-
Name
Cyclohexapentylose
- EINECS 233-007-4
- CAS No. 10016-20-3
- Article Data43
- CAS DataBase
- Density 1.624 g/cm3
- Solubility H2O: 50 mg/mL
- Melting Point >278 °C (dec.)(lit.)
- Formula C36H60O30
- Boiling Point 1410.8 °C at 760 mmHg
- Molecular Weight 972.854
- Flash Point 807.1 °C
- Transport Information
- Appearance White crystalline powder
- Safety 26-36
- Risk Codes 36-36/37/38
-
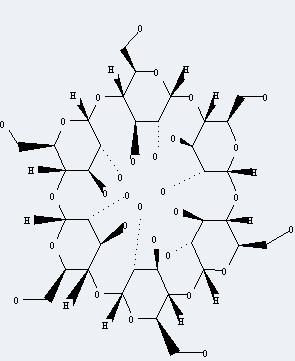
Molecular Structure
-
Hazard Symbols
 Xi
Xi
- Synonyms Cyclomaltohexose;Dextrin, a-cyclo;Dexy Pearl a-100;Isoeleat K 50;NSC 269470;Ringdex A;Stereoisomer of 5,10,15,20,25,30-hexakis(hydroxymethyl)-2,4,7,9,12,14,17,19,22,24,27,29-dodecaoxaheptacyclo[26.2.2.23,6.28,11.213,16.218,21.223,26]dotetracontane-31,32,33,34,35,36,37,38,39,40,41,42-dodecol;a-Cycloamylose;a-Dextrin;a-Schardinger dextrin;Cyclohexaamylose(6CI);Alfadex;Cavamax W 6;Cavamax W 6 Food;Celdex A 100;Cyclohexadextrin;
- PSA 474.90000
- LogP -13.05480
Synthetic route

-
-
10016-20-3
alpha cyclodextrin

| Conditions | Yield |
|---|---|
| With hydrogen; palladium dihydroxide In methanol; water at 20℃; for 12h; | 100% |

| Conditions | Yield |
|---|---|
| With sodium hydroxide; cyclodextrin-α(1-4)glucosyltransferase In water at 45℃; for 0.333333h; pH=6.0, sodium acetate buffer; | A 38% B 30% |
-
-
64887-49-6
α-cyclodextrin-Methyl Orange complex

-
A
-
547-58-0
methyl orange

-
B
-
10016-20-3
alpha cyclodextrin

| Conditions | Yield |
|---|---|
| In water at 25℃; Equilibrium constant; | |
| In methanol; water at 25℃; Equilibrium constant; | |
| In water; dimethyl sulfoxide at 25℃; Equilibrium constant; |

| Conditions | Yield |
|---|---|
| With phosphate buffer at 25℃; Equilibrium constant; |

| Conditions | Yield |
|---|---|
| With phosphate buffer at 25℃; Equilibrium constant; |

| Conditions | Yield |
|---|---|
| With phosphate buffer at 25℃; Equilibrium constant; |

| Conditions | Yield |
|---|---|
| With phosphate buffer at 25℃; Equilibrium constant; |

| Conditions | Yield |
|---|---|
| With phosphate buffer at 25℃; Equilibrium constant; |

| Conditions | Yield |
|---|---|
| With phosphate buffer at 25℃; Equilibrium constant; |

| Conditions | Yield |
|---|---|
| With phosphate buffer at 25℃; Equilibrium constant; |

| Conditions | Yield |
|---|---|
| With phosphate buffer at 25℃; Equilibrium constant; |

-
A
-
113894-26-1
3-nitro-4-(octanoyloxy)benzoic acid

-
B
-
10016-20-3
alpha cyclodextrin

| Conditions | Yield |
|---|---|
| With phosphate buffer at 25℃; Equilibrium constant; |
-
-
141484-63-1
C37H47N3(2+)*C36H60O30*2Br(1-)

-
A
-
141484-62-0
C37H47N3(2+)*2Br(1-)

-
B
-
10016-20-3
alpha cyclodextrin

| Conditions | Yield |
|---|---|
| In water-d2 at 30℃; Equilibrium constant; other temperatures; |

-
A
-
95598-14-4
3-t-butyl-1-methyl-1-nitrosothiourea

-
B
-
10016-20-3
alpha cyclodextrin

| Conditions | Yield |
|---|---|
| With acetate buffer at 37℃; Rate constant; dissociation constant and catalyzed rate constant of the inclusion complex is determined; |
-
-
114987-35-8
C36H60O30*C12H14N2

-
A
-
10016-20-3
alpha cyclodextrin

-
B
-
25128-26-1
1,1'-dimethyl-1,1'-dihydro-4,4'-bipyridyl

| Conditions | Yield |
|---|---|
| In water at 25℃; Equilibrium constant; |
-
-
85090-50-2
C36H60O30*C14H11N2O3(1-)

-
A
-
85090-40-0
4-(4-Hydroxy-2-methyl-phenylazo)-benzoic acid anion

-
B
-
10016-20-3
alpha cyclodextrin

| Conditions | Yield |
|---|---|
| In water at 19 - 25℃; Equilibrium constant; pH = 7.2; phosphate buffer; ionic strength of 0.15M,; |
-
-
77192-49-5
prostacyclin*α-cyclodextrin

-
A
-
58962-34-8
7-[(1R,2R,3R,5S)-3,5-Dihydroxy-2-((E)-(S)-3-hydroxy-oct-1-enyl)-cyclopentyl]-6-oxo-heptanoic acid

-
B
-
10016-20-3
alpha cyclodextrin

| Conditions | Yield |
|---|---|
| With phosphate buffer (pH=ca. 7); water at 10 - 30℃; Thermodynamic data; Kinetics; activation parameters: ΔS(excit.), ΔG(excit.), E investigated; |
-
-
69377-71-5
C36H60O30*C21H34O5

-
A
-
63557-55-1
6-keto-PGF1α methyl ester

-
B
-
10016-20-3
alpha cyclodextrin

| Conditions | Yield |
|---|---|
| With phosphate buffer (pH=ca. 7); water at 10 - 30℃; Thermodynamic data; Kinetics; activation parameters: ΔS(excit.), ΔG(excit.), E investigated; |
-
-
110237-97-3, 123930-57-4, 150520-16-4
hexakis-(2,3,6-tri-O-benzyl)-α-cyclodextrin

-
-
10016-20-3
alpha cyclodextrin

| Conditions | Yield |
|---|---|
| With hydrogen; palladium on activated charcoal In methanol; formic acid at 50℃; Yield given; |
-
-
113879-86-0
allyl O-(2,3,4-tri-O-benzyl-α-D-glucopyranosyl)-(1<*>4)-2,3,6-tri-O-benzyl-β-D-glucopyranoside

-
-
113842-96-9
allyl O-(4-O-acetyl-2,3,6-tri-O-benzyl-α-D-glucopyranosyl)-(1->4)-bis

-
-
10016-20-3
alpha cyclodextrin

| Conditions | Yield |
|---|---|
| Yield given. Multistep reaction; |
-
-
113842-86-7
O-(4-O-acetyl-2,3,6-tri-O-benzyl-α-D-glucopyranosyl)-(1->4)-2,3,6-tri-O-benzyl-β-D-glucopyranosyl fluoride

-
-
113842-97-0
O-(2,3,6-tri-O-benzyl-α-D-glucopyranosyl)-(1->4)-bis

-
-
10016-20-3
alpha cyclodextrin

| Conditions | Yield |
|---|---|
| Yield given. Multistep reaction; |

-
A
-
63442-80-8
sodium 4-pyren-1-ylbutyrate

-
B
-
10016-20-3
alpha cyclodextrin

| Conditions | Yield |
|---|---|
| In water at 25℃; Equilibrium constant; |

| Conditions | Yield |
|---|---|
| In water at 25℃; Equilibrium constant; |

-
-
10016-20-3
alpha cyclodextrin

| Conditions | Yield |
|---|---|
| In water at 25℃; Equilibrium constant; |

| Conditions | Yield |
|---|---|
| In water at 19.9℃; Equilibrium constant; |

-
-
10016-20-3
alpha cyclodextrin

| Conditions | Yield |
|---|---|
| In water at 25℃; Equilibrium constant; |

| Conditions | Yield |
|---|---|
| In water at 25℃; Equilibrium constant; Thermodynamic data; partial molal volume change, atmospheric pressure; |

| Conditions | Yield |
|---|---|
| In water Equilibrium constant; |

-
A
-
98928-28-0
5-(4-hydroxyphenylazo)naphthalene-1-sulphonate

-
B
-
10016-20-3
alpha cyclodextrin

| Conditions | Yield |
|---|---|
| In water at 25℃; Equilibrium constant; phosphate buffer pH 4.6; |

| Conditions | Yield |
|---|---|
| In water at 25℃; Equilibrium constant; phosphate buffer pH 11.8; |
-
-
10016-20-3
alpha cyclodextrin

-
-
131105-41-4
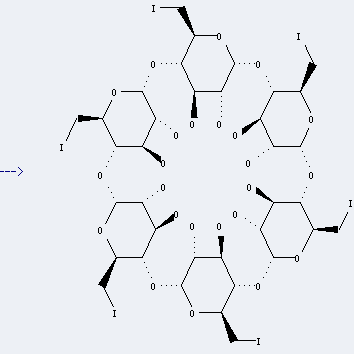
hexakis-(6-deoxy-6-iodo)-α-cyclodextrin

| Conditions | Yield |
|---|---|
| With iodine; triphenylphosphine In N,N-dimethyl-formamide at 70℃; for 20h; Inert atmosphere; | 100% |
| With tetraethylammonium iodide; 4-pyrrolidin-1-ylpyridine; ethanaminium,N-(difluoro-λ4-sulfanylidene)-N-ethyl-,tetrafluoroborate In N,N-dimethyl-formamide at 20℃; regioselective reaction; | 91% |
| With Iod; triphenylphosphine In N,N-dimethyl-formamide at 80℃; for 15h; | 80% |
-
-
108-24-7
acetic anhydride

-
-
10016-20-3
alpha cyclodextrin

-
-
23661-37-2
hexakis(2,3,6-tri-O-acetyl)-α-cyclomaltohexaose

| Conditions | Yield |
|---|---|
| In pyridine | 100% |
| With iodine at 20℃; for 24h; neat (no solvent); | 100% |
| With pyridine at 60℃; for 12h; | 100% |

| Conditions | Yield |
|---|---|
| In water for 13h; Reflux; | 100% |
| In water at 20℃; Reflux; | |
| In water at 20℃; Reflux; |
-
-
100-44-7
benzyl chloride

-
-
10016-20-3
alpha cyclodextrin

-
-
110237-97-3, 123930-57-4, 150520-16-4
hexakis-(2,3,6-tri-O-benzyl)-α-cyclodextrin

| Conditions | Yield |
|---|---|
| With sodium hydride In dimethyl sulfoxide at 20℃; for 22h; Inert atmosphere; Schlenk technique; | 99% |
| In dimethyl sulfoxide at 20℃; Inert atmosphere; | 98% |
| With sodium hydride In dimethyl sulfoxide at 20℃; for 18h; | 95% |

| Conditions | Yield |
|---|---|
| With iodine at 80℃; for 24h; neat (no solvent); | 99% |

| Conditions | Yield |
|---|---|
| With pyridine at 80℃; for 12h; | 98% |
| With pyridine for 24h; Ambient temperature; |

| Conditions | Yield |
|---|---|
| With (benzotriazo-1-yloxy)tris(dimethylamino)phosphonium hexafluorophosphate; N-ethyl-N,N-diisopropylamine In N,N-dimethyl-formamide at 4℃; | 98% |

-
-
106-31-0
butanoic acid anhydride

-
-
10016-20-3
alpha cyclodextrin

-
-
1258780-44-7
per-O-butyryl-α-cyclodextrin

| Conditions | Yield |
|---|---|
| With iodine at 80℃; for 24h; neat (no solvent); | 98% |
| With pyridine at 80℃; for 12h; | 98% |

| Conditions | Yield |
|---|---|
| With methanesulfonic acid In neat (no solvent) at 30 - 35℃; for 0.166667h; Green chemistry; | 98% |

| Conditions | Yield |
|---|---|
| With pyridine at 80℃; for 12h; | 98% |

| Conditions | Yield |
|---|---|
| for 24h; | 97.2% |
-
-
18162-48-6
tert-butyldimethylsilyl chloride

-
-
10016-20-3
alpha cyclodextrin

-
-
90289-32-0
Hexakis(2,6-di-O-tert-butyldimethylsilyl)-α-cyclodextrin

| Conditions | Yield |
|---|---|
| In pyridine; N,N-dimethyl-formamide at 100℃; for 18h; | 97% |
| With pyridine; dmap In N,N-dimethyl-formamide at 100℃; for 18h; | 86% |
| With pyridine; dmap In N,N-dimethyl-formamide at 100℃; for 18h; Inert atmosphere; | 75% |

-
-
768-94-5
1-Adamantanamine

-
-
10016-20-3
alpha cyclodextrin

- poly(ethylene glycol) 3500, bis[N-(1-adamantyl)aminocarbonyl]-terminated, inclusion complex with α-cyclodextrin, prepared with 1-adamantylamine and 2,4,6-trimethylpyridine
-
poly(ethylene glycol) 3500, bis[N-(1-adamantyl)aminocarbonyl]-terminated, inclusion complex with α-cyclodextrin, prepared with 1-adamantylamine and 2,4,6-trimethylpyridine

| Conditions | Yield |
|---|---|
| With 2,4,6-trimethyl-pyridine; (benzotriazo-1-yloxy)tris(dimethylamino)phosphonium hexafluorophosphate In N,N-dimethyl-formamide at 4℃; | 97% |

-
-
10016-20-3
alpha cyclodextrin

-
-
5538-51-2
O-acetylsalicyloyl chloride

-
-
1332459-75-2
6-[O-(2-acetoxybenzoyl)]-α-cyclodextrin

| Conditions | Yield |
|---|---|
| With pyridine In N,N-dimethyl-formamide; benzene at 0 - 20℃; regioselective reaction; | 97% |
-
-
10016-20-3
alpha cyclodextrin

-
-
53784-82-0
hexakis-(6-bromo-6-deoxy)-α-cyclodextrin

| Conditions | Yield |
|---|---|
| With tetrabutylammomium bromide; (chloro-phenylthio-methylene)dimethylammonium chloride In N,N-dimethyl-formamide at 20℃; regioselective reaction; | 96% |
| With tetraethylammonium bromide; 4-pyrrolidin-1-ylpyridine; ethanaminium,N-(difluoro-λ4-sulfanylidene)-N-ethyl-,tetrafluoroborate In N,N-dimethyl-formamide at 20℃; regioselective reaction; | 95% |
| With bromine; triphenylphosphine In N,N-dimethyl-formamide at 75 - 80℃; for 18h; | 86% |

-
-
665-66-7
amantadine hydrochloride

-
-
10016-20-3
alpha cyclodextrin

- poly(ethylene glycol) 3500, bis[N-(1-adamantyl)aminocarbonyl]-terminated, inclusion complex with α-cyclodextrin, prepared with 1-adamantylamine and 4-(dimethylamino)pyridine
-
poly(ethylene glycol) 3500, bis[N-(1-adamantyl)aminocarbonyl]-terminated, inclusion complex with α-cyclodextrin, prepared with 1-adamantylamine and 4-(dimethylamino)pyridine

| Conditions | Yield |
|---|---|
| With dmap; (benzotriazo-1-yloxy)tris(dimethylamino)phosphonium hexafluorophosphate In N,N-dimethyl-formamide at 4℃; | 96% |


-
-
665-66-7
amantadine hydrochloride

-
-
10016-20-3
alpha cyclodextrin

- poly(ethylene glycol) 3500, bis[N-(1-adamantyl)aminocarbonyl]-terminated, inclusion complex with α-cyclodextrin, prepared with 1-adamantylamine and proton sponge
-
poly(ethylene glycol) 3500, bis[N-(1-adamantyl)aminocarbonyl]-terminated, inclusion complex with α-cyclodextrin, prepared with 1-adamantylamine and proton sponge

| Conditions | Yield |
|---|---|
| With N,N,N',N'-tetramethyl-1,8-diaminonaphthalene; (benzotriazo-1-yloxy)tris(dimethylamino)phosphonium hexafluorophosphate In N,N-dimethyl-formamide at 4℃; | 95% |

-
-
999-97-3
1,1,1,3,3,3-hexamethyl-disilazane

-
-
10016-20-3
alpha cyclodextrin

| Conditions | Yield |
|---|---|
| With trimethylsilyl trifluoromethanesulfonate In dichloromethane at 20℃; for 0.166667h; Inert atmosphere; regioselective reaction; | 95% |

| Conditions | Yield |
|---|---|
| In water at 20℃; for 3h; | 95% |

| Conditions | Yield |
|---|---|
| In water at 20℃; for 3h; | 95% |
-
-
122710-25-2
(1-allyloxy-3-propoxybenzene)

-
-
10016-20-3
alpha cyclodextrin

-
A
-
122710-44-5
2-allyl-5-propoxy phenol

-
B
-
122710-43-4
2-allyl-3-propoxy phenol

| Conditions | Yield |
|---|---|
| In solid Irradiation; | A 94% B 5 % Chromat. |


| Conditions | Yield |
|---|---|
| In diethyl ether for 8h; Inert atmosphere; Sonication; Darkness; | 93% |
-
-
13435-12-6
N-Trimethylsilylacetamide

-
-
10016-20-3
alpha cyclodextrin

| Conditions | Yield |
|---|---|
| In N,N-dimethyl-formamide at 50℃; for 48h; | 92% |

| Conditions | Yield |
|---|---|
| With pyridine In N,N-dimethyl-formamide at 0 - 20℃; regioselective reaction; | 92% |
| With pyridine at -7 - 20℃; for 5.7h; Acylation; | 14% |
-
-
40138-16-7
2-formylbenzene boronic acid

-
-
10016-20-3
alpha cyclodextrin

| Conditions | Yield |
|---|---|
| Stage #1: O,O'-diaminododecanediol; alpha cyclodextrin In water at 20℃; for 0.25h; Stage #2: 2-formylbenzene boronic acid In water at 20℃; for 1h; | 92% |


-
-
10016-20-3
alpha cyclodextrin

- polypseudorotaxane based on linear polyethylenimine (Mw = 3200) with α-cyclodextrin (threading ratio (repeating unit of polyethylenimine/cyclodextrin) = 2.1); monomer(s): 2-methyl-2-oxazoline, α-cyclodextrin
-
polypseudorotaxane based on linear polyethylenimine (Mw = 3200) with α-cyclodextrin (threading ratio (repeating unit of polyethylenimine/cyclodextrin) = 2.1); monomer(s): 2-methyl-2-oxazoline, α-cyclodextrin

| Conditions | Yield |
|---|---|
| In phosphate buffer at 60℃; pH=11.0; | 91.2% |
-
-
10016-20-3
alpha cyclodextrin

-
-
173094-59-2
hexakis(6-chloro-6-deoxy)-α-cyclodextrin

| Conditions | Yield |
|---|---|
| With tetraethylammonium chloride; 4-pyrrolidin-1-ylpyridine; ethanaminium,N-(difluoro-λ4-sulfanylidene)-N-ethyl-,tetrafluoroborate In N,N-dimethyl-formamide at 20℃; regioselective reaction; | 91% |
| With methanesulfonyl chloride In N,N-dimethyl-formamide at 65℃; for 48h; | 90% |
| With methanesulfonyl chloride In N,N-dimethyl-formamide at 65℃; for 48h; | 80% |

| Conditions | Yield |
|---|---|
| In water at 20℃; for 3h; | 91% |
-
-
10016-20-3
alpha cyclodextrin

-
-
17557-23-2
neopentyl glycol di(3-azido-2-hydroxylpropan-1-ol)

| Conditions | Yield |
|---|---|
| In water at 20℃; for 10h; inclusion; | 90% |
Cyclohexapentylose History
Cyclohexapentylose Specification
The Cyclodextrin is an organic compound with the formula C36H60O30. The systematic name of this chemical is (1S, 3R, 5R, 6S, 8R, 10R, 11S, 13R, 15R, 16S, 18R, 20R, 21S, 23R, 25R, 26S, 28R, 30R, 31R, 32R, 33R, 34R, 35R, 36R, 37R, 38R, 39R, 40R, 41R, 42R)-5,10,15,20,25,30-hexakis(hydroxymethyl)-2,4,7,9,12,14,17,19,22,24,27,29-dodecaoxahe ptacyclo[26.2.2.23,6.28,11.213,16.218,21.223,26]dotetracontane-31,32,33,34,35,36,37,38,39,40,41,42-dodecol (non-preferred name). With the CAS registry number 10016-20-3, it is also named as a-Schardinger Dextrin. The product's categories are Industrial/Fine Chemicals; Biochemistry; Cyclodextrins; Functional Materials; Macrocycles for Host-Guest Chemistry; Oligosaccharides; Sugars; Dextrins, Sugar & Carbohydrates. Besides, it is a white crystalline powder, which should be stored in a closed and dry palce.
In the food industry cyclodextrins are employed for the preparation of cholesterol free products. Other food applications further include the ability to stabilize volatile or unstable compounds and the reduction of unwanted tastes and odour. Reportedly cyclodextrins are used in alcohol powder, a powder for mixing alcoholic drinks.
Physical properties about Cyclodextrin are: (1)# of Rule of 5 Violations: 3; (2)ACD/BCF (pH 5.5): 1; (3)ACD/BCF (pH 7.4): 1; (4)ACD/KOC (pH 5.5): 1; (5)ACD/KOC (pH 7.4): 1; (6)#H bond acceptors: 30; (7)#H bond donors: 18; (8)#Freely Rotating Bonds: 24; (9)Polar Surface Area: 474.9 Å2; (10)Index of Refraction: 1.591; (11)Molar Refractivity: 202.387 cm3; (12)Molar Volume: 598.936 cm3; (13)Polarizability: 80.232×10-24cm3; (14)Surface Tension: 73.864 dyne/cm; (15)Density: 1.624 g/cm3; (16)Flash Point: 807.051 °C; (17)Enthalpy of Vaporization: 241.394 kJ/mol; (18)Boiling Point: 1410.846 °C at 760 mmHg.
Uses of Cyclodextrin: it can be used to produce hexakis(6-deoxy-6-iodo)cyclomaltohexaose at temperature of 80 °C. It will need reagent iod, triphenylphosφne and solvent dimethylformamide with reaction time of 15 hours. The yield is about 80%.
When you are using this chemical, please be cautious about it as the following:
This chemical is irritating to eyes, respiratory system and skin. When you are using it, wear suitable protective clothing. In case of contact with eyes, rinse immediately with plenty of water and seek medical advice.
You can still convert the following datas into molecular structure:
(1)SMILES: C([C@@H]1[C@@H]2[C@@H]([C@H]([C@H](O1)O[C@@H]3[C@H](O[C@@H]([C@@H]([C@H]3O)O)O[C@@H]4[C@H](O[C@@H]([C@@H]([C@H]4O)O)O[C@@H]5[C@H](O[C@@H]([C@@H]([C@H]5O)O)O[C@@H]6[C@H](O[C@@H]([C@@H]([C@H]6O)O)O[C@@H]7[C@H](O[C@H](O2)[C@@H]([C@H]7O)O)CO)CO)CO)CO)CO)O)O)O
(2)InChI: InChI=1/C36H60O30/c37-1-7-25-13(43)19(49)31(55-7)62-26-8(2-38)57-33(21(51)15(26)45)64-28-10(4-40)59-35(23(53)17(28)47)66-30-12(6-42)60-36(24(54)18(30)48)65-29-11(5-41)58-34(22(52)16(29)46)63-27-9(3-39)56-32(61-25)20(50)14(27)44/h7-54H,1-6H2/t7-,8-,9-,10-,11-,12-,13-,14-,15-,16-,17-,18-,19-,20-,21-,22-,23-,24-,25-,26-,27-,28-,29-,30-,31-,32-,33-,34-,35-,36-/m1/s1
(3)InChIKey: HFHDHCJBZVLPGP-RWMJIURBBY
(4)Std. InChI: InChI=1S/C36H60O30/c37-1-7-25-13(43)19(49)31(55-7)62-26-8(2-38)57-33(21(51)15(26)45)64-28-10(4-40)59-35(23(53)17(28)47)66-30-12(6-42)60-36(24(54)18(30)48)65-29-11(5-41)58-34(22(52)16(29)46)63-27-9(3-39)56-32(61-25)20(50)14(27)44/h7-54H,1-6H2/t7-,8-,9-,10-,11-,12-,13-,14-,15-,16-,17-,18-,19-,20-,21-,22-,23-,24-,25-,26-,27-,28-,29-,30-,31-,32-,33-,34-,35-,36-/m1/s1
(5)Std. InChIKey: HFHDHCJBZVLPGP-RWMJIURBSA-N
The toxicity data is as follows:
| Organism | Test Type | Route | Reported Dose (Normalized Dose) | Effect | Source |
|---|---|---|---|---|---|
| rat | LD50 | intraperitoneal | 1gm/kg (1000mg/kg) | Oyo Yakuri. Pharmacometrics. Vol. 26, Pg. 287, 1983. | |
| rat | LD50 | intravenous | 788mg/kg (788mg/kg) | American Journal of Pathology. Vol. 83, Pg. 367, 1976. |
Related Products
- Cyclohexapentylose
- 100-16-3
- 100163-29-9
- 1001645-58-4
- 100165-88-6
- 10017-39-7
- 100-17-4
- 10017-44-4
- 1001754-72-8
- 1001754-82-0
- 1001757-50-1
Hot Products
About|Contact|Cas|Product Name|Molecular|Country|Encyclopedia
Message|New Cas|MSDS|Service|Advertisement|CAS DataBase|Article Data|Manufacturers | Chemical Catalog
©2008 LookChem.com,License: ICP
NO.:Zhejiang16009103
complaints:service@lookchem.com Desktop View