-
Name
Deferoxamine
- EINECS 200-738-5
- CAS No. 70-51-9
- Article Data10
- CAS DataBase
- Density 1.212g/cm3
- Solubility
- Melting Point 139°C
- Formula C25H48 N6 O8
- Boiling Point 627.9°C (rough estimate)
- Molecular Weight 560.692
- Flash Point
- Transport Information
- Appearance
- Safety Poison by intravenous route. Moderately toxic by ingestion, intraperitoneal and subcutaneous routes. Human systemic effects: changes in hearing acuity, eye hemorrhage, optic nerve neuropathy, thrombocytopenia, visual field changes. Human mutation data reported. When heated to decomposition it emits toxic fumes of NOx.
- Risk Codes
-
Molecular Structure
- Hazard Symbols
- Synonyms Butanediamide,N'-[5-[[4-[[5-(acetylhydroxyamino)pentyl]amino]-1,4-dioxobutyl]hydroxyamino]pentyl]-N-(5-aminopentyl)-N-hydroxy-(9CI); Propionohydroxamic acid,N-[5-[3-[(5-aminopentyl)hydroxycarbamoyl]propionamido]pentyl]-3-[[5-(N-hydroxyacetamido)pentyl]carbamoyl]-(8CI); 3,9,14,20,25-Pentaazatriacontane-2,10,13,21,24-pentone,30-amino-3,14,25-trihydroxy-; 30-Amino-3,14,25-trihydroxy-3,9,14,20,25pentaazatriacontane-2,10,13,21,24-pentaone; Deferoxamin; Deferoxamine;Deferoxamine B; Deferriferrioxamine B; Deferrioxamine; Deferrioxamine B;Desferan; Desferex; Desferin; Desferioxamine B; Desferrin; Desferrioxamine;Desferrioxamine B;N-[5-[3-[(5-Aminopentyl)hydroxycarbamoyl]propionamido]pentyl]-3-[[5-(N-hydroxyacetamido)pentyl]carbamoyl]propionohydroxamicacid; NSC 527604
- PSA 205.84000
- LogP 2.40420
Synthetic route

-
-
70-51-9
deferoxamine

| Conditions | Yield |
|---|---|
| With palladium 10% on activated carbon; hydrogen; acetic acid In methanol at 25℃; under 760.051 Torr; for 0.333333h; | 50% |
-
-
402913-84-2
1-Carbobenzoxyamino-6,17-dihydroxy-7,10,18,21-tetraoxy-27-(N-acetyl-hydroxyamino)-6,11,17,22-tetraaza-hepteikosan

-
-
70-51-9
deferoxamine

| Conditions | Yield |
|---|---|
| With hydrogenchloride; hydrogen; palladium on activated charcoal In methanol |
-
-
112139-65-8
N'-<5-<<4-<<5-pentyl>amino>-1,4-dioxobutyl>(phenylmethoxy)amino>pentyl>-N-(4-cyanobutyl)-N-(phenylmethoxy)butanediamide

-
-
70-51-9
deferoxamine

| Conditions | Yield |
|---|---|
| With hydrogenchloride; hydrogen; palladium on activated charcoal In methanol |
-
-
129245-21-2
N-(5-aminopentyl)-N-(tert-butoxycarbonyl)-O-benzylhydroxylamine

-
-
70-51-9
deferoxamine

| Conditions | Yield |
|---|---|
| Multi-step reaction with 7 steps 1: 99 percent / diphenyl phosphorazidate, triethylamine / dimethylformamide / 17 h / 0 - 20 °C 2: 87 percent / trifluoroacetic acid (TFA) / CH2Cl2 / 0.75 h / 0 - 20 °C 3: 96 percent / pyridine 4: 95 percent / diphenyl phosphorazidate, triethylamine / dimethylformamide / 17 h / 0 - 20 °C 5: 100 percent / trifluoroacetic acid (TFA) / CH2Cl2 6: 91 percent / pyridine / 12 h 7: H2, HCl / 10percent Pd/C / methanol View Scheme |
-
-
129245-24-5
5,16-bis(benzyloxy)-20-cyano-4,12,15-trioxo-5,11,16-triazaeicosanoic acid

-
-
70-51-9
deferoxamine

| Conditions | Yield |
|---|---|
| Multi-step reaction with 4 steps 1: 95 percent / diphenyl phosphorazidate, triethylamine / dimethylformamide / 17 h / 0 - 20 °C 2: 100 percent / trifluoroacetic acid (TFA) / CH2Cl2 3: 91 percent / pyridine / 12 h 4: H2, HCl / 10percent Pd/C / methanol View Scheme |
-
-
130946-41-7
27--6,17-bis(benzyloxy)-7,10,18,21-tetraoxo-6,11,17,22-tetraazaheptacosanenitrile

-
-
70-51-9
deferoxamine

| Conditions | Yield |
|---|---|
| Multi-step reaction with 2 steps 1: 91 percent / pyridine / 12 h 2: H2, HCl / 10percent Pd/C / methanol View Scheme |
-
-
130946-40-6
27--6,17-bis(benzyloxy)-7,10,18,21-tetraoxo-6,11,17,22-tetraazaheptacosanenitrile

-
-
70-51-9
deferoxamine

| Conditions | Yield |
|---|---|
| Multi-step reaction with 3 steps 1: 100 percent / trifluoroacetic acid (TFA) / CH2Cl2 2: 91 percent / pyridine / 12 h 3: H2, HCl / 10percent Pd/C / methanol View Scheme |
-
-
112139-60-3
N-(4-Cyanobutyl)-N-(benzyloxy)succinamic Acid

-
-
70-51-9
deferoxamine

| Conditions | Yield |
|---|---|
| Multi-step reaction with 7 steps 1: 99 percent / diphenyl phosphorazidate, triethylamine / dimethylformamide / 17 h / 0 - 20 °C 2: 87 percent / trifluoroacetic acid (TFA) / CH2Cl2 / 0.75 h / 0 - 20 °C 3: 96 percent / pyridine 4: 95 percent / diphenyl phosphorazidate, triethylamine / dimethylformamide / 17 h / 0 - 20 °C 5: 100 percent / trifluoroacetic acid (TFA) / CH2Cl2 6: 91 percent / pyridine / 12 h 7: H2, HCl / 10percent Pd/C / methanol View Scheme |
-
-
129245-23-4
N-(4-cyanobutyl)-3-<<5-<(benzyloxy)amino>pentyl>carbamoyl>-O-benzylpropionohydroxamic acid

-
-
70-51-9
deferoxamine

| Conditions | Yield |
|---|---|
| Multi-step reaction with 5 steps 1: 96 percent / pyridine 2: 95 percent / diphenyl phosphorazidate, triethylamine / dimethylformamide / 17 h / 0 - 20 °C 3: 100 percent / trifluoroacetic acid (TFA) / CH2Cl2 4: 91 percent / pyridine / 12 h 5: H2, HCl / 10percent Pd/C / methanol View Scheme |
-
-
129245-22-3
N-(4-cyanobutyl)-3-<<5-<(benzyloxy)-tert-butoxy-carbonylamino>pentyl>carbamoyl>-O-benzylpropionohydroxamic acid

-
-
70-51-9
deferoxamine

| Conditions | Yield |
|---|---|
| Multi-step reaction with 6 steps 1: 87 percent / trifluoroacetic acid (TFA) / CH2Cl2 / 0.75 h / 0 - 20 °C 2: 96 percent / pyridine 3: 95 percent / diphenyl phosphorazidate, triethylamine / dimethylformamide / 17 h / 0 - 20 °C 4: 100 percent / trifluoroacetic acid (TFA) / CH2Cl2 5: 91 percent / pyridine / 12 h 6: H2, HCl / 10percent Pd/C / methanol View Scheme |
-
-
91905-05-4
1-(Carbobenzoxyamino)-5-(hydroxyamino)pentane

-
-
70-51-9
deferoxamine

| Conditions | Yield |
|---|---|
| Multi-step reaction with 4 steps 1: Py 2: acetic anhydride / 5 h / 95 - 100 °C 3: (i) H2, Pd-C, MeOH, (ii) /BRN= 898057/ 4: H2, aq. HCl / Pd-C / methanol View Scheme | |
| Multi-step reaction with 6 steps 1: Py 2: acetic anhydride / 5 h / 95 - 100 °C 3: (i) NaOMe, MeOH, (ii) /BRN= 898057/, THF 4: (i) aq. NH4Cl, Zn, EtOH, (ii) /BRN= 385737/, Py 5: (i) H2, Pd-C, MeOH, (ii) /BRN= 898057/ 6: H2, aq. HCl / Pd-C / methanol View Scheme |
-
-
92034-20-3
1-Carbobenzoxyamino-5-nitro-pentan

-
-
70-51-9
deferoxamine

| Conditions | Yield |
|---|---|
| Multi-step reaction with 5 steps 1: aq. NH4Cl, Zn / ethanol 2: Py 3: acetic anhydride / 5 h / 95 - 100 °C 4: (i) H2, Pd-C, MeOH, (ii) /BRN= 898057/ 5: H2, aq. HCl / Pd-C / methanol View Scheme | |
| Multi-step reaction with 7 steps 1: aq. NH4Cl, Zn / ethanol 2: Py 3: acetic anhydride / 5 h / 95 - 100 °C 4: (i) NaOMe, MeOH, (ii) /BRN= 898057/, THF 5: (i) aq. NH4Cl, Zn, EtOH, (ii) /BRN= 385737/, Py 6: (i) H2, Pd-C, MeOH, (ii) /BRN= 898057/ 7: H2, aq. HCl / Pd-C / methanol View Scheme |
-
-
106410-46-2
N-(5-benzyloxycarbonylamino-pentyl)-N-hydroxy-succinamic acid

-
-
70-51-9
deferoxamine

| Conditions | Yield |
|---|---|
| Multi-step reaction with 3 steps 1: acetic anhydride / 5 h / 95 - 100 °C 2: (i) H2, Pd-C, MeOH, (ii) /BRN= 898057/ 3: H2, aq. HCl / Pd-C / methanol View Scheme | |
| Multi-step reaction with 5 steps 1: acetic anhydride / 5 h / 95 - 100 °C 2: (i) NaOMe, MeOH, (ii) /BRN= 898057/, THF 3: (i) aq. NH4Cl, Zn, EtOH, (ii) /BRN= 385737/, Py 4: (i) H2, Pd-C, MeOH, (ii) /BRN= 898057/ 5: H2, aq. HCl / Pd-C / methanol View Scheme |
-
-
95228-06-1
1-Carbobenzoxyamino-6-hydroxy-16-nitro-7,10-dioxo-6,11-diaza-hexadecan

-
-
70-51-9
deferoxamine

| Conditions | Yield |
|---|---|
| Multi-step reaction with 3 steps 1: (i) aq. NH4Cl, Zn, EtOH, (ii) /BRN= 385737/, Py 2: (i) H2, Pd-C, MeOH, (ii) /BRN= 898057/ 3: H2, aq. HCl / Pd-C / methanol View Scheme |
-
-
95748-46-2
N(1)-Benzyloxycarbonyl-6,17-dihydroxy-7,10,18-trioxo-6,11,17-triazanonadecylamin

-
-
70-51-9
deferoxamine

| Conditions | Yield |
|---|---|
| Multi-step reaction with 2 steps 1: (i) H2, Pd-C, MeOH, (ii) /BRN= 898057/ 2: H2, aq. HCl / Pd-C / methanol View Scheme |

-
-
70-51-9
deferoxamine

| Conditions | Yield |
|---|---|
| Multi-step reaction with 6 steps 1: aq. NaOH 2: aq. NH4Cl, Zn / ethanol 3: Py 4: acetic anhydride / 5 h / 95 - 100 °C 5: (i) H2, Pd-C, MeOH, (ii) /BRN= 898057/ 6: H2, aq. HCl / Pd-C / methanol View Scheme | |
| Multi-step reaction with 8 steps 1: aq. NaOH 2: aq. NH4Cl, Zn / ethanol 3: Py 4: acetic anhydride / 5 h / 95 - 100 °C 5: (i) NaOMe, MeOH, (ii) /BRN= 898057/, THF 6: (i) aq. NH4Cl, Zn, EtOH, (ii) /BRN= 385737/, Py 7: (i) H2, Pd-C, MeOH, (ii) /BRN= 898057/ 8: H2, aq. HCl / Pd-C / methanol View Scheme |
-
-
94622-86-3
[5-(3,6-dioxo-[1,2]oxazinan-2-yl)-pentyl]-carbamic acid benzyl ester

-
-
70-51-9
deferoxamine

| Conditions | Yield |
|---|---|
| Multi-step reaction with 2 steps 1: (i) H2, Pd-C, MeOH, (ii) /BRN= 898057/ 2: H2, aq. HCl / Pd-C / methanol View Scheme | |
| Multi-step reaction with 4 steps 1: (i) NaOMe, MeOH, (ii) /BRN= 898057/, THF 2: (i) aq. NH4Cl, Zn, EtOH, (ii) /BRN= 385737/, Py 3: (i) H2, Pd-C, MeOH, (ii) /BRN= 898057/ 4: H2, aq. HCl / Pd-C / methanol View Scheme |
-
-
144108-69-0
N-acetyl-N-hydroxy-1,5-diaminopentane

-
-
70-51-9
deferoxamine

| Conditions | Yield |
|---|---|
| With DesD Enzymatic reaction; |

| Conditions | Yield |
|---|---|
| Multi-step reaction with 6 steps 1: sodium hydride / N,N-dimethyl-formamide / 0 - 25 °C / Inert atmosphere 2: sodium azide / N,N-dimethyl-formamide / 3 h / 80 °C 3: trifluoroacetic acid / dichloromethane / 0.33 h / 0 - 20 °C 4: pyridine / 100 °C 5: 1-ethyl-(3-(3-dimethylamino)propyl)-carbodiimide hydrochloride; dmap / dichloromethane / 25 °C / Inert atmosphere 6: acetic acid; palladium 10% on activated carbon; hydrogen / methanol / 0.33 h / 25 °C / 760.05 Torr View Scheme | |
| Multi-step reaction with 8 steps 1.1: sodium hydride / N,N-dimethyl-formamide / 0 - 25 °C / Inert atmosphere 2.1: sodium azide / N,N-dimethyl-formamide / 3 h / 80 °C 3.1: trifluoroacetic acid / dichloromethane / 0.33 h / 0 - 20 °C 4.1: pyridine / 100 °C 5.1: 1-ethyl-(3-(3-dimethylamino)propyl)-carbodiimide hydrochloride; dmap / dichloromethane / 3 h / 25 °C / Inert atmosphere 6.1: triphenylphosphine / tetrahydrofuran / 1 h / 80 °C 6.2: 1 h / 80 °C 7.1: 1-ethyl-(3-(3-dimethylamino)propyl)-carbodiimide hydrochloride; dmap / dichloromethane / 25 °C / Inert atmosphere 8.1: acetic acid; palladium 10% on activated carbon; hydrogen / methanol / 0.33 h / 25 °C / 760.05 Torr View Scheme | |
| Multi-step reaction with 9 steps 1.1: sodium hydride / N,N-dimethyl-formamide / 0 - 25 °C / Inert atmosphere 2.1: sodium azide / N,N-dimethyl-formamide / 3 h / 80 °C 3.1: trifluoroacetic acid / dichloromethane / 0.33 h / 0 - 20 °C 4.1: dmap / dichloromethane / 0.25 h / 0 - 20 °C 5.1: triphenylphosphine / tetrahydrofuran / 1 h / 80 °C 5.2: 1 h / 80 °C 6.1: 1-ethyl-(3-(3-dimethylamino)propyl)-carbodiimide hydrochloride; dmap / dichloromethane / 3 h / 25 °C / Inert atmosphere 7.1: triphenylphosphine / tetrahydrofuran / 1 h / 80 °C 7.2: 1 h / 80 °C 8.1: 1-ethyl-(3-(3-dimethylamino)propyl)-carbodiimide hydrochloride; dmap / dichloromethane / 25 °C / Inert atmosphere 9.1: acetic acid; palladium 10% on activated carbon; hydrogen / methanol / 0.33 h / 25 °C / 760.05 Torr View Scheme |
-
-
83966-23-8
1-Amino-5-pentane

-
-
70-51-9
deferoxamine

| Conditions | Yield |
|---|---|
| Multi-step reaction with 4 steps 1.1: 1-ethyl-(3-(3-dimethylamino)propyl)-carbodiimide hydrochloride; dmap / dichloromethane / 3 h / 25 °C / Inert atmosphere 2.1: triphenylphosphine / tetrahydrofuran / 1 h / 80 °C 2.2: 1 h / 80 °C 3.1: 1-ethyl-(3-(3-dimethylamino)propyl)-carbodiimide hydrochloride; dmap / dichloromethane / 25 °C / Inert atmosphere 4.1: acetic acid; palladium 10% on activated carbon; hydrogen / methanol / 0.33 h / 25 °C / 760.05 Torr View Scheme |

-
-
70-51-9
deferoxamine

| Conditions | Yield |
|---|---|
| Multi-step reaction with 5 steps 1: sodium azide / N,N-dimethyl-formamide / 3 h / 80 °C 2: trifluoroacetic acid / dichloromethane / 0.33 h / 0 - 20 °C 3: pyridine / 100 °C 4: 1-ethyl-(3-(3-dimethylamino)propyl)-carbodiimide hydrochloride; dmap / dichloromethane / 25 °C / Inert atmosphere 5: acetic acid; palladium 10% on activated carbon; hydrogen / methanol / 0.33 h / 25 °C / 760.05 Torr View Scheme | |
| Multi-step reaction with 7 steps 1.1: sodium azide / N,N-dimethyl-formamide / 3 h / 80 °C 2.1: trifluoroacetic acid / dichloromethane / 0.33 h / 0 - 20 °C 3.1: pyridine / 100 °C 4.1: 1-ethyl-(3-(3-dimethylamino)propyl)-carbodiimide hydrochloride; dmap / dichloromethane / 3 h / 25 °C / Inert atmosphere 5.1: triphenylphosphine / tetrahydrofuran / 1 h / 80 °C 5.2: 1 h / 80 °C 6.1: 1-ethyl-(3-(3-dimethylamino)propyl)-carbodiimide hydrochloride; dmap / dichloromethane / 25 °C / Inert atmosphere 7.1: acetic acid; palladium 10% on activated carbon; hydrogen / methanol / 0.33 h / 25 °C / 760.05 Torr View Scheme | |
| Multi-step reaction with 8 steps 1.1: sodium azide / N,N-dimethyl-formamide / 3 h / 80 °C 2.1: trifluoroacetic acid / dichloromethane / 0.33 h / 0 - 20 °C 3.1: dmap / dichloromethane / 0.25 h / 0 - 20 °C 4.1: triphenylphosphine / tetrahydrofuran / 1 h / 80 °C 4.2: 1 h / 80 °C 5.1: 1-ethyl-(3-(3-dimethylamino)propyl)-carbodiimide hydrochloride; dmap / dichloromethane / 3 h / 25 °C / Inert atmosphere 6.1: triphenylphosphine / tetrahydrofuran / 1 h / 80 °C 6.2: 1 h / 80 °C 7.1: 1-ethyl-(3-(3-dimethylamino)propyl)-carbodiimide hydrochloride; dmap / dichloromethane / 25 °C / Inert atmosphere 8.1: acetic acid; palladium 10% on activated carbon; hydrogen / methanol / 0.33 h / 25 °C / 760.05 Torr View Scheme |

-
-
70-51-9
deferoxamine

| Conditions | Yield |
|---|---|
| Multi-step reaction with 4 steps 1: trifluoroacetic acid / dichloromethane / 0.33 h / 0 - 20 °C 2: pyridine / 100 °C 3: 1-ethyl-(3-(3-dimethylamino)propyl)-carbodiimide hydrochloride; dmap / dichloromethane / 25 °C / Inert atmosphere 4: acetic acid; palladium 10% on activated carbon; hydrogen / methanol / 0.33 h / 25 °C / 760.05 Torr View Scheme | |
| Multi-step reaction with 6 steps 1.1: trifluoroacetic acid / dichloromethane / 0.33 h / 0 - 20 °C 2.1: pyridine / 100 °C 3.1: 1-ethyl-(3-(3-dimethylamino)propyl)-carbodiimide hydrochloride; dmap / dichloromethane / 3 h / 25 °C / Inert atmosphere 4.1: triphenylphosphine / tetrahydrofuran / 1 h / 80 °C 4.2: 1 h / 80 °C 5.1: 1-ethyl-(3-(3-dimethylamino)propyl)-carbodiimide hydrochloride; dmap / dichloromethane / 25 °C / Inert atmosphere 6.1: acetic acid; palladium 10% on activated carbon; hydrogen / methanol / 0.33 h / 25 °C / 760.05 Torr View Scheme | |
| Multi-step reaction with 7 steps 1.1: trifluoroacetic acid / dichloromethane / 0.33 h / 0 - 20 °C 2.1: dmap / dichloromethane / 0.25 h / 0 - 20 °C 3.1: triphenylphosphine / tetrahydrofuran / 1 h / 80 °C 3.2: 1 h / 80 °C 4.1: 1-ethyl-(3-(3-dimethylamino)propyl)-carbodiimide hydrochloride; dmap / dichloromethane / 3 h / 25 °C / Inert atmosphere 5.1: triphenylphosphine / tetrahydrofuran / 1 h / 80 °C 5.2: 1 h / 80 °C 6.1: 1-ethyl-(3-(3-dimethylamino)propyl)-carbodiimide hydrochloride; dmap / dichloromethane / 25 °C / Inert atmosphere 7.1: acetic acid; palladium 10% on activated carbon; hydrogen / methanol / 0.33 h / 25 °C / 760.05 Torr View Scheme |

-
-
70-51-9
deferoxamine

| Conditions | Yield |
|---|---|
| Multi-step reaction with 5 steps 1.1: triphenylphosphine / tetrahydrofuran / 1 h / 80 °C 1.2: 1 h / 80 °C 2.1: 1-ethyl-(3-(3-dimethylamino)propyl)-carbodiimide hydrochloride; dmap / dichloromethane / 3 h / 25 °C / Inert atmosphere 3.1: triphenylphosphine / tetrahydrofuran / 1 h / 80 °C 3.2: 1 h / 80 °C 4.1: 1-ethyl-(3-(3-dimethylamino)propyl)-carbodiimide hydrochloride; dmap / dichloromethane / 25 °C / Inert atmosphere 5.1: acetic acid; palladium 10% on activated carbon; hydrogen / methanol / 0.33 h / 25 °C / 760.05 Torr View Scheme |

-
-
70-51-9
deferoxamine

| Conditions | Yield |
|---|---|
| Multi-step reaction with 3 steps 1.1: triphenylphosphine / tetrahydrofuran / 1 h / 80 °C 1.2: 1 h / 80 °C 2.1: 1-ethyl-(3-(3-dimethylamino)propyl)-carbodiimide hydrochloride; dmap / dichloromethane / 25 °C / Inert atmosphere 3.1: acetic acid; palladium 10% on activated carbon; hydrogen / methanol / 0.33 h / 25 °C / 760.05 Torr View Scheme |

-
-
112139-64-7
N-(5-aminopentyl)-3-<<5-<(benzyloxy)acetylamino>pentyl>carbamoyl>-O-benzylpropionohydroxamic acid

-
-
70-51-9
deferoxamine

| Conditions | Yield |
|---|---|
| Multi-step reaction with 2 steps 1: 1-ethyl-(3-(3-dimethylamino)propyl)-carbodiimide hydrochloride; dmap / dichloromethane / 25 °C / Inert atmosphere 2: acetic acid; palladium 10% on activated carbon; hydrogen / methanol / 0.33 h / 25 °C / 760.05 Torr View Scheme |

-
-
70-51-9
deferoxamine

| Conditions | Yield |
|---|---|
| Multi-step reaction with 3 steps 1: pyridine / 100 °C 2: 1-ethyl-(3-(3-dimethylamino)propyl)-carbodiimide hydrochloride; dmap / dichloromethane / 25 °C / Inert atmosphere 3: acetic acid; palladium 10% on activated carbon; hydrogen / methanol / 0.33 h / 25 °C / 760.05 Torr View Scheme | |
| Multi-step reaction with 5 steps 1.1: pyridine / 100 °C 2.1: 1-ethyl-(3-(3-dimethylamino)propyl)-carbodiimide hydrochloride; dmap / dichloromethane / 3 h / 25 °C / Inert atmosphere 3.1: triphenylphosphine / tetrahydrofuran / 1 h / 80 °C 3.2: 1 h / 80 °C 4.1: 1-ethyl-(3-(3-dimethylamino)propyl)-carbodiimide hydrochloride; dmap / dichloromethane / 25 °C / Inert atmosphere 5.1: acetic acid; palladium 10% on activated carbon; hydrogen / methanol / 0.33 h / 25 °C / 760.05 Torr View Scheme | |
| Multi-step reaction with 6 steps 1.1: dmap / dichloromethane / 0.25 h / 0 - 20 °C 2.1: triphenylphosphine / tetrahydrofuran / 1 h / 80 °C 2.2: 1 h / 80 °C 3.1: 1-ethyl-(3-(3-dimethylamino)propyl)-carbodiimide hydrochloride; dmap / dichloromethane / 3 h / 25 °C / Inert atmosphere 4.1: triphenylphosphine / tetrahydrofuran / 1 h / 80 °C 4.2: 1 h / 80 °C 5.1: 1-ethyl-(3-(3-dimethylamino)propyl)-carbodiimide hydrochloride; dmap / dichloromethane / 25 °C / Inert atmosphere 6.1: acetic acid; palladium 10% on activated carbon; hydrogen / methanol / 0.33 h / 25 °C / 760.05 Torr View Scheme |

-
-
70-51-9
deferoxamine

| Conditions | Yield |
|---|---|
| Multi-step reaction with 4 steps 1.1: 1-ethyl-(3-(3-dimethylamino)propyl)-carbodiimide hydrochloride; dmap / dichloromethane / 3 h / 25 °C / Inert atmosphere 2.1: triphenylphosphine / tetrahydrofuran / 1 h / 80 °C 2.2: 1 h / 80 °C 3.1: 1-ethyl-(3-(3-dimethylamino)propyl)-carbodiimide hydrochloride; dmap / dichloromethane / 25 °C / Inert atmosphere 4.1: acetic acid; palladium 10% on activated carbon; hydrogen / methanol / 0.33 h / 25 °C / 760.05 Torr View Scheme |

| Conditions | Yield |
|---|---|
| at 20℃; for 24h; | 100% |


| Conditions | Yield |
|---|---|
| With triethylamine In N,N-dimethyl-formamide for 15h; Heating; | 90% |
-
-
4044-65-9
1 ,4-phenylenediisothiocyanate

-
-
70-51-9
deferoxamine

-
-
1222468-90-7
N-[5-({3-[5-(acetyl-hydroxy-amino)-pentylcarbamoyl]-propionyl}-hydroxy-amino)-pentyl]-N'-hydroxy-N'-{5-[3-(4-isothiocyanato-phenyl)-thioureido]-pentyl}-succinamide

| Conditions | Yield |
|---|---|
| With triethylamine In chloroform; water; isopropyl alcohol at 20℃; for 1.5h; | 89% |

| Conditions | Yield |
|---|---|
| With triethylamine In methanol at 23℃; for 6h; Inert atmosphere; | 88% |

| Conditions | Yield |
|---|---|
| Stage #1: 3-(4-nitrophenyl)-1-adamantanecarboxylic acid With 1-hydroxy-pyrrolidine-2,5-dione; 1-ethyl-(3-(3-dimethylamino)propyl)-carbodiimide hydrochloride In N,N-dimethyl-formamide at 25℃; for 24h; Inert atmosphere; Stage #2: deferoxamine With sodium hydroxide In methanol at 70℃; for 3h; Inert atmosphere; | 71% |

| Conditions | Yield |
|---|---|
| Stage #1: 4-pentylbicyclo[2.2.2]octane-1-carboxylic acid With 1-hydroxy-pyrrolidine-2,5-dione; 1-ethyl-(3-(3-dimethylamino)propyl)-carbodiimide hydrochloride In N,N-dimethyl-formamide at 25℃; for 24h; Inert atmosphere; Stage #2: deferoxamine With sodium hydroxide In methanol at 70℃; for 3h; Inert atmosphere; | 66% |

| Conditions | Yield |
|---|---|
| Stage #1: C22H23NO7 With benzotriazol-1-yloxyl-tris-(pyrrolidino)-phosphonium hexafluorophosphate In N,N-dimethyl-formamide for 0.166667h; Cooling with ice; Stage #2: deferoxamine With 4-methyl-morpholine; potassium hydroxide In water; N,N-dimethyl-formamide at 20℃; for 96h; | 58% |

| Conditions | Yield |
|---|---|
| Stage #1: 3-bromoadamantane-1-carboxylic acid With 1-hydroxy-pyrrolidine-2,5-dione; 1-ethyl-(3-(3-dimethylamino)propyl)-carbodiimide hydrochloride In N,N-dimethyl-formamide at 25℃; for 24h; Inert atmosphere; Stage #2: deferoxamine With sodium hydroxide In methanol at 70℃; for 3h; Inert atmosphere; | 52% |

| Conditions | Yield |
|---|---|
| Stage #1: 3-chloroadamantane-1-carboxylic acid With 1-hydroxy-pyrrolidine-2,5-dione; 1-ethyl-(3-(3-dimethylamino)propyl)-carbodiimide hydrochloride In N,N-dimethyl-formamide at 25℃; for 24h; Inert atmosphere; Stage #2: deferoxamine With sodium hydroxide In methanol at 70℃; for 3h; Inert atmosphere; | 47% |
-
-
70-51-9
deferoxamine

| Conditions | Yield |
|---|---|
| With sodium hydroxide In methanol at 70℃; for 3h; | 44% |

| Conditions | Yield |
|---|---|
| Stage #1: (3-bromo-1-adamantyl)acetic acid With 1-hydroxy-pyrrolidine-2,5-dione; 1-ethyl-(3-(3-dimethylamino)propyl)-carbodiimide hydrochloride In N,N-dimethyl-formamide at 25℃; for 24h; Inert atmosphere; Stage #2: deferoxamine With sodium hydroxide In methanol at 70℃; for 3h; Inert atmosphere; | 35% |

| Conditions | Yield |
|---|---|
| Stage #1: (3-hydroxyadamantan-1-yl)acetic acid With 1-hydroxy-pyrrolidine-2,5-dione; 1-ethyl-(3-(3-dimethylamino)propyl)-carbodiimide hydrochloride In N,N-dimethyl-formamide at 25℃; for 24h; Inert atmosphere; Stage #2: deferoxamine With sodium hydroxide In methanol at 70℃; for 3h; Inert atmosphere; | 34% |

| Conditions | Yield |
|---|---|
| Stage #1: acide dimethyl-3,5 adamantylacetique With 1-hydroxy-pyrrolidine-2,5-dione; 1-ethyl-(3-(3-dimethylamino)propyl)-carbodiimide hydrochloride In N,N-dimethyl-formamide at 25℃; for 24h; Inert atmosphere; Stage #2: deferoxamine With sodium hydroxide In methanol at 70℃; for 3h; Inert atmosphere; | 28% |

| Conditions | Yield |
|---|---|
| Stage #1: 3,5,7-trimethyladamantanecarboxylic acid With 1-hydroxy-pyrrolidine-2,5-dione; 1-ethyl-(3-(3-dimethylamino)propyl)-carbodiimide hydrochloride In N,N-dimethyl-formamide at 25℃; for 24h; Inert atmosphere; Stage #2: deferoxamine With sodium hydroxide In methanol at 70℃; for 3h; Inert atmosphere; | 27% |

| Conditions | Yield |
|---|---|
| Stage #1: 3,5-dimethyl-1-adamantanecarboxylic acid With 1-hydroxy-pyrrolidine-2,5-dione; 1-ethyl-(3-(3-dimethylamino)propyl)-carbodiimide hydrochloride In N,N-dimethyl-formamide at 25℃; for 24h; Inert atmosphere; Stage #2: deferoxamine With sodium hydroxide In methanol at 70℃; for 3h; Inert atmosphere; | 24% |

| Conditions | Yield |
|---|---|
| Stage #1: 1-Adamantanecarboxylic acid With 1-hydroxy-pyrrolidine-2,5-dione; 1-ethyl-(3-(3-dimethylamino)propyl)-carbodiimide hydrochloride In N,N-dimethyl-formamide at 25℃; for 24h; Inert atmosphere; Stage #2: deferoxamine With sodium hydroxide In methanol at 70℃; for 3h; Inert atmosphere; | 22% |
-
-
70-51-9
deferoxamine


| Conditions | Yield |
|---|---|
| In tetrahydrofuran; aq. phosphate buffer at 20℃; for 14h; pH=8; Inert atmosphere; | 21% |

| Conditions | Yield |
|---|---|
| Stage #1: Cyclohexanecarboxylic acid With 1-hydroxy-pyrrolidine-2,5-dione; 1-ethyl-(3-(3-dimethylamino)propyl)-carbodiimide hydrochloride In N,N-dimethyl-formamide at 25℃; for 24h; Inert atmosphere; Stage #2: deferoxamine With sodium hydroxide In methanol at 70℃; for 3h; Inert atmosphere; | 21% |

| Conditions | Yield |
|---|---|
| With iron(III) chloride In chloroform for 0.5h; pH=8.5; | 20% |

| Conditions | Yield |
|---|---|
| Stage #1: 3-noradamantanecarboxylic acid With 1-hydroxy-pyrrolidine-2,5-dione; 1-ethyl-(3-(3-dimethylamino)propyl)-carbodiimide hydrochloride In N,N-dimethyl-formamide at 25℃; for 24h; Inert atmosphere; Stage #2: deferoxamine With sodium hydroxide In methanol at 70℃; for 3h; Inert atmosphere; | 19% |

| Conditions | Yield |
|---|---|
| Stage #1: endo-bicyclo<3,3,1>nonan-3-carboxylic acid With 1-hydroxy-pyrrolidine-2,5-dione; 1-ethyl-(3-(3-dimethylamino)propyl)-carbodiimide hydrochloride In N,N-dimethyl-formamide at 25℃; for 24h; Inert atmosphere; Stage #2: deferoxamine With sodium hydroxide In methanol at 70℃; for 3h; Inert atmosphere; | 17% |
-
-
70-51-9
deferoxamine

-
-
120-74-1, 934-30-5, 1195-12-6, 20507-53-3, 58001-99-3, 67999-50-2, 74645-32-2, 108266-75-7, 67999-53-5
(+)-(1R,4R,5S)-bicyclo<2.2.1>hept-2-ene-5-carboxylic acid

| Conditions | Yield |
|---|---|
| Stage #1: (+)-(1R,4R,5S)-bicyclo<2.2.1>hept-2-ene-5-carboxylic acid With 1-hydroxy-pyrrolidine-2,5-dione; 1-ethyl-(3-(3-dimethylamino)propyl)-carbodiimide hydrochloride In N,N-dimethyl-formamide at 25℃; for 24h; Inert atmosphere; Stage #2: deferoxamine With sodium hydroxide In methanol at 70℃; for 3h; Inert atmosphere; | 17% |
-
-
70-51-9
deferoxamine

| Conditions | Yield |
|---|---|
| With N-ethyl-N,N-diisopropylamine In N,N-dimethyl-formamide at 40 - 45℃; for 7h; Inert atmosphere; | 15% |
-
-
70-51-9
deferoxamine

| Conditions | Yield |
|---|---|
| Stage #1: 11-hydroxy-2,2-dimethyl-4,12-dioxo-3,8-dioxa-5,11-diazapentadecan-15-oic acid With di(succinimido) carbonate; triethylamine In N,N-dimethyl-formamide for 4h; Inert atmosphere; Stage #2: deferoxamine In N,N-dimethyl-formamide Inert atmosphere; Stage #3: With trifluoroacetic acid In dichloromethane for 2h; Inert atmosphere; | 12% |

| Conditions | Yield |
|---|---|
| Stage #1: (1R,4R)-bicyclo[2.2.1]hept-5-ene-2-carboxylic acid With 1-hydroxy-pyrrolidine-2,5-dione; 1-ethyl-(3-(3-dimethylamino)propyl)-carbodiimide hydrochloride In N,N-dimethyl-formamide at 25℃; for 24h; Inert atmosphere; Stage #2: deferoxamine With sodium hydroxide In methanol at 70℃; for 3h; Inert atmosphere; | 11% |

| Conditions | Yield |
|---|---|
| Stage #1: di(succinimido) carbonate; methyl (1S,4E,6R)-6-{[(4-{[(2-{2-[2-(2,5-dioxo-2,5-dihydro-1H-pyrrol-1-yl)ethoxy]ethoxy}ethyl)carbamoyl] (hydroxy)methyl}phenyl)(methyl)carbamoyl]oxy}-1-hydroxycyclooct-4-ene-1-carboxylate With N-ethyl-N,N-diisopropylamine In dimethyl sulfoxide at 20℃; for 96h; Stage #2: deferoxamine In dimethyl sulfoxide at 20℃; for 1h; | 11% |


| Conditions | Yield |
|---|---|
| Stage #1: bicyclo<2.2.1>heptane-2-carboxylic acid With 1-hydroxy-pyrrolidine-2,5-dione; 1-ethyl-(3-(3-dimethylamino)propyl)-carbodiimide hydrochloride In N,N-dimethyl-formamide at 25℃; for 24h; Inert atmosphere; Stage #2: deferoxamine With sodium hydroxide In methanol at 70℃; for 3h; Inert atmosphere; | 8% |

| Conditions | Yield |
|---|---|
| Stage #1: 2-((1S,4R)-bicyclo[2.2.1]heptan-2-yl)acetic acid With 1-hydroxy-pyrrolidine-2,5-dione; 1-ethyl-(3-(3-dimethylamino)propyl)-carbodiimide hydrochloride In N,N-dimethyl-formamide at 25℃; for 24h; Inert atmosphere; Stage #2: deferoxamine With sodium hydroxide In methanol at 70℃; for 3h; Inert atmosphere; | 8% |
Deferoxamine Chemical Properties
Molecular Formula: C25H48N6O8
Molar mass: 560.684 g/mol
EINECS: 200-738-5
Density: 1.212 g/cm3
Index of Refraction: 1.537
Structure of Deferoxamine (CAS NO.70-51-9):
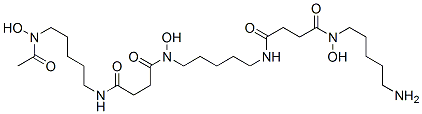
Deferoxamine Uses
Deferoxamine (CAS NO.70-51-9) is used to treat acute iron poisoning, especially in small children and is also frequently used to treat hemochromatosis, a disease of iron accumulation that can be either genetic or acquired. Another, it has also been used in the treatment of a patient with aceruloplasminemia.
Deferoxamine Toxicity Data With Reference
| Organism | Test Type | Route | Reported Dose (Normalized Dose) | Effect | Source |
|---|---|---|---|---|---|
| child | TDLo | multiple routes | 440mg/kg/6D-I (440mg/kg) | SENSE ORGANS AND SPECIAL SENSES: "RETINAL CHANGES (PIGMENTARY DEPOSITIONS, RETINITIS, OTHER): EYE" | Archives of Disease in Childhood. Vol. 63, Pg. 250, 1988. |
| child | TDLo | subcutaneous | 12gm/kg/17W-I (12000mg/kg) | SENSE ORGANS AND SPECIAL SENSES: OPTIC NERVE NEUROPATHY: EYE SENSE ORGANS AND SPECIAL SENSES: CHANGE IN ACUITY: EAR | New England Journal of Medicine. Vol. 314, Pg. 869, 1986. |
| human | TDLo | subcutaneous | 37gm/kg/2Y-I (37000mg/kg) | SENSE ORGANS AND SPECIAL SENSES: OPTIC NERVE NEUROPATHY: EYE | New England Journal of Medicine. Vol. 314, Pg. 869, 1986. |
| man | TDLo | intravenous | 86mg/kg/1H-C (86mg/kg) | BLOOD: THROMBOCYTOPENIA | American Journal of Kidney Diseases. Vol. 6, Pg. 254, 1985. |
| mouse | LD50 | intraperitoneal | 1680mg/kg (1680mg/kg) | Kiso to Rinsho. Clinical Report. Vol. 4, Pg. 99, 1970. | |
| mouse | LD50 | intravenous | 250mg/kg (250mg/kg) | LUNGS, THORAX, OR RESPIRATION: DYSPNEA | United States Patent Document. Vol. #4863964, |
| mouse | LD50 | oral | 1340mg/kg (1340mg/kg) | Arzneimittel-Forschung. Drug Research. Vol. 17, Pg. 748, 1967. | |
| mouse | LD50 | subcutaneous | 1450mg/kg (1450mg/kg) | Iyakuhin Kenkyu. Study of Medical Supplies. Vol. 6, Pg. 119, 1975. | |
| rat | LD50 | intravenous | 329mg/kg (329mg/kg) | Toxicology and Applied Pharmacology. Vol. 18, Pg. 185, 1971. | |
| rat | LD50 | subcutaneous | 12240mg/kg (12240mg/kg) | Iyakuhin Kenkyu. Study of Medical Supplies. Vol. 6, Pg. 119, 1975. | |
| women | TDLo | intravenous | 40mg/kg (40mg/kg) | SENSE ORGANS AND SPECIAL SENSES: VISUAL FIELD CHANGES: EYE SENSE ORGANS AND SPECIAL SENSES: HEMORRHAGE: EYE | Nephron. Vol. 46, Pg. 211, 1987. |
Deferoxamine Safety Profile
Poison by intravenous route. Moderately toxic by ingestion, intraperitoneal and subcutaneous routes. Human systemic effects: changes in hearing acuity, eye hemorrhage, optic nerve neuropathy, thrombocytopenia, visual field changes. Human mutation data reported. When heated to decomposition it emits toxic fumes of NOx.
Deferoxamine Specification
Deferoxamine ,its cas register number is 70-51-9. It also can be called Desferrioxamine B ; Desferoxamine B ; DFO-B ; DFOA ; DFB or Desferal . It acts by binding free iron in the bloodstream and enhancing its elimination in the urine. Deferoxamine (CAS NO.70-51-9) may modulate expression and release of inflammatory mediators by specific cell types. A recent study shows that it speeds healing of nerve damage. Its administration after acute intoxication may color the urine a pinkish red, a phenomenon termed "vin rose urine".
Related Products
- Deferoxamine
- Deferoxamine mesylate
- 70519-79-8
- 705-21-5
- 70521-71-0
- 70521-73-2
- 70521-74-3
- 70521-75-4
- 70522-05-3
- 70523-22-7
- 70523-24-9
- 705-24-8
Hot Products
About|Contact|Cas|Product Name|Molecular|Country|Encyclopedia
Message|New Cas|MSDS|Service|Advertisement|CAS DataBase|Article Data|Manufacturers | Chemical Catalog
©2008 LookChem.com,License: ICP
NO.:Zhejiang16009103
complaints:service@lookchem.com Desktop View