Cas|MSDS|Service|Advertisement|CAS DataBase|Article Data|Manufacturers | Chemical Catalog
©2008 LookChem.com,License: ICP
NO.:Zhejiang16009103
complaints:service@lookchem.com Desktop View