-
Name
Diclofenac sodium
- EINECS 239-346-4
- CAS No. 15307-79-6
- Article Data20
- CAS DataBase
- Density 0.781 g/cm3
- Solubility Water solubility: 50 mg/mL
- Melting Point 288-290 °C
- Formula C14H10Cl2NNaO2
- Boiling Point 412 °C at 760 mmHg
- Molecular Weight 318.135
- Flash Point 203 °C
- Transport Information UN 2811 6.1/PG 3
- Appearance white or off-white powder
- Safety 22-36/37-45
- Risk Codes 25
-
Molecular Structure
-
Hazard Symbols
 T,
T, Xi
Xi
- Synonyms Neriodin;Dichronic;Hyanalgese D;Diclofenac sodium [USAN:JAN];Solaraze;Sodium (2-((2,6-dichlorophenyl)amino)phenyl)acetate;Voltaren;Sodium (o-(2,6-dichloroanilino)phenyl)acetate;Naclof;
- PSA 52.16000
- LogP 3.10240
Synthetic route
-
-
15307-78-5
2-[(2,6-dichlorophenyl)amino]phenylacetic acid methyl ester

-
-
15307-79-6
diclofenac sodium

| Conditions | Yield |
|---|---|
| With sodium hydroxide In water; N,N-dimethyl-formamide at 80℃; for 24h; | 96.1% |
-
-
15362-40-0
1-(2,6-dichlorophenyl)indolin-2-one

-
-
15307-79-6
diclofenac sodium

| Conditions | Yield |
|---|---|
| With sodium hydroxide In ethanol; water for 4h; Heating; | 93% |
| With sodium dithionite; water; sodium hydroxide for 6h; Reflux; | 92.3% |
| With sodium hydroxide In ethanol for 3h; Heating; | 85% |
| With N-benzyl-N,N,N-triethylammonium chloride; sodium hydroxide In toluene for 10h; Reflux; | 83.6% |
| With sodium hydroxide for 2h; Temperature; Reflux; | 4.035 g |

-
-
15307-79-6
diclofenac sodium

| Conditions | Yield |
|---|---|
| With water; sodium hydroxide In methanol for 6h; Solvent; Reflux; | 86% |

| Conditions | Yield |
|---|---|
| With 1-methyl-pyrrolidin-2-one; copper; potassium carbonate at 120℃; for 22h; | 56% |
-
-
2444-36-2
2'-chloro-benzeneacetic acid

-
-
608-31-1
2,6-Dichloroaniline

-
-
71-36-3
butan-1-ol

-
-
15307-79-6
diclofenac sodium

| Conditions | Yield |
|---|---|
| With sodium hydroxide; potassium iodide; copper(I) iodide; potassium carbonate In N,N-dimethyl-formamide | 42.9% |


| Conditions | Yield |
|---|---|
| Stage #1: 2'-chloro-benzeneacetic acid; 2,6-Dichloroaniline With copper(l) iodide; iodine; potassium carbonate; potassium iodide In N,N-dimethyl-formamide for 12h; Inert atmosphere; Schlenk technique; Reflux; Stage #2: With sodium hydroxide In water for 4h; Reflux; | 31% |
-
-
66156-75-0
2-(2-((2,6-dichlorophenyl)amino)phenyl)-2-oxoacetic acid

-
-
15307-79-6
diclofenac sodium

| Conditions | Yield |
|---|---|
| With sodium methylate; hydrazine hydrate In 2-methoxy-ethanol 1.) from 60 deg C to 150 deg C, 2.) 150 deg C, 1 h; Yield given; |
-
-
15307-93-4
N-phenyl-2,6-dichloroaniline

-
-
15307-79-6
diclofenac sodium

| Conditions | Yield |
|---|---|
| Multi-step reaction with 5 steps 1: benzene / 6 h / Heating 2: 91 percent / m-chloroperbenzoic acid / CH2Cl2 / 1 h / Ambient temperature 3: 84 percent / p-toluenesulfonic acid / benzene / 0.5 h / Heating 4: 97 percent / Zn / acetic acid / 4 h / Heating 5: 85 percent / 20percent NaOH / ethanol / 3 h / Heating View Scheme | |
| Multi-step reaction with 5 steps 1: benzene / 6 h / Heating 2: N-chlorosuccinimide / CCl4 / 1 h / Ambient temperature 3: SnCl4 / CCl4 / 0.83 h / Ambient temperature 4: 97 percent / Zn / acetic acid / 4 h / Heating 5: 85 percent / 20percent NaOH / ethanol / 3 h / Heating View Scheme | |
| Multi-step reaction with 4 steps 1: benzene / 2 h / Ambient temperature 2: AlCl3 / various solvent(s) / 20 h / Ambient temperature 3: 1 N NaOH / ethanol; H2O / 0.17 h / Heating 4: hydrazine hydrate, NaOCH3 / 2-methoxy-ethanol / 1.) from 60 deg C to 150 deg C, 2.) 150 deg C, 1 h View Scheme | |
| Multi-step reaction with 3 steps 1: 66 percent / 16 h / Heating 2: 75.4 percent / AlCl3 / 2 h / 160 °C 3: 93 percent / 2 N NaOH / ethanol; H2O / 4 h / Heating View Scheme | |
| Multi-step reaction with 3 steps 1: toluene / 3 h / 120 °C 2: triethylamine hydrochloride-aluminum trichloride / 4.5 h / 20 - 60 °C 3: sodium hydroxide / 2 h / Reflux View Scheme |
-
-
83281-93-0
N-(2,6-dichlorophenyl)-α-(methylthio)acetanilide

-
-
15307-79-6
diclofenac sodium

| Conditions | Yield |
|---|---|
| Multi-step reaction with 4 steps 1: 91 percent / m-chloroperbenzoic acid / CH2Cl2 / 1 h / Ambient temperature 2: 84 percent / p-toluenesulfonic acid / benzene / 0.5 h / Heating 3: 97 percent / Zn / acetic acid / 4 h / Heating 4: 85 percent / 20percent NaOH / ethanol / 3 h / Heating View Scheme | |
| Multi-step reaction with 4 steps 1: N-chlorosuccinimide / CCl4 / 1 h / Ambient temperature 2: SnCl4 / CCl4 / 0.83 h / Ambient temperature 3: 97 percent / Zn / acetic acid / 4 h / Heating 4: 85 percent / 20percent NaOH / ethanol / 3 h / Heating View Scheme |
-
-
83281-94-1
1-(2,6-dichlorophenyl)-3-(methylthio)oxindole

-
-
15307-79-6
diclofenac sodium

| Conditions | Yield |
|---|---|
| Multi-step reaction with 2 steps 1: 97 percent / Zn / acetic acid / 4 h / Heating 2: 85 percent / 20percent NaOH / ethanol / 3 h / Heating View Scheme |
-
-
83281-96-3
N-(2,6-dichlorophenyl)-α-(methylsulfinyl)acetanilide

-
-
15307-79-6
diclofenac sodium

| Conditions | Yield |
|---|---|
| Multi-step reaction with 3 steps 1: 84 percent / p-toluenesulfonic acid / benzene / 0.5 h / Heating 2: 97 percent / Zn / acetic acid / 4 h / Heating 3: 85 percent / 20percent NaOH / ethanol / 3 h / Heating View Scheme |

-
-
15307-79-6
diclofenac sodium

| Conditions | Yield |
|---|---|
| Multi-step reaction with 3 steps 1: SnCl4 / CCl4 / 0.83 h / Ambient temperature 2: 97 percent / Zn / acetic acid / 4 h / Heating 3: 85 percent / 20percent NaOH / ethanol / 3 h / Heating View Scheme |
-
-
24542-74-3
1-(2,6-Dichlorphenyl)-2,3-indoldion

-
-
15307-79-6
diclofenac sodium

| Conditions | Yield |
|---|---|
| Multi-step reaction with 2 steps 1: 1 N NaOH / ethanol; H2O / 0.17 h / Heating 2: hydrazine hydrate, NaOCH3 / 2-methoxy-ethanol / 1.) from 60 deg C to 150 deg C, 2.) 150 deg C, 1 h View Scheme |
-
-
24542-55-0
[(2,6-Dichloro-phenyl)-phenyl-amino]-oxo-acetyl chloride

-
-
15307-79-6
diclofenac sodium

| Conditions | Yield |
|---|---|
| Multi-step reaction with 3 steps 1: AlCl3 / various solvent(s) / 20 h / Ambient temperature 2: 1 N NaOH / ethanol; H2O / 0.17 h / Heating 3: hydrazine hydrate, NaOCH3 / 2-methoxy-ethanol / 1.) from 60 deg C to 150 deg C, 2.) 150 deg C, 1 h View Scheme |
-
-
15308-01-7
2-chloro-N-(2',6'-dichlorophenyl)-N-phenylacetamide

-
-
15307-79-6
diclofenac sodium

| Conditions | Yield |
|---|---|
| Multi-step reaction with 2 steps 1: 75.4 percent / AlCl3 / 2 h / 160 °C 2: 93 percent / 2 N NaOH / ethanol; H2O / 4 h / Heating View Scheme |

-
-
15307-79-6
diclofenac sodium

| Conditions | Yield |
|---|---|
| Multi-step reaction with 3 steps 1: tert-butylhypochlorite / dichloromethane / 10 h / 25 °C / Cooling with ice 2: aluminum (III) chloride / dichloromethane / 12 h / 20 °C / Cooling with ice 3: N-benzyl-N,N,N-triethylammonium chloride; sodium hydroxide / toluene / 10 h / Reflux View Scheme |
-
-
608-31-1
2,6-Dichloroaniline

-
-
15307-79-6
diclofenac sodium

| Conditions | Yield |
|---|---|
| Multi-step reaction with 4 steps 1: calcium chloride; pyridine / 4 h / Reflux; Cooling with ice 2: tert-butylhypochlorite / dichloromethane / 10 h / 25 °C / Cooling with ice 3: aluminum (III) chloride / dichloromethane / 12 h / 20 °C / Cooling with ice 4: N-benzyl-N,N,N-triethylammonium chloride; sodium hydroxide / toluene / 10 h / Reflux View Scheme |
-
-
103-80-0
phenylacetyl chloride

-
-
15307-79-6
diclofenac sodium

| Conditions | Yield |
|---|---|
| Multi-step reaction with 4 steps 1: calcium chloride; pyridine / 4 h / Reflux; Cooling with ice 2: tert-butylhypochlorite / dichloromethane / 10 h / 25 °C / Cooling with ice 3: aluminum (III) chloride / dichloromethane / 12 h / 20 °C / Cooling with ice 4: N-benzyl-N,N,N-triethylammonium chloride; sodium hydroxide / toluene / 10 h / Reflux View Scheme |
-
-
3559-26-0
(2-benzoylamino-phenyl)-acetic acid methyl ester

-
-
15307-79-6
diclofenac sodium

| Conditions | Yield |
|---|---|
| Multi-step reaction with 3 steps 1.1: thionyl chloride / 3 h / Reflux 1.2: 3 h / Cooling with ice; Reflux 2.1: diphenylether / 3 h / Reflux 3.1: sodium hydroxide; water / methanol / 6 h / Reflux View Scheme |
-
-
30095-98-8
methyl (2-nitrophenyl)acetate

-
-
15307-79-6
diclofenac sodium

| Conditions | Yield |
|---|---|
| Multi-step reaction with 5 steps 1.1: palladium on activated charcoal; hydrogen / toluene / 5 °C / 760.05 Torr 2.1: triethylamine / toluene / 2 h / 0 °C 3.1: thionyl chloride / 3 h / Reflux 3.2: 3 h / Cooling with ice; Reflux 4.1: diphenylether / 3 h / Reflux 5.1: sodium hydroxide; water / methanol / 6 h / Reflux View Scheme |
-
-
101-41-7
benzeneacetic acid methyl ester

-
-
15307-79-6
diclofenac sodium

| Conditions | Yield |
|---|---|
| Multi-step reaction with 6 steps 1.1: nitric acid / dichloromethane / 24 h / 0 - 20 °C 2.1: palladium on activated charcoal; hydrogen / toluene / 5 °C / 760.05 Torr 3.1: triethylamine / toluene / 2 h / 0 °C 4.1: thionyl chloride / 3 h / Reflux 4.2: 3 h / Cooling with ice; Reflux 5.1: diphenylether / 3 h / Reflux 6.1: sodium hydroxide; water / methanol / 6 h / Reflux View Scheme |
-
-
35613-44-6
2-aminophenylacetic acid methyl ester

-
-
15307-79-6
diclofenac sodium

| Conditions | Yield |
|---|---|
| Multi-step reaction with 4 steps 1.1: triethylamine / toluene / 2 h / 0 °C 2.1: thionyl chloride / 3 h / Reflux 2.2: 3 h / Cooling with ice; Reflux 3.1: diphenylether / 3 h / Reflux 4.1: sodium hydroxide; water / methanol / 6 h / Reflux View Scheme |
-
-
7173-51-5
didecyldimethylammonium chloride

-
-
15307-79-6
diclofenac sodium

-
-
934544-42-0
diclofenac didecyldimethylammonium salt

| Conditions | Yield |
|---|---|
| In water at 20℃; for 0.5h; Product distribution / selectivity; | 100% |
-
-
15307-79-6
diclofenac sodium

-
-
10108-64-2
cadmium(II) chloride

-
-
149753-43-5
{Cd(C6H3Cl2NHC6H4CH2COO)2(H2O)}

| Conditions | Yield |
|---|---|
| With H2O; KOH In water byproducts: NaCl; mixing aq. soln. of the components, adjusting to pH 5.5-7.5 with KOH and stirring at room temp. for 4-6 h; filtn., washing with water, drying over silica gel (vac.) and over P4O10 at 90°C (vac.); | 100% |
-
-
15307-79-6
diclofenac sodium

-
-
7646-85-7
zinc(II) chloride

-
-
149753-42-4
{Zn(C6H3Cl2NHC6H4CH2COO)2(H2O)}

| Conditions | Yield |
|---|---|
| With H2O; KOH In water byproducts: NaCl; mixing aq. soln. of the components, adjusting to pH 5.5-7.5 with KOH and stirring at room temp. for 4-6 h; filtn., washing with water, drying over silica gel (vac.) and over P4O10 at 90°C (vac.); | 100% |
-
-
15307-79-6
diclofenac sodium

-
-
15307-86-5
[2-(2,6-dichloroanilino)phenyl]acetic acid

| Conditions | Yield |
|---|---|
| With hydrogenchloride In water; acetone at 20 - 45℃; for 3h; pH=1.4 - 1.6; Solvent; | 99% |
| With hydrogenchloride In water Cooling with ice; | 90% |
| With hydrogenchloride In water at 0 - 20℃; | 70% |
-
-
15307-79-6
diclofenac sodium

-
-
1401311-49-6
1-(2,6-dichlorophenyl)-1,3-dihydro-indol-2-one-[3,3,5,7-D4]

| Conditions | Yield |
|---|---|
| With water-d2; hydrogen chloride at 160℃; for 0.333333h; Microwave irradiation; | 96% |
-
-
67-48-1
choline chloride

-
-
15307-79-6
diclofenac sodium

-
-
148439-51-4
(2-hydroxyethyl)tri-methylammonium [o-(2,6-dichloroanilino)phenyl]acetate

| Conditions | Yield |
|---|---|
| In ethanol at 20℃; for 1h; | 96% |
-
-
15307-79-6
diclofenac sodium

-
-
572-09-8
2,3,4,6-tetra-O-acetyl-α-D-glucopyranosyl bromide

-
-
87906-12-5
1-O-<2-<(2,6-dichlorophenyl)amino>phenylacetyl>-2,3,4,6-tetra-O-acetyl-β-D-glucopyranose

| Conditions | Yield |
|---|---|
| citrimide In water; 1,2-dichloro-ethane Ambient temperature; | 95% |
-
-
5292-43-3
bromoacetic acid tert-butyl ester

-
-
15307-79-6
diclofenac sodium

-
-
139272-68-7
2-((2,6-dichlorophenyl)amino)phenylacetoxyacetic acid tert-butyl ester

| Conditions | Yield |
|---|---|
| With potassium iodide In ethanol at 61 - 65℃; for 3h; Temperature; Large scale; | 94.45% |
| With potassium iodide In acetonitrile at 30℃; for 0.25h; Microwave irradiation; | 91.6% |
| With tetra(n-butyl)ammonium hydrogensulfate; potassium iodide In water; acetone at 60 - 65℃; for 2h; | 17.3 g |
| In N,N-dimethyl-formamide at 20 - 45℃; |
-
-
3068-88-0, 32082-74-9, 36536-46-6, 65058-82-4
4-methyloxetan-2-one

-
-
15307-79-6
diclofenac sodium

| Conditions | Yield |
|---|---|
| In dimethyl sulfoxide at 20℃; | 94% |
-
-
15307-79-6
diclofenac sodium

| Conditions | Yield |
|---|---|
| With trifluoroacetic acid-d1 at 110℃; for 16h; Sealed tube; | 94% |
-
-
553-26-4
4,4'-bipyridine

-
-
15307-79-6
diclofenac sodium

| Conditions | Yield |
|---|---|
| In water; acetonitrile at 50℃; | 94% |

-
-
15307-79-6
diclofenac sodium

-
-
169614-77-1
3-(bromomethyl)-4-methyl-1,2,5-oxadiazole-2-oxide

| Conditions | Yield |
|---|---|
| In N,N-dimethyl-formamide at 20 - 25℃; for 2h; | 93% |
-
-
103-74-2
2-(2-Hydroxyethyl)pyridine

-
-
15307-79-6
diclofenac sodium

-
-
6046-93-1
copper(II) acetate monohydrate

| Conditions | Yield |
|---|---|
| In methanol at 50℃; | 91% |

-
-
15307-79-6
diclofenac sodium

-
-
2390-68-3
N,N-didecyl-N,N-dimethylammonium bromide

-
-
934544-42-0
diclofenac didecyldimethylammonium salt

| Conditions | Yield |
|---|---|
| With sodium hydrogencarbonate; sodium hydroxide In methanol; water at 20℃; for 0.5h; | 91% |
-
-
15307-79-6
diclofenac sodium

-
-
74-88-4
methyl iodide

-
-
15307-78-5
2-[(2,6-dichlorophenyl)amino]phenylacetic acid methyl ester

| Conditions | Yield |
|---|---|
| In N,N-dimethyl-formamide | 90% |
| In acetone Heating; |

| Conditions | Yield |
|---|---|
| In water at 20℃; | 90% |
-
-
107-59-5
tert-Butyl chloroacetate

-
-
15307-79-6
diclofenac sodium

-
-
139272-68-7
2-((2,6-dichlorophenyl)amino)phenylacetoxyacetic acid tert-butyl ester

| Conditions | Yield |
|---|---|
| With sodium iodide In acetonitrile at 77 - 78℃; for 2h; | 89.6% |
| In acetonitrile for 4h; Reflux; | |
| With potassium iodide In N,N-dimethyl-formamide for 2h; Reflux; Large scale; | 25.5 g |
-
-
15307-79-6
diclofenac sodium

| Conditions | Yield |
|---|---|
| In N,N-dimethyl-formamide at 20 - 25℃; for 2h; | 89% |
-
-
15307-79-6
diclofenac sodium

-
-
175609-21-9
5-(bromomethyl)benzofuroxan

-
-
883738-54-3
given name is incorrect

| Conditions | Yield |
|---|---|
| With N-Bromosuccinimide; dibenzoyl peroxide In N,N-dimethyl-formamide at 25℃; for 2h; | 89% |
-
-
103-74-2
2-(2-Hydroxyethyl)pyridine

-
-
15307-79-6
diclofenac sodium

| Conditions | Yield |
|---|---|
| In methanol at 50℃; | 89% |

-
-
67-56-1
methanol

-
-
15307-79-6
diclofenac sodium

| Conditions | Yield |
|---|---|
| In water at 20℃; | 89% |

-
-
288-32-4
1H-imidazole

-
-
15307-79-6
diclofenac sodium

| Conditions | Yield |
|---|---|
| In methanol; water at 20℃; | 89% |

-
-
934544-25-9
didecyldimethylammonium acesulfamate

-
-
15307-79-6
diclofenac sodium

-
-
934544-42-0
diclofenac didecyldimethylammonium salt

| Conditions | Yield |
|---|---|
| In acetone at 60℃; for 2h; Product distribution / selectivity; | 88% |

| Conditions | Yield |
|---|---|
| In water | 87% |
| In methanol at 20℃; for 1h; Darkness; | 85% |
| With ammonia In ethanol; water at 20℃; for 0.25h; pH=8; |
-
-
258841-42-8
isopropyloxycarbonyloxymethyl iodide

-
-
15307-79-6
diclofenac sodium

-
-
1264635-62-2
1-[(1-methylethoxy)carbonyloxy]methyl 2-[(2,6-dichlorophenyl)amino]phenylacetate

| Conditions | Yield |
|---|---|
| Stage #1: diclofenac sodium With sodium carbonate In N,N-dimethyl acetamide at 30℃; for 0.166667h; Inert atmosphere; Stage #2: isopropyloxycarbonyloxymethyl iodide In N,N-dimethyl acetamide at -10℃; for 0.75h; Inert atmosphere; | 86.4% |

| Conditions | Yield |
|---|---|
| In chloroform byproducts: sodium chloride; under inert atmosphere; 1 equiv. of sodium salt of ligand refluxed withSn compd. in dry CHCl3 for 6-7 h; cooled to room temp.; filtered; solvent removed from filtrate by rotary evaporator; crystd. from CH2Cl2-hexane; elem. anal.; | 86% |
-
-
7154-73-6
1-(2-aminoethyl)pyrrolidine

-
-
15307-79-6
diclofenac sodium

-
-
1596252-87-7
[Ni(diclofenac)2(1-(2-aminoethyl)pyrrolidine)2]

| Conditions | Yield |
|---|---|
| Stage #1: nickel(II) chloride hexahydrate; diclofenac sodium In ethanol at 50℃; Stage #2: 1-(2-aminoethyl)pyrrolidine In ethanol at 20℃; | 86% |


| Conditions | Yield |
|---|---|
| In water at 20℃; for 3h; | 86% |

Diclofenac sodium Chemical Properties
Molecular Formula: C14H10Cl2NNaO2
Molecular Weight: 318.13 g/mol
EINECS: 239-346-4
Melting point: 256-257 °C
Storage tempreture: 288-290 °C
Water solubility: 50 mg/mL
Flash Point: 203 °C
Enthalpy of Vaporization: 70.07 kJ/mol
Boiling Point: 412 °C at 760 mmHg
Vapour Pressure: 1.59E-07 mmHg at 25 °C
Appearance: Off-white crystalline solid
Structure of Diclofenac sodium (CAS NO.15307-79-6):
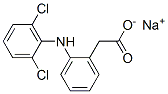
IUPAC Name: Sodium 2-[2-(2,6-dichloroanilino)phenyl]acetate
Product Category of Diclofenac sodium (CAS NO.15307-79-6): Active Pharmaceutical Ingredients;Organics;Intermediates & Fine Chemicals;Steroids;API's;Lipid signaling
Diclofenac sodium Toxicity Data With Reference
| Organism | Test Type | Route | Reported Dose (Normalized Dose) | Effect | Source |
|---|---|---|---|---|---|
| dog | LD50 | intravenous | 42mg/kg (42mg/kg) | Kiso to Rinsho. Clinical Report. Vol. 6, Pg. 1521, 1972. | |
| dog | LD50 | oral | 59mg/kg (59mg/kg) | Kiso to Rinsho. Clinical Report. Vol. 6, Pg. 1521, 1972. | |
| mouse | LD50 | intraperitoneal | 74mg/kg (74mg/kg) | Pharmaceutical Chemistry Journal Vol. 27, Pg. 343, 1993. | |
| mouse | LD50 | intravenous | 116mg/kg (116mg/kg) | Iyakuhin Kenkyu. Study of Medical Supplies. Vol. 5, Pg. 106, 1974. | |
| mouse | LD50 | oral | 95mg/kg (95mg/kg) | Pharmaceutical Chemistry Journal Vol. 23, Pg. 579, 1989. | |
| mouse | LD50 | subcutaneous | 390mg/kg (390mg/kg) | Drugs in Japan Vol. 6, Pg. 311, 1982. | |
| mouse | LD50 | unreported | 380mg/kg (380mg/kg) | Pharmaceutical Chemistry Journal Vol. 21, Pg. 275, 1987. | |
| rabbit | LD50 | intravenous | > 100mg/kg (100mg/kg) | Kiso to Rinsho. Clinical Report. Vol. 6, Pg. 1521, 1972. | |
| rabbit | LD50 | oral | 157mg/kg (157mg/kg) | Kiso to Rinsho. Clinical Report. Vol. 6, Pg. 1521, 1972. | |
| rat | LD50 | intraperitoneal | 25mg/kg (25mg/kg) | Drugs in Japan Vol. 6, Pg. 311, 1982. | |
| rat | LD50 | intravenous | 117mg/kg (117mg/kg) | Iyakuhin Kenkyu. Study of Medical Supplies. Vol. 5, Pg. 106, 1974. | |
| rat | LD50 | oral | 53mg/kg (53mg/kg) | BEHAVIORAL: ALTERED SLEEP TIME (INCLUDING CHANGE IN RIGHTING REFLEX) BEHAVIORAL: ATAXIA LUNGS, THORAX, OR RESPIRATION: RESPIRATORY STIMULATION | Toho Igakkai Zasshi. Journal of Medical Society of Toho University. Vol. 28, Pg. 99, 1981. |
| rat | LD50 | rectal | 85400ug/kg (85.4mg/kg) | Yakuri to Chiryo. Pharmacology and Therapeutics. Vol. 14, Pg. 2259, 1986. | |
| rat | LD50 | subcutaneous | 83mg/kg (83mg/kg) | Iyakuhin Kenkyu. Study of Medical Supplies. Vol. 5, Pg. 106, 1974. | |
| women | TDLo | intramuscular | 9mg/kg/1W-I (9mg/kg) | KIDNEY, URETER, AND BLADDER: "CHANGES IN TUBULES (INCLUDING ACUTE RENAL FAILURE, ACUTE TUBULAR NECROSIS)" BLOOD: NORMOCYTIC ANEMIA | American Journal of Hematology. Vol. 58, Pg. 142, 1998. |
| women | TDLo | intramuscular | 15mg/kg/5D-I (15mg/kg) | GASTROINTESTINAL: "HYPERMOTILITY, DIARRHEA" KIDNEY, URETER, AND BLADDER: "CHANGES IN TUBULES (INCLUDING ACUTE RENAL FAILURE, ACUTE TUBULAR NECROSIS)" GASTROINTESTINAL: OTHER CHANGES | Lancet. Vol. 340, Pg. 126, 1992. |
| women | TDLo | oral | 30mg/kg/10D-I (30mg/kg) | GASTROINTESTINAL: NAUSEA OR VOMITING | Postgraduate Medical Journal. Vol. 69, Pg. 486, 1993. |
| women | TDLo | oral | 112mg/kg/8W-I (112mg/kg) | GASTROINTESTINAL: "HYPERMOTILITY, DIARRHEA" SKIN AND APPENDAGES (SKIN): "DERMATITIS, OTHER: AFTER SYSTEMIC EXPOSURE" GASTROINTESTINAL: OTHER CHANGES | Archives of Internal Medicine. Vol. 152, Pg. 625, 1992. |
| women | TDLo | oral | 180mg/kg/13W- (180mg/kg) | BEHAVIORAL: ANOREXIA (HUMAN SKIN AND APPENDAGES (SKIN): "DERMATITIS, OTHER: AFTER SYSTEMIC EXPOSURE" KIDNEY, URETER, AND BLADDER: PROTEINURIS | British Medical Journal. Vol. 295, Pg. 182, 1987. |
| women | TDLo | oral | 183mg/kg/26W- (183mg/kg) | LIVER: "HEPATITIS, FIBROUS (CIRRHOSIS, POST-NECROTIC SCARRING)" LIVER: "JAUNDICE, OTHER OR UNCLASSIFIED" | British Journal of Clinical Practice. Vol. 43, Pg. 125, 1989. |
| women | TDLo | oral | 270mg/kg/90D- (270mg/kg) | GASTROINTESTINAL: ULCERATION OR BLEEDING FROM LARGE INTESTINE GASTROINTESTINAL: OTHER CHANGES BLOOD: CHANGES IN ERYTHROCYTE (RBC) COUNT | Archives of Internal Medicine. Vol. 152, Pg. 625, 1992. |
| women | TDLo | oral | 300mg/kg/17W- (300mg/kg) | LIVER: LIVER FUNCTION TESTS IMPAIRED | Clinical Rheumatology. Vol. 11, Pg. 120, 1992. |
| women | TDLo | oral | 2190mg/kg/2Y- (2190mg/kg) | GASTROINTESTINAL: "HYPERMOTILITY, DIARRHEA" | American Journal of Gastroenterology. Vol. 90, Pg. 1871, 1995. |
| women | TDLo | unreported | 364mg/kg/26W- (364mg/kg) | KIDNEY, URETER, AND BLADDER: INTERSTITIAL NEPHRITIS KIDNEY, URETER, AND BLADDER: URINE VOLUME DECREASED KIDNEY, URETER, AND BLADDER: PROTEINURIS | Wiener Klinische Wochenschrift. Vol. 111, Pg. 523, 1999. |
Diclofenac sodium Safety Profile
The Hazard Codes of Diclofenac sodium (CAS NO.15307-79-6):  T,
T,  Xi
Xi
Risk Statements:
25: Toxic if swallowed
36/37/38: Irritating to eyes, respiratory system and skin
Safety Statements:
22: Do not breathe dust
26: In case of contact with eyes, rinse immediately with plenty of water and seek medical advice
36: Wear suitable protective clothing
45: In case of accident or if you feel unwell, seek medical advice immediately (show label where possible)
36/37: Wear suitable protective clothing and gloves
Diclofenac sodium Specification
Diclofenac sodium , its cas register number is 15307-79-6. It also can be called 2-[(2,6-Dichlorophenyl)amino]benzeneacetic acid sodium salt ; Voltaren ; and Diclofenac .
About|Contact|Cas|Product Name|Molecular|Country|Encyclopedia
Message|New Cas|MSDS|Service|Advertisement|CAS DataBase|Article Data|Manufacturers | Chemical Catalog
©2008 LookChem.com,License: ICP
NO.:Zhejiang16009103
complaints:service@lookchem.com Desktop View