-
Name
Hecogenin
- EINECS 207-392-4
- CAS No. 467-55-0
- Article Data24
- CAS DataBase
- Density 1.16 g/cm3
- Solubility
- Melting Point 268 °C
- Formula C27H42O4
- Boiling Point 548.9 °C at 760 mmHg
- Molecular Weight 430.628
- Flash Point 177.8 °C
- Transport Information
- Appearance White solid.
- Safety
- Risk Codes R36/37/38
-
Molecular Structure
-
Hazard Symbols
 Xi
Xi
- Synonyms 5a,25D-Spirostan-12-one, 3b-hydroxy- (7CI);5a-Spirostan-12-one, 3b-hydroxy-, (25R)- (8CI);(+)-Hecogenin;(25R)-3b-Hydroxy-5a-spirostan-12-one;(3b,5a,25R)-3-Hydroxyspirostan-12-one;12-Oxotigogenin;Gekogenin;Hocogenin;NSC 115921;
- PSA 55.76000
- LogP 4.97280
Synthetic route

| Conditions | Yield |
|---|---|
| With methanol; potassium hydroxide In dichloromethane at 20℃; for 18h; | 99% |
| With methanol; potassium carbonate In tetrahydrofuran for 24h; | 97% |
| With potassium carbonate In tetrahydrofuran; methanol at 20℃; for 12h; Inert atmosphere; | 95% |

-
-
467-55-0
hecogenin

| Conditions | Yield |
|---|---|
| With sodium perborate tetrahydrate In tetrahydrofuran; water for 3h; | 79% |

-
-
467-55-0
hecogenin

| Conditions | Yield |
|---|---|
| With sulfuric acid | 44.8% |
-
-
108747-55-3
12-methylenetigogenin

-
-
467-55-0
hecogenin

| Conditions | Yield |
|---|---|
| With sodium periodate; potassium carbonate; tert-butyl alcohol Reagens 4: Kaliumpermanganat; |

| Conditions | Yield |
|---|---|
| With pyridine; sodium borate | |
| With tetrahydrofuran; lithium aluminium tetrahydride; diethyl ether Behandeln des erhaltenen Reaktionsprodukts in Pyridin mit Bernsteinsaeure-anhydrid und mit Chrom(VI)-oxid und wss. Essigsaeure, danach mit methanol. Kalilauge; | |
| Aspergillus niger; |

| Conditions | Yield |
|---|---|
| With hydrogenchloride; ethanol |
-
-
78179-47-2
(25R)-12α-(Aminomethyl)-5α-spirostan-3β,12β-diol

-
A
-
72166-67-7
(25R)-3β-Hydroxy-C-homo-5α-spirostan-12-on

-
B
-
72166-68-8
(25R)-3β-Hydroxy-C-homo-5α-spirostan-12-on

-
D
-
467-55-0
hecogenin

| Conditions | Yield |
|---|---|
| With sodium azide; sodium nitrite Yield given. Multistep reaction. Yields of byproduct given. Title compound not separated from byproducts; |


-
-
467-55-0
hecogenin

| Conditions | Yield |
|---|---|
| With hydrogenchloride In ethanol at 100℃; for 3.5h; |

-
C
-
467-55-0
hecogenin

| Conditions | Yield |
|---|---|
| With hydrogenchloride In butan-1-ol at 70℃; for 2.5h; | A 13.5 mg B 16.5 mg C 15 mg |


-
-
467-55-0
hecogenin

| Conditions | Yield |
|---|---|
| With hydrogenchloride In ethanol at 100℃; for 3.5h; | |
| Multi-step reaction with 2 steps 1: 20 mg / 1M HCl / butan-1-ol / 2.5 h / 70 °C 2: 2M HCl / ethanol / 3.5 h / 100 °C View Scheme | |
| Multi-step reaction with 2 steps 1: 20 mg / 1M HCl / butan-1-ol / 2.5 h / 70 °C 2: 15 mg / 1M HCl / butan-1-ol / 2.5 h / 70 °C View Scheme |

| Conditions | Yield |
|---|---|
| With hydrogenchloride In butan-1-ol at 70℃; for 2.5h; | A 10 mg B 13.5 mg C 20 mg D 10 mg |

-
-
168960-81-4
25(R)-5α-spirostan-3β-ol-12-one 3-O-β-D-glucopyranosyl-(1->2)-[β-D-xylopyranosyl-(1->3)]-O-β-D-glucopyranosyl-(1->4)-β-D-galactopyranoside

-
-
467-55-0
hecogenin

| Conditions | Yield |
|---|---|
| With hydrogenchloride In 1,4-dioxane; water for 2h; Heating; | 4.9 mg |

| Conditions | Yield |
|---|---|
| With hydrogenchloride In 1,4-dioxane at 100℃; for 3h; Product distribution; Hydrolysis; | A 20.8 mg B 8.1 mg C 5.8 mg D 11.8 mg |


-
-
467-55-0
hecogenin

| Conditions | Yield |
|---|---|
| With hydrogenchloride; methanol |
-
-
7647-01-0
hydrogenchloride

-
-
17990-60-2
(25R)-3β-hydroxy-5α-spirostan-12-one-(2,4-dinitro-phenylhydrazone)

-
-
467-55-0
hecogenin


-
-
467-55-0
hecogenin

| Conditions | Yield |
|---|---|
| With hydrogenchloride In 1,4-dioxane at 98℃; for 2h; | 3.4 mg |

-
-
467-55-0
hecogenin

| Conditions | Yield |
|---|---|
| In ethanol; sulfuric acid at 100℃; for 6h; | 2 mg |
-
-
38673-31-3
(25R)-12-ethylenedioxy-5α-spirostan-3β-ol

-
-
467-55-0
hecogenin

| Conditions | Yield |
|---|---|
| Multi-step reaction with 2 steps 1: Jones' reagent 2: Aspergillus niger View Scheme |

-
-
467-55-0
hecogenin

| Conditions | Yield |
|---|---|
| Multi-step reaction with 3 steps 1: aq. KOH 2: Jones' reagent 3: Aspergillus niger View Scheme |

| Conditions | Yield |
|---|---|
| Multi-step reaction with 2 steps 1: palladium/charcoal; dioxane; methanol. KOH-solution 2: sodium borate; pyridine View Scheme |

-
-
467-55-0
hecogenin

| Conditions | Yield |
|---|---|
| Multi-step reaction with 3 steps 1: ethanol; acetic acid; zinc-powder 2: palladium/charcoal; dioxane; methanol. KOH-solution 3: sodium borate; pyridine View Scheme |

-
-
467-55-0
hecogenin

| Conditions | Yield |
|---|---|
| Multi-step reaction with 4 steps 1: sodium iodide; acetone / Behandeln des Reaktionsprodukts in Aceton und Dioxan mit Chrom(II)-chlorid in wss. Salzsaeure 2: ethanol; acetic acid; zinc-powder 3: palladium/charcoal; dioxane; methanol. KOH-solution 4: sodium borate; pyridine View Scheme |

-
-
467-55-0
hecogenin

| Conditions | Yield |
|---|---|
| Multi-step reaction with 2 steps 2: ethanol; aqueous hydrochloric acid View Scheme |
-
-
28180-63-4
(25R)-2α,3α-epoxy-5α-spirostan-12-one

-
-
467-55-0
hecogenin

| Conditions | Yield |
|---|---|
| Multi-step reaction with 2 steps 1: benzene; diethyl ether; lithium alanate / Behandeln einer Loesung des Reaktionsprodukts in Essigsaeure mit Chrom(VI)-oxid und wss. Essigsaeure 2: tetrahydrofuran; lithium alanate; diethyl ether / Behandeln des erhaltenen Reaktionsprodukts in Pyridin mit Bernsteinsaeure-anhydrid und mit Chrom(VI)-oxid und wss. Essigsaeure, danach mit methanol. Kalilauge View Scheme |
-
-
1174898-06-6
(25R)-3β-[(O-β-D-glucopyranosyl-(1->2)-O-[β-D-glucopyranosyl-(1->3)]-O-β-D-glucopyranosyl-(1->4)-β-D-galactopyranosyl)oxy]-5α-spirostan-12-one

-
-
467-55-0
hecogenin

| Conditions | Yield |
|---|---|
| With hydrogenchloride; water In 1,4-dioxane at 95℃; for 2h; Inert atmosphere; | 1.2 mg |
-
-
1499187-81-3
(25R)-3β-[(O-α-L-arabinopyranosyl-(1 → 2)-O-[β-D-xylopyranosyl-(1 → 3)]-O-β-D-glucopyranosyl-(1 → 4)-O-[α-L-rhamnopyranosyl-(1 → 2)]-β-D-galactopyranosyl)oxy]-5α-spirostan-12-one

-
A
-
58-86-6
D-xylose

-
B
-
5328-37-0
L-arabinose

-
C
-
3615-41-6
L-Rhamnose

-
D
-
50-99-7
D-glucose

-
E
-
59-23-4
D-Galactose

-
F
-
467-55-0
hecogenin

| Conditions | Yield |
|---|---|
| With naringinase; potassium acetate; acetic acid In aq. buffer at 20℃; for 49h; pH=4.3; Enzymatic reaction; | A n/a B n/a C n/a D n/a E n/a F 3.1 mg |


-
-
467-55-0
hecogenin

| Conditions | Yield |
|---|---|
| Multi-step reaction with 2 steps 1.1: 2,2'-azobis(isobutyronitrile); tris-(trimethylsilyl)silane / N,N-dimethyl acetamide; 1,4-dioxane / 48 h / Inert atmosphere; Schlenk technique; Irradiation 1.2: 2 h / Inert atmosphere; Schlenk technique; Irradiation 2.1: sodium perborate tetrahydrate / tetrahydrofuran; water / 3 h View Scheme |

| Conditions | Yield |
|---|---|
| With Jones reagent In acetone at 20℃; for 0.333333h; | 99% |
| Multi-step reaction with 4 steps 1: pyridine / Ambient temperature 2: BF3-Et2O / CH2Cl2 / Ambient temperature 3: aq. KOH 4: Jones' reagent View Scheme | |
| With pyridinium chlorochromate In dichloromethane at 0 - 20℃; for 3h; |

| Conditions | Yield |
|---|---|
| With pyridine at 20℃; | 95% |
-
-
467-55-0
hecogenin

-
-
545-77-7, 16653-52-4, 38673-26-6, 90375-72-7, 90457-38-8, 107297-25-6
3β,12β-dihydroxy-(25R)-5α-spirostane

| Conditions | Yield |
|---|---|
| With ammonia; lithium In tetrahydrofuran at -78 - -33℃; for 5h; Reduction; | 95% |
| With diethyl ether; acetic acid; platinum Hydrogenation; | |
| Stage #1: hecogenin With sodium tetrahydroborate In tetrahydrofuran; methanol at 0℃; for 2h; Stage #2: With hydrogenchloride In tetrahydrofuran; methanol; water stereoselective reaction; |
-
-
98-59-9
p-toluenesulfonyl chloride

-
-
467-55-0
hecogenin

-
-
60433-68-3
(25R)-12-oxo-5α-spirostan-3β-yl tosylate

| Conditions | Yield |
|---|---|
| With pyridine In dichloromethane at 4℃; for 24h; | 94% |
| With pyridine at 20℃; | 93% |
| With pyridine | |
| With pyridine |

| Conditions | Yield |
|---|---|
| With scandium tris(trifluoromethanesulfonate) In chloroform at 55℃; for 20h; | 93% |
-
-
149707-75-5
2,3,4,6-tetra-O-benzoyl-D-glucopyranosyl trichloroacetimidate

-
-
467-55-0
hecogenin

-
-
1373440-74-4
hecogenyl 2,3,4,6-tetra-O-benzoyl-β-D-glucopyranoside

| Conditions | Yield |
|---|---|
| With trimethylsilyl trifluoromethanesulfonate In dichloromethane at 20℃; for 1h; Inert atmosphere; Molecular sieve; | 91% |
-
-
108-24-7
acetic anhydride

-
-
467-55-0
hecogenin

-
-
245124-65-6
(25R)-23-acetyl-22,26-epoxy-12-oxo-5α-cholesta-22-ene-3β,16β-diyl diacetate

| Conditions | Yield |
|---|---|
| With boron trifluoride diethyl etherate at 20℃; for 0.833333h; Ring cleavage; acetylation; | 87% |
| With boron trifluoride diethyl etherate at 20℃; for 0.166667h; | 87% |
-
-
321125-08-0
2-O-(4-methoxybenzoyl)-3,4-di-O-triethylsilyl-β-D-xylopyranosyl-(1→3)-2-O-acetyl-4-O-triethylsilyl-α,β-L-arabinopyranosyl trichloroacetimidate

-
-
467-55-0
hecogenin

| Conditions | Yield |
|---|---|
| With trimethylsilyl trifluoromethanesulfonate; 4 Angstroem MS In dichloromethane at -20℃; | 86% |
-
-
108-24-7
acetic anhydride

-
-
467-55-0
hecogenin

-
-
1228298-02-9
(25R)-26-hydroxy-12,22-dioxo-5α-cholestan-3β,16β-diyl diacetate

| Conditions | Yield |
|---|---|
| Stage #1: acetic anhydride; hecogenin With boron trifluoride diethyl etherate In dichloromethane at 0℃; Stage #2: With water In dichloromethane Cooling with ice; | 86% |
| With boron trifluoride diethyl etherate In dichloromethane at 0℃; for 0.25h; | 86% |
| With boron trifluoride diethyl etherate In dichloromethane at 0℃; for 0.5h; |
-
-
18162-48-6
tert-butyldimethylsilyl chloride

-
-
467-55-0
hecogenin

-
-
909880-00-8
(25R)-3β-(tert-butyl(dimethyl)silyloxy)-5α-spirostan-12-one

| Conditions | Yield |
|---|---|
| With 1H-imidazole In N,N-dimethyl-formamide for 3h; | 84% |
-
-
20295-82-3
O-carboxymethylhydroxylamine hydrochloride

-
-
467-55-0
hecogenin

| Conditions | Yield |
|---|---|
| With pyridine at 20℃; | 84% |
-
-
146728-55-4
3,4,6-tri-O-acetyl-2-deoxy-2-phthalimido-(α/β)-D-glucopyranosyl trichloroacetimidate

-
-
467-55-0
hecogenin

-
-
869884-28-6
hecogenin 3,4,6-tri-O-acetyl-2-deoxy-2-phthalimido-β-D-glucopyranoside

| Conditions | Yield |
|---|---|
| With trimethylsilyl trifluoromethanesulfonate In dichloromethane at -20℃; for 1h; Molecular sieve; | 82% |

| Conditions | Yield |
|---|---|
| With triethylamine In dichloromethane at 0 - 20℃; Inert atmosphere; | 80% |
| With triethylamine In dichloromethane at 0 - 20℃; Inert atmosphere; | 80% |
-
-
467-55-0
hecogenin

-
-
1432631-48-5
(25R)-3α-amino-5α-spirostan-12-one

| Conditions | Yield |
|---|---|
| Stage #1: hecogenin With methanesulfonyl chloride; triethylamine In dichloromethane Stage #2: With sodium azide In dimethyl sulfoxide at 70℃; Stage #3: With 10 wt% Pd(OH)2 on carbon; hydrogen | 79% |
| Multi-step reaction with 3 steps 1: pyridine 2: sodium azide / 60 °C 3: triphenylphosphine; water / tetrahydrofuran / 20 °C View Scheme |

| Conditions | Yield |
|---|---|
| With dmap In dichloromethane at 55℃; for 3h; | 79% |

| Conditions | Yield |
|---|---|
| With pyridine at 20℃; | 78% |
| In ethanol at 20℃; |
-
-
38901-29-0
N-(2-(trifluoromethyl)phenyl)hydrazinecarbothioamide

-
-
467-55-0
hecogenin

| Conditions | Yield |
|---|---|
| With pyridine at 20℃; | 75% |

| Conditions | Yield |
|---|---|
| With pyridine at 20℃; | 75% |
-
-
73305-12-1
4-(2'-nitrophenyl)-3-thiosemicarbazide

-
-
467-55-0
hecogenin

| Conditions | Yield |
|---|---|
| With pyridine at 20℃; | 72% |

| Conditions | Yield |
|---|---|
| Stage #1: hecogenin With pyridine; dmap; triethylamine at 20℃; for 0.333333h; Stage #2: 2-furancarbonyl chloride | 71% |
-
-
21615-34-9
2-Methoxybenzoyl chloride

-
-
467-55-0
hecogenin

| Conditions | Yield |
|---|---|
| Stage #1: hecogenin With pyridine; dmap; triethylamine at 20℃; for 0.333333h; Stage #2: 2-Methoxybenzoyl chloride | 70% |

| Conditions | Yield |
|---|---|
| With pyridine In toluene at 20 - 50℃; | 69.5% |

| Conditions | Yield |
|---|---|
| With 3-chloro-benzenecarboperoxoic acid In dichloromethane at 0 - 20℃; for 120h; Baeyer-Villiger oxidation; Darkness; | 69% |
-
-
467-55-0
hecogenin

-
-
10007-76-8
(25R)-5α-spirostan-3β,12β-diol diacetate

| Conditions | Yield |
|---|---|
| With sodium tetrahydroborate In 1,4-dioxane; methanol at 10℃; for 0.5h; | 68% |
| Multi-step reaction with 2 steps 1: 95 percent / liquid NH3; Li / tetrahydrofuran / 5 h / -78 - -33 °C 2: 90 percent / NEt3; DMAP / CH2Cl2 / 5.5 h View Scheme | |
| Multi-step reaction with 2 steps 1: NaBH4 / dioxane; methanol / 0.5 h / 10 °C 2: 8.2 g / pyridine View Scheme | |
| Multi-step reaction with 2 steps 1: platinum; acetic acid; diethyl ether / Hydrogenation View Scheme |

| Conditions | Yield |
|---|---|
| Stage #1: hecogenin With pyridine; dmap; triethylamine at 20℃; for 0.333333h; Stage #2: 4-cyanobenzoyl chlorIde | 68% |
-
-
42135-75-1
4-(2'-chlorophenyl)-3-thiosemicarbazide

-
-
467-55-0
hecogenin

| Conditions | Yield |
|---|---|
| With pyridine at 20℃; | 65% |

| Conditions | Yield |
|---|---|
| With dmap In dichloromethane at 0 - 20℃; | 64% |

| Conditions | Yield |
|---|---|
| Stage #1: hecogenin With pyridine; dmap; triethylamine at 20℃; for 0.333333h; Stage #2: 4-nitro-benzoyl chloride | 63% |
Hecogenin Specification
The Hecogenin, with the CAS registry number 467-55-0, is also known as (25R)-3b-Hydroxy-5a-spirostan-12-one. It belongs to the product categories of Steroids; Biochemistry; Steroids (Others). Its EINECS registry number is 207-392-4. This chemical's molecular formula is C27H42O4 and molecular weight is 430.62. What's more, its systematic name is called (3β,5α,25R)-3-Hydroxyspirostan-12-one.
Physical properties about Hecogenin are: (1)ACD/LogP: 4.22; (2)# of Rule of 5 Violations: 0; (3)ACD/LogD (pH 5.5): 4.22; (4)ACD/LogD (pH 7.4): 4.22; (5)ACD/BCF (pH 5.5): 947.44; (6)ACD/BCF (pH 7.4): 947.44; (7)ACD/KOC (pH 5.5): 4701.29; (8)ACD/KOC (pH 7.4): 4701.29; (9)#H bond acceptors: 4; (10)#H bond donors: 1; (11)#Freely Rotating Bonds: 1; (12)Polar Surface Area: 44.76 Å2; (13)Index of Refraction: 1.558; (14)Molar Refractivity: 119.77 cm3; (15)Molar Volume: 371.1 cm3; (16)Surface Tension: 46.3 dyne/cm; (17)Density: 1.16 g/cm3; (18)Flash Point: 177.8 °C; (19)Enthalpy of Vaporization: 95.25 kJ/mol; (20)Boiling Point: 548.9 °C at 760 mmHg; (21)Vapour Pressure: 2.45E-14 mmHg at 25 °C.
Preparation of Hecogenin: this chemical can be prepared by a-D-Glucopyranosyl(1->4)a-D-glucopyranosyl(1->4)a-D-glucopyranosyl(1->3)hecogenin. This reaction needs reagent (NH2)2SO4. The yield is 44.8 %.
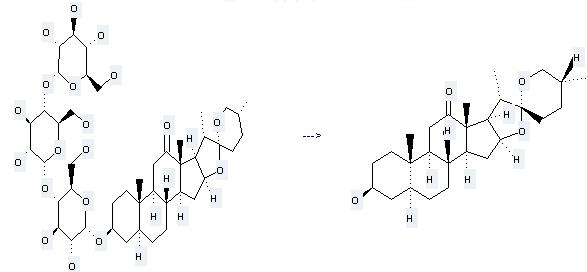
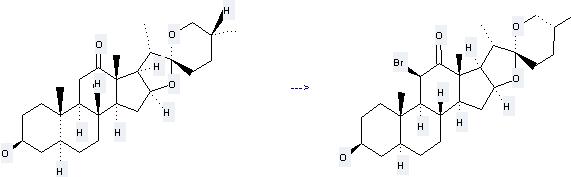
Uses of Hecogenin: (1) it is used in the preparation of steroidal hormones; (2) it is used to produce other chemicals. For example, it can produce 3b-Hydroxy-11b-bromo-12-oxo-(25R)-5a-spirostane. The reaction occurs with reagents Br2, HCl and solvents methanol, CH2Cl2. The yield is 59 %.
You can still convert the following datas into molecular structure:
(1) SMILES: O=C2C[C@@H]1[C@@]6(C)CC[C@H](O)C[C@@H]6CC[C@H]1[C@H]5[C@]2([C@@H]4[C@@H](O[C@@]3(OC[C@@H](CC3)C)[C@H]4C)C5)C
(2) InChI: InChI=1S/C27H42O4/c1-15-7-10-27(30-14-15)16(2)24-22(31-27)12-21-19-6-5-17-11-18(28)8-9-25(17,3)20(19)13-23(29)26(21,24)4/h15-22,24,28H,5-14H2,1-4H3/t15-,16+,17+,18+,19-,20+,21+,22+,24+,25+,26-,27-/m1/s1
(3) InChIKey: QOLRLLFJMZLYQJ-LOBDNJQFSA-N
About|Contact|Cas|Product Name|Molecular|Country|Encyclopedia
Message|New Cas|MSDS|Service|Advertisement|CAS DataBase|Article Data|Manufacturers | Chemical Catalog
©2008 LookChem.com,License: ICP
NO.:Zhejiang16009103
complaints:service@lookchem.com Desktop View