-
Name
Diisopinocampheylborane
- EINECS
- CAS No. 21947-87-5
- Article Data18
- CAS DataBase
- Density
- Solubility
- Melting Point
- Formula C20H35B
- Boiling Point 351.475oC at 760 mmHg
- Molecular Weight 286.309
- Flash Point 166.367oC
- Transport Information
- Appearance
- Safety
- Risk Codes
-
Molecular Structure
- Hazard Symbols
- Synonyms Borane,bis(2,6,6-trimethylbicyclo[3.1.1]hept-3-yl)-, [1S-[1a,2b,3a(1R*,2S*,3R*,5R*),5a]]-;Borane, di-3-pinanyl-, (+)-(8CI);(+)-Diisopinocampheylborane;d-Diisopinocampheylborane;
- PSA 0.00000
- LogP 5.40410
Synthetic route
-
-
7785-26-4
(-)-α-pinene

-
-
21947-87-5
L-diisopinocampheylborane

| Conditions | Yield |
|---|---|
| With borane N-ethyl-N-isopropylaniline complex In 1,4-dioxane hydroboration; | 99% |
| With dimethylsulfide borane complex In tetrahydrofuran at 20℃; Inert atmosphere; | 73% |
| With sodium tetrahydroborate; boron trifluoride diethyl etherate |
-
-
7785-26-4
(-)-α-pinene

-
-
13292-87-0
dimethylsulfide borane complex

-
-
21947-87-5
L-diisopinocampheylborane

| Conditions | Yield |
|---|---|
| In tetrahydrofuran at 20℃; for 16h; Inert atmosphere; | 82% |
| In tetrahydrofuran at 0℃; for 0.5h; | 60% |
| In tetrahydrofuran at 0℃; for 0.5h; Inert atmosphere; | 52% |
| In tetrahydrofuran | |
| In tetrahydrofuran at 0℃; |
-
-
13292-87-0
dimethylsulfide borane complex

-
-
127-91-3
(1R,5R)-(+)-β-pinene

-
-
21947-87-5
L-diisopinocampheylborane

| Conditions | Yield |
|---|---|
| In tetrahydrofuran pinene was slowly added to THF soln. of BMS at 0°C (N2); stirring for 2.5 h; evapn.; |

| Conditions | Yield |
|---|---|
| In tetrahydrofuran at 0 - 20℃; for 2h; Inert atmosphere; stereoselective reaction; |
-
-
15095-61-1
1-benzyl-3-methyl-1H-imidazol-3-ium iodide

-
-
21947-87-5
L-diisopinocampheylborane

| Conditions | Yield |
|---|---|
| With potassium hexamethylsilazane In tetrahydrofuran at 25℃; Inert atmosphere; Schlenk technique; | 83% |
-
-
100-52-7
benzaldehyde

-
-
21947-87-5
L-diisopinocampheylborane

-
-
155818-57-8
3-phenyl-1-(tributylstannyl)-1,2-propadiene

| Conditions | Yield |
|---|---|
| In diethyl ether Sn compd. in Et2O treated with B compd. at 0°C for 5 h, C6H5CHO added at -78°C, stirred at -78°C for 8 h; NaHCO3 and H2O2 added at 0°C; 94 % ee; | 76% |

-
-
104-53-0
3-phenyl-propionaldehyde

-
-
21947-87-5
L-diisopinocampheylborane

-
-
155818-57-8
3-phenyl-1-(tributylstannyl)-1,2-propadiene

| Conditions | Yield |
|---|---|
| In diethyl ether Sn compd. in Et2O treated with B compd. at 0°C for 5 h, C6H5(CH2)2CHO added at -78°C, stirred at -78°C for 8 h; NaHCO3 and H2O2 added at 0°C; 94 % ee; | 71% |

-
-
21947-87-5
L-diisopinocampheylborane

| Conditions | Yield |
|---|---|
| With potassium hexamethylsilazane In tetrahydrofuran at 25℃; Inert atmosphere; Schlenk technique; | 60% |
-
-
65039-06-7
N-methyl-N'-phenyl imidazolium iodide

-
-
21947-87-5
L-diisopinocampheylborane

| Conditions | Yield |
|---|---|
| With potassium hexamethylsilazane In tetrahydrofuran at 25℃; Inert atmosphere; Schlenk technique; | 56% |
-
-
4333-62-4
1,3-dimethylimidazolim iodide

-
-
21947-87-5
L-diisopinocampheylborane

| Conditions | Yield |
|---|---|
| With potassium hexamethylsilazane In tetrahydrofuran at 20℃; for 20h; Sealed tube; Inert atmosphere; Glovebox; | 37% |
| With potassium hexamethylsilazane In tetrahydrofuran at 25℃; for 20h; Inert atmosphere; Schlenk technique; | 37% |
-
-
21947-87-5
L-diisopinocampheylborane

| Conditions | Yield |
|---|---|
| With potassium hexamethylsilazane In tetrahydrofuran at 20℃; for 20h; Sealed tube; Inert atmosphere; Glovebox; | 34% |
-
-
21947-87-5
L-diisopinocampheylborane

| Conditions | Yield |
|---|---|
| With potassium hexamethylsilazane In tetrahydrofuran at 25℃; Inert atmosphere; Schlenk technique; | 34% |

| Conditions | Yield |
|---|---|
| With Pinene In tetrahydrofuran at -25℃; for 30h; asymmetric hydroboration; Title compound not separated from byproducts; |


| Conditions | Yield |
|---|---|
| With Pinene In tetrahydrofuran at -25℃; for 76h; asymmetric hydroboration; Title compound not separated from byproducts; |

-
-
17065-24-6
1-ethoxycyclopentene

-
-
21947-87-5
L-diisopinocampheylborane

| Conditions | Yield |
|---|---|
| With Pinene In tetrahydrofuran at -25℃; for 80h; asymmetric hydroboration; Title compound not separated from byproducts; |


| Conditions | Yield |
|---|---|
| With Pinene In tetrahydrofuran at -15℃; for 120h; asymmetric hydroboration; Title compound not separated from byproducts; |

-
-
21947-87-5
L-diisopinocampheylborane


| Conditions | Yield |
|---|---|
| With Pinene In tetrahydrofuran at -10℃; for 72h; asymmetric hydroboration; Title compound not separated from byproducts; |

-
-
21947-87-5
L-diisopinocampheylborane

-
-
479579-58-3
2-allenyl-4,4,5,5-tetraphenyl-1,3,2-dioxaborolane

| Conditions | Yield |
|---|---|
| In dichloromethane at 0℃; for 2.5h; | |
| In dichloromethane at 0 - 23℃; |
-
-
21947-87-5
L-diisopinocampheylborane

-
-
169517-16-2
2-allenyl-[1,3,2]-dioxaborinane

| Conditions | Yield |
|---|---|
| In dichloromethane at 0 - 20℃; |
-
-
21947-87-5
L-diisopinocampheylborane

-
-
479579-58-3
2-allenyl-4,4,5,5-tetraphenyl-1,3,2-dioxaborolane

| Conditions | Yield |
|---|---|
| In dichloromethane at 0℃; for 2h; |

| Conditions | Yield |
|---|---|
| In diethyl ether at 0℃; for 2h; | |
| In tetrahydrofuran at 20℃; for 4h; Inert atmosphere; |
-
-
603040-81-9
(R)-1-methoxy-4-((pent-4-yn-2-yloxy)methyl)benzene

-
-
21947-87-5
L-diisopinocampheylborane

| Conditions | Yield |
|---|---|
| In tetrahydrofuran at -35 - 20℃; for 2.5h; |
-
-
21947-87-5
L-diisopinocampheylborane

-
-
106356-53-0
B-allyldiisopinocampheylborane

| Conditions | Yield |
|---|---|
| Multi-step reaction with 2 steps 1: diethyl ether / 2 h / 0 °C 2: diethyl ether / 1 h / 20 °C View Scheme |
-
-
21947-87-5
L-diisopinocampheylborane

| Conditions | Yield |
|---|---|
| Multi-step reaction with 2 steps 1: CH2Cl2 / 2 h / 0 °C 2: CH2Cl2 / 18 h / -78 °C View Scheme |
-
-
21947-87-5
L-diisopinocampheylborane

| Conditions | Yield |
|---|---|
| Multi-step reaction with 2 steps 1: CH2Cl2 / 2 h / 0 °C 2: CH2Cl2 / 2.5 h / -78 °C View Scheme |

| Conditions | Yield |
|---|---|
| Norbornene is hydroborated with Ipc2BH at -25°C (83% ee).; |
-
-
21947-87-5
L-diisopinocampheylborane

-
-
175892-48-5
B-bromodiisopinocampheylborane

| Conditions | Yield |
|---|---|
| With hydrogen bromide In pentane 0°C;; |
-
-
21947-87-5
L-diisopinocampheylborane

-
-
175892-49-6
B-iododiisopinocampheylborane

| Conditions | Yield |
|---|---|
| With iodine In pentane 0°C;; |
-
-
21947-87-5
L-diisopinocampheylborane

-
-
112246-73-8
B-chlorodiisopinocampheylborane

| Conditions | Yield |
|---|---|
| With hydrogenchloride In pentane 0°C;; |

| Conditions | Yield |
|---|---|
| With sodium hydroxide; acetaldehyde In tetrahydrofuran mixt. of B compd. and pinene was kept at 0°C (N2) for 3 d; cooling to -25°C, addn. of cis-butene; stirring for 6 h; treatment with AcH with stirring at 25°C for 36 h, evapn., addn. of pentane, treatment with aq. NaOH; treatment with aq. HCl, extn. with Et2O, drying in vac.; |
Diisopinocampheylborane Chemical Properties
Molecular Structure of Diisopinocampheylborane (CAS No.21947-87-5):
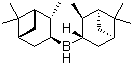
Molecular Formula: C20H35B
Molecular Weight: 286.30
CAS No: 21947-87-5
IUPAC Name: bis {(1S,2S,3S,5R)-2,6,6-Trimethylbicyclo [3.1.1] heptan-3-yl} borane
Diisopinocampheylborane History
Diisopinocampheylborane was reported in 1961 by Zweifel and Brown in a pioneering demonstration of asymmetric synthesis using boranes.
Diisopinocampheylborane Uses
Diisopinocampheylborane (CAS No.21947-87-5) is mainly used for the synthesis of chiral secondary alcohols.
Diisopinocampheylborane Production
Diisopinocampheylborane is now more commonly generated from borane-methyl sulfide (BMS).
Diisopinocampheylborane Specification
Diisopinocampheylborane (CAS No.21947-87-5), its synonyms are (+)-Diisopinocampheylborane ; Bis[(1S,2R,3S,5S)-2,6,6-trimethylbicyclo[3.1.1]hept-3-yl]borane .
Related Products
- Diisopinocampheylborane
- 21947-98-8
- 21948-10-7
- 219485-21-9
- 21948-70-9
- 219492-12-3
- 219503-81-8
- 219508-19-7
- 219508-62-0
- 219509-79-2
- 219511-71-4
Hot Products
About|Contact|Cas|Product Name|Molecular|Country|Encyclopedia
Message|New Cas|MSDS|Service|Advertisement|CAS DataBase|Article Data|Manufacturers | Chemical Catalog
©2008 LookChem.com,License: ICP
NO.:Zhejiang16009103
complaints:service@lookchem.com Desktop View