-
Name
Dimethylamine hydrochloride
- EINECS 208-046-5
- CAS No. 506-59-2
- Article Data135
- CAS DataBase
- Density 0.64 g/cm3
- Solubility 3000 g/L (20 °C) in water
- Melting Point 170-173 ºC (lit.)
- Formula C2H7N.HCl
- Boiling Point 6.1 ºC at 760 mmHg
- Molecular Weight 81.5452
- Flash Point
- Transport Information
- Appearance white crystals
- Safety 26-36/37-37/39-36
- Risk Codes 22-36/37/38
-
Molecular Structure
-
Hazard Symbols
 Xn
Xn
- Synonyms Methanamine, N-methyl-, hydrochloride (9CI);Dimethylammonium chloride;
- PSA 12.03000
- LogP 1.02850
Synthetic route

| Conditions | Yield |
|---|---|
| With dichloromethane; hydrogen; palladium on activated charcoal In methanol at 20℃; for 24h; atmospheric pressure; | 99% |
| With 1,1,2-trichloroethane; 10% palladium on activated carbon; hydrogen In methanol under 760.051 Torr; for 0.666667h; chemoselective reaction; | 98% |
-
-
94947-35-0
({(CH3)2NH}2B(Cl)C6H5)(1+)*Cl(1-)=({(CH3)2NH}2B(Cl)C6H5)Cl

-
A
-
1196-44-7
chloro(dimethylamino)phenylborane

-
B
-
506-59-2
N,N-dimethylammonium chloride

| Conditions | Yield |
|---|---|
| In neat (no solvent) pyrolysis from 150 to 200°C;; | A 94.1% B n/a |
| In neat (no solvent) pyrolysis from 150 to 200°C;; | A 94.1% B n/a |
-
-
57802-40-1
pentachloro-2H-pyrrole

-
-
2083-91-2
N,N-Dimethyltrimethylsilylamine

-
A
-
506-59-2
N,N-dimethylammonium chloride

| Conditions | Yield |
|---|---|
| In tetrahydrofuran | A n/a B 91% C n/a |


-
-
93766-28-0
N,N-dimethyl-N',N''-bisphosphorous triamide

-
A
-
506-59-2
N,N-dimethylammonium chloride

| Conditions | Yield |
|---|---|
| at 50℃; for 1h; | A 89% B 91% |

-
-
71-41-0
pentan-1-ol

-
-
3397-76-0, 100508-13-2
N,N-Dimethylformamide Hydrochloride

-
A
-
543-59-9
1-Chloropentane

-
B
-
638-49-3
n-pentyl formate

-
C
-
506-59-2
N,N-dimethylammonium chloride

| Conditions | Yield |
|---|---|
| at 100℃; for 0.833333h; Product distribution; different alcohols; | A n/a B 90% C n/a |

-
-
93304-26-8
H(C5H5)Mo(CO)2(P(OC2H5)2N(CH3)2)

-
A
-
93304-27-9
H(C5H5)Mo(CO)2(P(OC2H5)2Cl)

-
B
-
506-59-2
N,N-dimethylammonium chloride

| Conditions | Yield |
|---|---|
| With HCl In toluene dry gaseous HCl bubbled through soln. of educt in toluene at -20°C; elem. anal.; | A 70% B 90% |
-
-
7721-01-9
tantalum pentachloride

-
-
124-40-3
dimethyl amine

-
-
77071-69-3
mer,cis-[TaCl3(NMe2)2(NHMe2)]

-
-
77071-71-7
TaCl2(N(CH3)2)3(HN(CH3)2)

-
-
77071-70-6
(TaCl2(N(CH3)2)2(HN(CH3)2))2O

-
D
-
506-59-2
N,N-dimethylammonium chloride

| Conditions | Yield |
|---|---|
| In benzene dimethylamine added to frozen soln.TaCl5 in benzene at -178°C and slowly allowed to warm to room temp. and soln. stirred for 24 h under N2 atm.; Me2NH2Cl filtered, filtrate concd., pentane added slowly, crystals Ta(NMe2)2Cl3(HNMe2) filtered, filtrate allowed to stand for 10 days - Ta(NMe2)3Cl2(HNMe2) and (TaCl2(NMe2)2(HNMe2))2O; elem. anal.; | A 85% B 1.6% C 6.3% D n/a |

-
-
124-40-3
dimethyl amine

-
-
5032-91-7
pentafluorophenyl dichlorophosphine

-
A
-
506-59-2
N,N-dimethylammonium chloride

-
B
-
5032-95-1
Bis-dimethylamino-pentafluorphenyl-phosphin

| Conditions | Yield |
|---|---|
| In diethyl ether byproducts: HCl; in presence of a trap for HCl; | A n/a B 81.5% |
| In diethyl ether byproducts: HCl; in presence of a trap for HCl; | A n/a B 81.5% |

-
-
124-40-3
dimethyl amine

-
-
698-16-8
benzohydroximoyl chloride

-
A
-
65986-63-2
(E)-N,N-Dimethylbenzamid-oxim

-
B
-
506-59-2
N,N-dimethylammonium chloride

| Conditions | Yield |
|---|---|
| In diethyl ether for 48h; Ambient temperature; | A 80% B n/a |

-
-
1630-79-1
tetrakis(dimethylamido)diborane

-
A
-
64541-76-0
dichlorobis(dimethylamino)diborane(4)

-
B
-
506-59-2
N,N-dimethylammonium chloride

| Conditions | Yield |
|---|---|
| With HCl In diethyl ether | A 79.3% B n/a |
-
-
126306-16-9
bis(dimethylamino)cyclohexylphosphine(pentacarbonyl)chromium(0)

-
A
-
126306-27-2
chlorodimethylaminocyclohexylphosphine(pentacarbonyl)chromium(0)

-
B
-
506-59-2
N,N-dimethylammonium chloride

| Conditions | Yield |
|---|---|
| With hydrogenchloride In pentane Under stirring a strong stream of hydrogenhalogenide is bubbled for 10 min through the cooled (0°C) soln. of phosphine complex (inert atmosphere).; Solvent is evapd. at room temp. in vac., residue is digested with pentane, dialkylamminhydrohalogenide is filtered off, filtrate is dried to dryness in vac., recrystn. from pentane (-78°C), elem. anal.; | A 79% B n/a |
-
-
72868-70-3
bis(dimethylamino)phenylphosphine(pentacarbonyl)chromium(0)

-
A
-
126306-23-8
chlorodimethylaminophenylphosphine(pentacarbonyl)chromium(0)

-
B
-
506-59-2
N,N-dimethylammonium chloride

| Conditions | Yield |
|---|---|
| With hydrogenchloride In pentane Under stirring a strong stream of hydrogenhalogenide is bubbled for 10 min through the cooled (0°C) soln. of phosphins complex (inert atmosphere).; Solvent is evapd. at room temp. in vac., residue is digested with pentane, dialkylamminhydrohalogenide is filtered off, filtrate is dried to dryness in vac., chromy. (silica gel, pentane), elem. anal.; | A 74% B n/a |
-
-
3356-73-8
2-piperidino-2,4,4,6,6-pentacholorocyclotriphosphazatriene

-
-
124-40-3
dimethyl amine

-
A
-
506-59-2
N,N-dimethylammonium chloride


| Conditions | Yield |
|---|---|
| In tetrahydrofuran | A n/a B 30% C 70% |

-
-
3720-88-5
penatchloromonoisopropylaminocyclotriphosphazatriene

-
-
124-40-3
dimethyl amine

-
A
-
506-59-2
N,N-dimethylammonium chloride


| Conditions | Yield |
|---|---|
| A n/a B 70% C 30% | |
| at 30℃; | A n/a B 70% C 30% |

-
-
3998-23-0
Pentachlor--triphosphonitril

-
-
124-40-3
dimethyl amine

-
A
-
506-59-2
N,N-dimethylammonium chloride


| Conditions | Yield |
|---|---|
| Thermodynamic data; Kinetics; ΔH (excit.), ΔS (excit.); | A n/a B 70% C 30% |
| at 30℃; | A n/a B 70% C 30% |

-
-
77215-38-4
Dibutyl-(2,4,4,6,6-pentachloro-2λ5,4λ5,6λ5-[1,3,5,2,4,6]triazatriphosphinin-2-yl)-amine

-
-
124-40-3
dimethyl amine

-
A
-
506-59-2
N,N-dimethylammonium chloride


| Conditions | Yield |
|---|---|
| at 18 - 30℃; | A n/a B 30% C 70% |

-
-
650-52-2
bis(trifluoromethyl)chlorophosphine

-
-
124-40-3
dimethyl amine

-
A
-
432-01-9
dimethylamino-bis-trifluoromethyl-phosphine

-
B
-
506-59-2
N,N-dimethylammonium chloride

| Conditions | Yield |
|---|---|
| In gaseous matrix 0°C; | A 70% B n/a |

-
-
680-31-9
N,N,N,N,N,N-hexamethylphosphoric triamide

-
-
931-59-9
benzenesulfenyl chloride

-
A
-
1212-08-4
S-Phenyl benzenethiosulfonate

-
B
-
506-59-2
N,N-dimethylammonium chloride

| Conditions | Yield |
|---|---|
| In dichloromethane at 20℃; for 4h; Inert atmosphere; | A 70% B n/a |

-
-
680-31-9
N,N,N,N,N,N-hexamethylphosphoric triamide

-
-
933-01-7
4-chloro-benzenesulfenyl chloride

-
A
-
1146-44-7
4,4'-dichlorophenyl thiosulfonate

-
B
-
506-59-2
N,N-dimethylammonium chloride

| Conditions | Yield |
|---|---|
| In dichloromethane at 20℃; for 4h; Inert atmosphere; | A 70% B n/a |

-
-
26459-63-2
hexakis(dimethylamino)tetraborane(6)

-
B
-
506-59-2
N,N-dimethylammonium chloride

| Conditions | Yield |
|---|---|
| With hydrogenchloride In diethyl ether -20°C; | A 65% B n/a |
-
-
32882-73-8
{(CH3)3Si}2NB{N(CH3)2}2

-
A
-
32882-72-7
Bis(trimethylsilyl)aminochlorodimethylaminoboran

-
B
-
506-59-2
N,N-dimethylammonium chloride

| Conditions | Yield |
|---|---|
| With HCl In toluene sepn. of dimethylamine hydrochloride and toluene, distn.; | A 65% B n/a |

| Conditions | Yield |
|---|---|
| In dichloromethane under N2 to Mo(HB(Me2pyz)3)(NO)Cl2 dissolved in dichloromethane added dimethylamine and mixt. stirred for 30 min; volume soln. reduced in vacuo, treated with Et2O, (NH2Me2)Cl filtered off, filtrate slowly evapd.; elem. anal.; | A 61% B n/a |


| Conditions | Yield |
|---|---|
| for 6h; | A n/a B 60% |

-
-
73939-32-9
(η5-chlorodivinylborane)(η5-cyclopentadienyl)cobalt

-
-
124-40-3
dimethyl amine

-
A
-
11486-73-0
(η5-(dimethylamino)divinylborane)(η5-cyclopentadienyl)cobalt

-
B
-
506-59-2
N,N-dimethylammonium chloride

| Conditions | Yield |
|---|---|
| In diethyl ether Under nitrogen, dimethylamine is bubbled through a stirred soln. of Co-complex in ether, ppt. of dimethylamine*HCl forms, addn. of amine is continued for 15 min.; Filtn., redn. of solvent under high vac., extn. with pentane, coolg. of the red soln. to -78°C, filtn., drying under high vac, elem. anal.; | A 54% B n/a |


-
B
-
506-59-2
N,N-dimethylammonium chloride

| Conditions | Yield |
|---|---|
| With hydrogenchloride In diethyl ether -20°C for 2 h; distn. at 78-82°C/1 Torr; | A 53% B n/a |
-
-
26459-62-1
1,1,3,3-tetrakis(dimethylamino)-2-dimethylamino-triboran(5)

-
B
-
506-59-2
N,N-dimethylammonium chloride

| Conditions | Yield |
|---|---|
| With hydrogenchloride In diethyl ether -20°C; distn. at 70-72°C/1 Torr; | A 48% B n/a |
-
-
832-53-1
pentafluorobenzenesulonyl chloride

-
-
124-40-3
dimethyl amine

-
A
-
36650-05-2
2,3,4,5,6-pentafluoro-N,N-dimethylbenzenesulfonamide

-
B
-
506-59-2
N,N-dimethylammonium chloride

| Conditions | Yield |
|---|---|
| In diethyl ether 20°C,20 h,in presence of Et3N; | A 42.5% B n/a |
| In diethyl ether 20°C,20 h,in presence of Et3N; | A 42.5% B n/a |

-
-
10294-34-5
boron trichloride

-
-
124-40-3
dimethyl amine

-
A
-
1113-31-1
dichlorodimethylaminoborane

-
B
-
506-59-2
N,N-dimethylammonium chloride

| Conditions | Yield |
|---|---|
| A 35% B n/a |

-
-
1630-79-1
tetrakis(dimethylamido)diborane

-
A
-
7360-75-0
tris(dimethylamino)monochlorodiborane(4)

-
B
-
506-59-2
N,N-dimethylammonium chloride

| Conditions | Yield |
|---|---|
| With hydrogenchloride In diethyl ether -20°C; distn.; | A 28% B n/a |
| With HCl In diethyl ether -20°C; distn.; | A 28% B n/a |
| With HCl In diethyl ether -20°C; |
-
-
126306-18-1
bis(dimethylamino)-t-butylphosphine(pentacarbonyl)chromium(0)

-
A
-
126289-56-3
chlorodimethylamino-t-butylphosphine(pentacarbonyl)chromium(0)

-
B
-
506-59-2
N,N-dimethylammonium chloride

| Conditions | Yield |
|---|---|
| With hydrogenchloride In pentane Under stirring a strong stream of hydrogenhalogenide is bubbled for 10 min through the cooled (0°C) soln. of phosphine complex (inert atmosphere).; Solvent is evapd. at room temp. in vac., residue is digested with pentane, dialkylamminhydrohalogenide is filtered off, filtrate is dried to dryness in vac., recrystn. from pentane (-78°C), not isolated.; | A 10% B n/a |
-
-
934-37-2
2-methylimidazo[1,2-a]pyridine

-
-
50-00-0
formaldehyd

-
-
506-59-2
N,N-dimethylammonium chloride

-
-
133395-11-6
3-<(dimethylamino)methyl>-2-methylimidazo<1,2-a>pyridine

| Conditions | Yield |
|---|---|
| In methanol for 1.5h; Heating; | 100% |

-
-
506-59-2
N,N-dimethylammonium chloride

-
-
90905-31-0
2-(methylsulfanyl)pyrimidine-5-carbaldehyde

-
-
55551-49-0
2-(N,N-dimethylamino)pyrimidine-5-carbaldehyde

| Conditions | Yield |
|---|---|
| With sodium carbonate In ethanol Heating; | 100% |
-
-
506-59-2
N,N-dimethylammonium chloride

-
-
13263-97-3
7-(dimethylamino)-3-(β-D-ribofuranosyl)pyrazolo<4,3-d>pyrimidine

| Conditions | Yield |
|---|---|
| With pyridine at 57℃; for 22h; | 100% |
-
-
75-15-0
carbon disulfide

-
-
506-59-2
N,N-dimethylammonium chloride

-
-
128-04-1
sodium dimethyldithiocarbamate

| Conditions | Yield |
|---|---|
| With sodium hydroxide In methanol for 3h; Ambient temperature; | 100% |
-
-
506-59-2
N,N-dimethylammonium chloride

-
-
165180-39-2
6,8-difluoro-2-(3-fluoro-4-pivaloylaminophenyl)-7-methanesulfonyloxymethyl-5-pivaloylamino-4H-1-benzopyran-4-one

-
-
165180-41-6
7-dimethylaminomethyl-6,8-difluoro-2-(3-fluoro-4-pivaloylaminophenyl)-5-pivaloylamino-4H-1-benzopyran-4-one

| Conditions | Yield |
|---|---|
| With potassium carbonate In N,N-dimethyl-formamide for 0.75h; Ambient temperature; | 100% |
| With sodium chloride; potassium carbonate In N-methyl-acetamide; water | 100% |
| With sodium chloride; potassium carbonate In N-methyl-acetamide; water | 100% |
-
-
506-59-2
N,N-dimethylammonium chloride

-
-
2243-83-6
2-naphthaloyl chloride

-
-
13577-85-0
N,N-dimethyl-2-naphthylcarboxamide

| Conditions | Yield |
|---|---|
| With sodium hydroxide In diethyl ether; water; ethyl acetate at 0℃; for 1h; | 100% |
| With pyridine In dichloromethane for 20h; Substitution; | 572 mg |
-
-
506-59-2
N,N-dimethylammonium chloride

-
-
297751-32-7
3-[1-(benzenesulfonyl)-1H-indol-4-yl]propionic acid ethyl ester

-
-
297751-33-8
N,N-dimethyl-4-[1-(benzenesulfonyl)-1H-indol-4-yl]propionamide

| Conditions | Yield |
|---|---|
| With trimethylaluminum In hexane; benzene for 18h; Heating; | 100% |
| With hydrogenchloride; trimethylaluminum In benzene |
-
-
506-59-2
N,N-dimethylammonium chloride

-
-
221087-71-4
2-(2,5-dimethylpyrrolyl)-6-(4-(2-carboxymethyloxy)-1-naphthyl)pyridine

-
-
221087-73-6
2-(2,5-dimethylpyrrolyl)-6-((4-(N,N-dimethylcarboxamido)methyloxy)-1-naphthyl)pyridine

| Conditions | Yield |
|---|---|
| With benzotriazol-1-ol; N-(3-dimethylaminopropyl)-N-ethylcarbodiimide; triethylamine In acetonitrile at 20℃; for 80h; | 100% |
-
-
506-59-2
N,N-dimethylammonium chloride

-
-
221087-93-0
2-(2,5-dimethylpyrrolyl)-6-[4-carboxymethoxy-5,6,7,8-tetrahydronaphthalen-1-yl]pyridine

-
-
221087-95-2
2-(2,5-dimethylpyrrolyl)-6-[4-(N,N-dimethylcarboxamido)methoxy-5,6,7,8-tetrahydronaphthalen-1-yl]pyridine

| Conditions | Yield |
|---|---|
| With benzotriazol-1-ol; N-(3-dimethylaminopropyl)-N-ethylcarbodiimide; triethylamine In acetonitrile at 20℃; | 100% |
-
-
506-59-2
N,N-dimethylammonium chloride

-
-
76-05-1
trifluoroacetic acid

-
-
711020-56-3
2-[(2S)-2-(3,4-dichlorophenyl)-4-oxobutyl]-3-methyl-1-oxo-1,3,4,6-tetrahydro-2H-naphtho[1,2-f][1,4]oxazocine-7-carbonitrile

| Conditions | Yield |
|---|---|
| With sodium cyanoborohydride; acetic acid; triethylamine In methanol | 100% |

-
-
506-59-2
N,N-dimethylammonium chloride

-
-
147471-66-7
(S)-4-tert-Butoxycarbonylamino-5-ethoxycarbonyloxy-5-oxo-pentanoic acid benzyl ester

-
-
841233-80-5
benzyl N-tert-butyloxycarbonyl-L-γ-glutamate α-N,N-dimethylamide

| Conditions | Yield |
|---|---|
| With triethylamine In dichloromethane at 20℃; for 20h; | 100% |
-
-
506-59-2
N,N-dimethylammonium chloride

| Conditions | Yield |
|---|---|
| Stage #1: N,N-dimethylammonium chloride With trimethylaluminum In toluene at 20℃; for 1h; Stage #2: 2'-trifluoromethanesulfonyloxy-[1,1']binaphthalenyl-2-carboxylic acid methyl ester In toluene for 3h; Heating; Further stages.; | 100% |
-
-
227449-41-4
2-methylsulfanyl-4-(3-trifluoromethylanilino)pyrimidine-5-carboxylic acid

-
-
506-59-2
N,N-dimethylammonium chloride

-
-
863029-03-2
N,N-dimethyl-2-methylsulfanyl-4-(3-trifluoromethylanilino)pyrimidine-5-carboxamide

| Conditions | Yield |
|---|---|
| Stage #1: 2-methylsulfanyl-4-(3-trifluoromethylanilino)pyrimidine-5-carboxylic acid With benzotriazol-1-ol; 1-ethyl-(3-(3-dimethylamino)propyl)-carbodiimide hydrochloride In N,N-dimethyl-formamide at 20℃; for 4h; Stage #2: N,N-dimethylammonium chloride In N,N-dimethyl-formamide at 20℃; for 14h; | 100% |
-
-
506-59-2
N,N-dimethylammonium chloride

-
-
126093-01-4
methyl 4-<<<4-<<<4-<<(tert-butyloxy)carbonyl>amino>-1-methyl-pyrrol-2-yl>carbonyl>amino>-1-methyl-pyrrol-2-yl>carbonyl>amino>-1-methyl-pyrrole-2-carboxylic acid

-
-
292073-28-0
{5-[5-(5-dimethylcarbamoyl-1-methyl-1H-pyrrol-3-ylcarbamoyl)-1-methyl-1H-pyrrol-3-ylcarbamoyl]-1-methyl-1H-pyrrol-3-yl}-carbamic acid tert-butyl ester

| Conditions | Yield |
|---|---|
| With dmap; 1-ethyl-(3-(3-dimethylamino)propyl)-carbodiimide hydrochloride In tetrahydrofuran; N,N-dimethyl-formamide at 25℃; for 14h; | 100% |
-
-
774213-95-5
[3-cyclohexyl-6-(methoxycarbonyl)-2-phenyl-1H-pyrrolo[2,3-b]pyridin-1-yl]acetic acid

-
-
506-59-2
N,N-dimethylammonium chloride

-
-
774213-96-6
methyl 3-cyclohexyl-1-[2-(dimethylamino)-2-oxoethyl]-2-phenyl-1H-pyrrolo[2,3-b]pyridine-6-carboxylate

| Conditions | Yield |
|---|---|
| With N-ethyl-N,N-diisopropylamine; N-[(dimethylamino)-3-oxo-1H-1,2,3-triazolo[4,5-b]pyridin-1-yl-methylene]-N-methylmethanaminium hexafluorophosphate In N,N-dimethyl-formamide at 20℃; | 100% |
| With N-ethyl-N,N-diisopropylamine; HATU In DMF (N,N-dimethyl-formamide) at 20℃; for 1.5h; | 100% |
-
-
1015174-82-9
1-(2-methoxyethyl)-2-methyl-8-phenyl-1,6,7,8-tetrahydrochromeno[7,8-d]imidazole-5-carboxylic acid

-
-
506-59-2
N,N-dimethylammonium chloride

| Conditions | Yield |
|---|---|
| With O-(1H-benzotriazol-1-yl)-N,N,N',N'-tetramethyluronium hexafluorophosphate; triethylamine In N,N-dimethyl-formamide at 0 - 20℃; for 12h; | 100% |
-
-
918650-03-0
(5S)-1-(tert-butoxycarbonyl)-5-(methoxycarbonyl)-2-methyl-4,5-dihydro-1H-pyrrole-3-carboxylic acid

-
-
506-59-2
N,N-dimethylammonium chloride

| Conditions | Yield |
|---|---|
| With 4-methyl-morpholine; benzotriazol-1-ol; 1-ethyl-(3-(3-dimethylamino)propyl)-carbodiimide hydrochloride In dichloromethane at 20℃; for 4h; | 100% |
-
-
728015-44-9
4-(3-cyano-phenyl)-7-ethyl-3-(methylsulfonyl)-pyrrolo[1,2-b]pyridazine-2-carboxylic acid

-
-
506-59-2
N,N-dimethylammonium chloride

| Conditions | Yield |
|---|---|
| With benzotriazol-1-ol; 1-ethyl-(3-(3-dimethylamino)propyl)-carbodiimide hydrochloride In DMF (N,N-dimethyl-formamide) at 20℃; for 3h; | 100% |

-
-
506-59-2
N,N-dimethylammonium chloride

| Conditions | Yield |
|---|---|
| In benzene Heating / reflux; | 100% |
-
-
17417-09-3
2-fluoro-5-nitrobenzonitrile

-
-
506-59-2
N,N-dimethylammonium chloride

-
-
17417-10-6
2-cyano-4-nitro-N,N-dimethylaniline

| Conditions | Yield |
|---|---|
| With potassium hydrogencarbonate In water; N,N-dimethyl-formamide | 100% |
-
-
506-59-2
N,N-dimethylammonium chloride

-
-
79463-77-7
N-cyanodiphenylcarbonimidate

-
-
167494-04-4
O-phenyl-N-cyano-N',N'-dimethylurea

| Conditions | Yield |
|---|---|
| In tetrahydrofuran | 100% |
-
-
170364-87-1
1-N-[1-N-(3-chloropropyl)-piperidin-4-yl]-indole

-
-
506-59-2
N,N-dimethylammonium chloride

| Conditions | Yield |
|---|---|
| With potassium carbonate In ethanol; water | 100% |

-
-
506-59-2
N,N-dimethylammonium chloride

| Conditions | Yield |
|---|---|
| With potassium carbonate In tetrahydrofuran; acetonitrile at 95℃; for 10h; | 100% |
| With potassium carbonate In tetrahydrofuran; acetonitrile at 95℃; for 10h; | 100% |
-
-
2604-39-9
4-amino-2-chloro-5-nitropyridine

-
-
506-59-2
N,N-dimethylammonium chloride

-
-
886435-69-4
N,N-dimethyl-5-nitropyridine-2,4-diamine

| Conditions | Yield |
|---|---|
| Stage #1: 4-amino-2-chloro-5-nitropyridine; N,N-dimethylammonium chloride With triethylamine In ethanol at 85℃; for 0.166667h; Microwave irradiation; Stage #2: With sodium hydroxide In water | 100% |
| With triethylamine In isopropyl alcohol at 100℃; for 12h; |
-
-
928774-42-9
2-methyl-8-[(5-methyl-3,4-dihydro-2H-chromen-4-yl)amino]imidazo[1,2-a]pyridine-6-carboxylic acid

-
-
506-59-2
N,N-dimethylammonium chloride

-
-
950595-98-9
N,N,2-trimethyl-8-[(5-methyl-3,4-dihydro-2H-chromen-4-yl)amino]imidazo[1,2-a]pyridine-6-carboxamide

| Conditions | Yield |
|---|---|
| With benzotriazol-1-ol; 1-ethyl-(3-(3-dimethylamino)propyl)-carbodiimide hydrochloride; triethylamine In dichloromethane at 0 - 20℃; for 24h; | 100% |
-
-
37366-09-9
dichloro(benzene)ruthenium(II) dimer

-
-
76189-55-4, 76189-56-5, 98327-87-8
(R)-(+)-2,2’-bis(diphenylphosphino)-1,1’-binaphthalene

-
-
506-59-2
N,N-dimethylammonium chloride

| Conditions | Yield |
|---|---|
| In 1,4-dioxane (Ar); heating a soln. of ruthenium complex with ammonium salt and phosphine in dioxane at 50°C for 2 h with stirring, refluxing with stirring for 12 h; cooling, evapn.; | 100% |

-
-
130004-33-0, 145926-28-9, 376354-47-1
(2,2'-bis(diphenylphosphino)-1,1'-binaphathalene)chloro(p-cymene)ruthenium chloride

-
-
506-59-2
N,N-dimethylammonium chloride

| Conditions | Yield |
|---|---|
| In tetrahydrofuran (Ar); heating a soln. of ruthenium complex with ammonium salt in THF for 6 h; evapn., washing; | 100% |
-
-
713530-55-3
4-hydroxy-N,N,1,2-tetramethyl-1H-benzimidazole-6-carboxamide

-
-
50-00-0
formaldehyd

-
-
506-59-2
N,N-dimethylammonium chloride

-
-
1034771-47-5
5-[(dimethylamino)methyl]-4-hydroxy-N,N,1,2-tetramethyl-1H-benzimidazole-6-carboxamide hydrochloride

| Conditions | Yield |
|---|---|
| With triethylamine In water; isopropyl alcohol at 35 - 45℃; for 2 - 6h; | 100% |

-
-
619-58-9
4-iodobenzoic acid

-
-
506-59-2
N,N-dimethylammonium chloride

-
-
24167-53-1
4-iodo-N,N-dimethylbenzamide

| Conditions | Yield |
|---|---|
| Stage #1: N,N-dimethylammonium chloride With triethylamine In dichloromethane for 0.5h; Stage #2: 4-iodobenzoic acid With 1-ethyl-(3-(3-dimethylamino)propyl)-carbodiimide hydrochloride In dichloromethane at 20℃; for 8h; | 100% |
-
-
62965-35-9
N-tert-butyloxycarbonyl-L-tert-leucine

-
-
506-59-2
N,N-dimethylammonium chloride

-
-
340161-34-4
(S)-tert-butyl 1-(dimethylamino)-3,3-dimethyl-1-oxobutan-2-ylcarbamate

| Conditions | Yield |
|---|---|
| Stage #1: N-tert-butyloxycarbonyl-L-tert-leucine With HBTU In dichloromethane for 0.0333333h; Inert atmosphere; Stage #2: N,N-dimethylammonium chloride With N-ethyl-N,N-diisopropylamine In dichloromethane for 1.5h; Inert atmosphere; | 100% |
| With triethylamine; dicyclohexyl-carbodiimide In dichloromethane at 20℃; for 12h; Cooling with ice; | 91% |
| With 1-ethyl-(3-(3-dimethylamino)propyl)-carbodiimide hydrochloride; N-ethyl-N,N-diisopropylamine In dichloromethane at 20℃; for 18h; | |
| With benzotriazol-1-ol; 1-ethyl-(3-(3-dimethylamino)propyl)-carbodiimide hydrochloride; N-ethyl-N,N-diisopropylamine In dichloromethane at 0 - 20℃; for 3h; |
Dimethylamine hydrochloride Specification
The Dimethylamine HCl, with the CAS registry number 506-59-2, is also known as Dimethylammonium chloride. It belongs to the product categories of Amine Salts; Nitrogen Compounds; Organic Building Blocks. Its EINECS registry number is 208-046-5. This chemical's molecular formula is C2H8ClN and molecular weight is 81.54. What's more, both its IUPAC name and systematic name are the same which is called N-Methylmethanamine hydrochloride. This chemical can be prepared by Dimethylamine with Hydrochloric acid.
Physical properties about Dimethylamine HCl are: (1)ACD/LogP: -0.43; (2)# of Rule of 5 Violations: 0; (3)ACD/LogD (pH 5.5): -3.53; (4)ACD/LogD (pH 7.4): -3.33; (5)ACD/BCF (pH 5.5): 1; (6)ACD/BCF (pH 7.4): 1; (7)ACD/KOC (pH 5.5): 1; (8)ACD/KOC (pH 7.4): 1; (9)#H bond acceptors: 1; (10)#H bond donors: 1; (11)#Freely Rotating Bonds: 0; (12)Polar Surface Area: 3.24 Å2; (13)Enthalpy of Vaporization: 26.4 kJ/mol; (14)Boiling Point: 6.1 °C at 760 mmHg; (15)Vapour Pressure: 1520 mmHg at 25 °C.
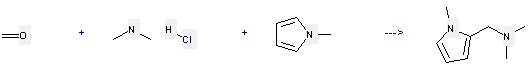
Uses of Dimethylamine HCl: (1) it is used as catalysator of acetylation analysis, raw materials of pharmaceutical and organic synthesis; (2) it is used to produce other chemicals. For example, it can react with 1-Methyl-pyrrole, Formaldehyde to get Dimethyl-(1-methyl-pyrrol-2-ylmethyl)-amine. The reaction occurs with solvent H2O. The reaction time is 6 hours. The yield is 70 %.
When you are dealing with this chemical, you should be very careful. This chemical is irritating to eyes, respiratory system and skin. If swallowed, it's harmful to health. Therefore, you should wear suitable protective clothing, gloves and eye/face protection. And in case of contacting with eyes, you should rinse immediately with plenty of water and seek medical advice.
You can still convert the following datas into molecular structure:
(1) SMILES: Cl.N(C)C
(2) InChI: InChI=1S/C2H7N.ClH/c1-3-2;/h3H,1-2H3;1H
(3) InChIKey: IQDGSYLLQPDQDV-UHFFFAOYSA-N
The toxicity data is as follows:
| Organism | Test Type | Route | Reported Dose (Normalized Dose) | Effect | Source |
|---|---|---|---|---|---|
| guinea pig | LD50 | oral | 1600mg/kg (1600mg/kg) | Gigiena i Sanitariya. For English translation, see HYSAAV. Vol. 32(6), Pg. 12, 1967. | |
| mouse | LD50 | intraperitoneal | 1570mg/kg (1570mg/kg) | LUNGS, THORAX, OR RESPIRATION: RESPIRATORY STIMULATION | Japanese Journal of Pharmacology. Vol. 17, Pg. 475, 1967. Link to PubMed |
| mouse | LD50 | intravenous | 1210mg/kg (1210mg/kg) | Archives Internationales de Pharmacodynamie et de Therapie. Vol. 112, Pg. 36, 1957. Link to PubMed | |
| mouse | LD50 | oral | 8100mg/kg (8100mg/kg) | Gigiena i Sanitariya. For English translation, see HYSAAV. Vol. 32(6), Pg. 12, 1967. | |
| mouse | LD50 | subcutaneous | 2gm/kg (2000mg/kg) | Archives Internationales de Pharmacodynamie et de Therapie. Vol. 112, Pg. 36, 1957. Link to PubMed | |
| rabbit | LD50 | oral | 1600mg/kg (1600mg/kg) | Gigiena i Sanitariya. For English translation, see HYSAAV. Vol. 32(6), Pg. 12, 1967. | |
| rat | LD50 | oral | 1070mg/kg (1070mg/kg) | Gigiena i Sanitariya. For English translation, see HYSAAV. Vol. 32(6), Pg. 12, 1967. |
About|Contact|Cas|Product Name|Molecular|Country|Encyclopedia
Message|New Cas|MSDS|Service|Advertisement|CAS DataBase|Article Data|Manufacturers | Chemical Catalog
©2008 LookChem.com,License: ICP
NO.:Zhejiang16009103
complaints:service@lookchem.com Desktop View