-
Name
Homovanillic acid
- EINECS 206-176-7
- CAS No. 306-08-1
- Article Data40
- CAS DataBase
- Density 1.307 g/cm3
- Solubility Soluble in water
- Melting Point 142-145 °C(lit.)
- Formula C9H10O4
- Boiling Point 368.7 °C at 760 mmHg
- Molecular Weight 182.176
- Flash Point 151.9 °C
- Transport Information
- Appearance white to beige crystalline powder
- Safety 26-36
- Risk Codes 36/37/38
-
Molecular Structure
-
Hazard Symbols
 Xi
Xi
- Synonyms Aceticacid, (4-hydroxy-3-methoxyphenyl)- (6CI,8CI);(4-Hydroxy-3-methoxyphenyl)aceticacid;3-Methoxy-4-hydroxyphenylacetic acid;4-Hydroxy-3-methoxybenzeneaceticacid;HVA;NSC 16682;Vanilacetic acid;
- PSA 66.76000
- LogP 1.02790
Synthetic route

| Conditions | Yield |
|---|---|
| With sodium hydroxide In ethanol; water at 80℃; for 48h; Inert atmosphere; | 89% |
| With potassium hydroxide In ethanol Heating; | 74% |
| With sodium hydroxide In 2-methoxy-ethanol |

| Conditions | Yield |
|---|---|
| With hydrogenchloride; tin(ll) chloride In water at 80℃; for 4h; | 88% |
| 75% | |
| With 5%-palladium/activated carbon; hydrogen; acetic acid In water |
-
-
13244-77-4
3-methoxy-4-hydroxy mandelic acid

-
-
306-08-1
Homovanillic acid

| Conditions | Yield |
|---|---|
| With formic acid; phosphoric acid; sodium hydrogensulfite In water at 100℃; under 3750.3 Torr; for 9h; | 71% |
-
-
6391-59-9
4-Acetoxy-3-methoxyphenylacetaldehyde

-
A
-
306-08-1
Homovanillic acid

-
B
-
5447-38-1
2-(4-acetoxy-3-methoxyphenyl)acetic acid

| Conditions | Yield |
|---|---|
| With potassium permanganate; magnesium sulfate; acetone |
-
-
60470-82-8, 39600-31-2
4-(4-acetoxy-3-methoxy-benzylidene)-2-methyl-4H-oxazol-5-one

-
-
306-08-1
Homovanillic acid

| Conditions | Yield |
|---|---|
| With sodium hydroxide anschliessend Behandeln mit wss. H2O2; |

| Conditions | Yield |
|---|---|
| With sodium hydroxide |
-
-
18692-68-7, 71413-82-6, 74857-79-7
m-methoxy-p-acetoxy-α-benzoylaminocinnamic acid azlactone

-
-
306-08-1
Homovanillic acid

| Conditions | Yield |
|---|---|
| folgend Behandeln mit Wasserstoffperoxyd in Eisessig; |

| Conditions | Yield |
|---|---|
| With potassium hydroxide |
-
-
873376-14-8
1-benzoyloxy-4-chloromethyl-2-methoxy-benzene

-
-
306-08-1
Homovanillic acid

| Conditions | Yield |
|---|---|
| With potassium cyanide anschliessend Erhitzen mit wss. Natronlauge; |
-
-
23513-13-5, 104186-09-6, 555-66-8
[6]-shogaol

-
A
-
306-08-1
Homovanillic acid

| Conditions | Yield |
|---|---|
| for 168h; biotransformation by aspergillus niger; | A 60 mg B 36 mg |
-
-
29973-91-9
4-benzyloxy-3-methoxyphenylacetic acid

-
-
306-08-1
Homovanillic acid

| Conditions | Yield |
|---|---|
| With hydrogen; palladium on activated charcoal In ethanol |
-
-
458-36-6
trans-coniferyl aldehyde

-
A
-
824-46-4
methoxyhydroquinone

-
B
-
121-34-6
3-methoxy-4-hydroxybenzoic acid

-
C
-
306-08-1
Homovanillic acid

-
D
-
121-33-5
vanillin

| Conditions | Yield |
|---|---|
| With peracetic acid In ethanol for 1h; pH=7; Product distribution; Further Variations:; Reagents; Oxidation; | A 10.1 % Chromat. B 13.6 % Chromat. C 10.9 % Chromat. D 7.1 % Chromat. |

-
-
6391-59-9
4-Acetoxy-3-methoxyphenylacetaldehyde

-
-
7487-88-9
magnesium sulfate

-
-
67-64-1
acetone

-
A
-
306-08-1
Homovanillic acid

-
B
-
5447-38-1
2-(4-acetoxy-3-methoxyphenyl)acetic acid


-
-
306-08-1
Homovanillic acid

| Conditions | Yield |
|---|---|
| With sodium hydroxide man gibt zu der Loesung erst Eisessig und dann H2O2; |
-
-
91054-36-3
(3-methoxy-4-oxo-cyclohexa-2,5-dienyliden)-acetonitrile

-
-
10034-85-2
hydrogen iodide

-
A
-
306-08-1
Homovanillic acid

| Conditions | Yield |
|---|---|
| 5-min. Erhitzen; |

-
-
284043-11-4
4-amino-butyric acid 2-(4-hydroxy-3-methoxy-phenyl)-2-oxo-ethyl ester

-
A
-
306-08-1
Homovanillic acid

-
B
-
18256-48-9
2,4'-dihydroxy-3'-methoxyacetophenone

-
C
-
498-02-2
1-(3-methoxy-4-hydroxyphenyl)ethanone

-
D
-
56-12-2
4-amino-n-butyric acid

| Conditions | Yield |
|---|---|
| With water Hydrolysis; reduction; rearrangement; UV-irradiation; |


-
A
-
56-86-0
L-glutamic acid

-
B
-
306-08-1
Homovanillic acid

-
C
-
18256-48-9
2,4'-dihydroxy-3'-methoxyacetophenone

-
D
-
498-02-2
1-(3-methoxy-4-hydroxyphenyl)ethanone

| Conditions | Yield |
|---|---|
| With water Hydrolysis; rearrangement; reduction; UV-irradiation; |


| Conditions | Yield |
|---|---|
| Multi-step reaction with 2 steps 1: 60 percent / dimethylformamide / 20 h / 130 °C 2: 74 percent / KOH / ethanol / Heating View Scheme | |
| Multi-step reaction with 2 steps 1: N,N-dimethyl-formamide / 24 h / 120 °C / Inert atmosphere 2: sodium hydroxide / ethanol; water / 48 h / 80 °C / Inert atmosphere View Scheme |
-
-
69638-06-8
α-bromo-3-methoxy-4-hydroxyacetophenone

-
-
306-08-1
Homovanillic acid

| Conditions | Yield |
|---|---|
| Multi-step reaction with 3 steps 1: DBU / dioxane / 12 h / 0 - 20 °C 2: TFA / 4 h / 0 °C 3: H2O / UV-irradiation View Scheme | |
| Multi-step reaction with 3 steps 1: 57 percent / DBU / benzene / 12 h / 20 °C 2: 95.3 percent / TFA / 4 h / 0 °C 3: H2O / UV-irradiation View Scheme |

| Conditions | Yield |
|---|---|
| Multi-step reaction with 4 steps 1: 47 percent / cupric bromide / CHCl3; ethyl acetate / 4 h / 20 °C 2: DBU / dioxane / 12 h / 0 - 20 °C 3: TFA / 4 h / 0 °C 4: H2O / UV-irradiation View Scheme | |
| Multi-step reaction with 4 steps 1: 47 percent / cupric bromide / CHCl3; ethyl acetate / 4 h / 20 °C 2: 57 percent / DBU / benzene / 12 h / 20 °C 3: 95.3 percent / TFA / 4 h / 0 °C 4: H2O / UV-irradiation View Scheme |
-
-
284043-13-6
2-(4-hydroxy-3-methoxyphenyl)-2-oxoethyl-4-[(tert-butoxycarbonyl)amino]butanoate

-
-
306-08-1
Homovanillic acid

| Conditions | Yield |
|---|---|
| Multi-step reaction with 2 steps 1: TFA / 4 h / 0 °C 2: H2O / UV-irradiation View Scheme |
-
-
284043-05-6
γ-O-(4-hydroxy-3-methoxyphenacetyl) t-butyl N-t-boc L-glutamate

-
-
306-08-1
Homovanillic acid

| Conditions | Yield |
|---|---|
| Multi-step reaction with 2 steps 1: 95.3 percent / TFA / 4 h / 0 °C 2: H2O / UV-irradiation View Scheme |
-
-
16209-54-4
methyl 4-benzyloxy-3-methoxyphenylethanoate

-
-
306-08-1
Homovanillic acid

| Conditions | Yield |
|---|---|
| Multi-step reaction with 2 steps 1: 10percent KOH / methanol; H2O 2: H2 / 10percent Pd-C / aq. ethanol View Scheme |
-
-
174840-51-8
4-(hydroxymethyl)-2-methoxyphenyl benzoate

-
-
306-08-1
Homovanillic acid

| Conditions | Yield |
|---|---|
| Multi-step reaction with 2 steps 1: calcium chloride; thionyl chloride 2: aqueous potassium cyanide / anschliessend Erhitzen mit wss. Natronlauge View Scheme |

| Conditions | Yield |
|---|---|
| Multi-step reaction with 2 steps 1: ethyl acetate; ozone / und anschliesende Hydrierung an Palladium/Kohle 2: potassium permanganate; magnesium sulfate; aqueous acetone View Scheme | |
| Multi-step reaction with 2 steps 1: KMnO4; water 2: NaOH-solution View Scheme |

| Conditions | Yield |
|---|---|
| Multi-step reaction with 3 steps 2: KMnO4; water 3: NaOH-solution View Scheme |
-
-
42973-53-5
2-methoxy-4-((methylamino)methyl)phenol

-
-
306-08-1
Homovanillic acid

| Conditions | Yield |
|---|---|
| Multi-step reaction with 2 steps 1: dimethylformamide 2: aq. NaOH / 2-methoxy-ethanol View Scheme |

| Conditions | Yield |
|---|---|
| Multi-step reaction with 3 steps 1: (i) EtOH, (ii) H2, PtO2 2: dimethylformamide 3: aq. NaOH / 2-methoxy-ethanol View Scheme | |
| Multi-step reaction with 3 steps 1: sodium tetrahydroborate / methanol / 24.5 h / 0 - 20 °C / Inert atmosphere 2: N,N-dimethyl-formamide / 24 h / 120 °C / Inert atmosphere 3: sodium hydroxide / ethanol; water / 48 h / 80 °C / Inert atmosphere View Scheme |
-
-
215872-63-2
8-O-4′-diferulic acid

-
A
-
1135-24-6
3-(4-hydroxy-3-methoxyphenyl)acrylic acid

-
B
-
621-54-5
3-(3-hydroxyphenyl)-propanoic acid

-
C
-
1135-23-5
3-(4-hydroxy-3-methoxyphenyl)propionic acid

-
D
-
331-39-5
caffeic acid

-
E
-
102-32-9
3,4-dihydroxyphenylacetate

-
F
-
306-08-1
Homovanillic acid

-
G
-
1078-61-1
dihydrocaffeic acid

-
H
-
23028-17-3
danshensu

-
I
-
2475-56-1
3-(4-hydroxy-3-methoxyphenyl)lactic acid

-
J
-
1081-71-6
3-(4-hydroxy-3-methoxyphenyl)-2-oxopropanoic acid

| Conditions | Yield |
|---|---|
| With D-glucose In dimethyl sulfoxide at 37℃; aq. buffer; anaerobic atmosphere; Enzymatic reaction; |

-
-
67-56-1
methanol

-
-
306-08-1
Homovanillic acid

-
-
15964-80-4
2-(4-hydroxy-3-methoxyphenyl)acetic acid methyl ester

| Conditions | Yield |
|---|---|
| With sulfuric acid for 3h; Reflux; | 100% |
| With toluene-4-sulfonic acid | |
| With sulfuric acid at 20℃; for 4h; Reflux; |
-
-
306-08-1
Homovanillic acid

-
-
75-26-3
isopropyl bromide

-
-
1256581-67-5
isopropyl 2-(4-isopropoxy-3-methoxyphenyl)acetate

| Conditions | Yield |
|---|---|
| With potassium carbonate In N,N-dimethyl-formamide for 24h; Reflux; Inert atmosphere; | 100% |
-
-
306-08-1
Homovanillic acid

-
-
108-46-3
recorcinol

-
-
40456-49-3
1-(2,4-dihydroxyphenyl)-2-(4'-hydroxy-3'-methoxyphenyl)ethanone

| Conditions | Yield |
|---|---|
| With boron trifluoride diethyl etherate at 60 - 70℃; for 1h; | 99% |
| With bis(trifluoromethanesulfonyl)amide In various solvent(s) at 90℃; for 0.0666667h; Friedel-Crafts reaction; microwave irradiation; | 70% |
| With boron trifluoride diethyl etherate In N,N-dimethyl-formamide at 120℃; for 0.166667h; Friedel-Crafts reaction; | 57% |

| Conditions | Yield |
|---|---|
| With sulfuric acid for 2h; Reflux; | 99% |
| With sulfuric acid | 99% |
| With sulfuric acid for 2h; Reflux; | 80% |
| With sulfuric acid Reflux; | |
| With sulfuric acid at 90℃; for 7h; |
-
-
306-08-1
Homovanillic acid

-
-
18162-48-6
tert-butyldimethylsilyl chloride

-
-
78324-15-9
tert-butyldimethyl 2-(4-(tert-butyldimethylsilyloxy)-3-methoxyphenyl)acetate

| Conditions | Yield |
|---|---|
| With 1H-imidazole In tetrahydrofuran at 20℃; for 14h; Inert atmosphere; | 98% |
| With 1H-imidazole In tetrahydrofuran at 20℃; for 14h; Inert atmosphere; | 64% |
| With 1H-imidazole In N,N-dimethyl-formamide at 0 - 20℃; for 3h; |

| Conditions | Yield |
|---|---|
| With Agaricus bisporus; oxygen In aq. phosphate buffer; dichloromethane at 25℃; for 24h; pH=7; | 98% |
-
-
306-08-1
Homovanillic acid

-
-
108-24-7
acetic anhydride

-
-
5447-38-1
2-(4-acetoxy-3-methoxyphenyl)acetic acid

| Conditions | Yield |
|---|---|
| With sulfuric acid | 97% |
| With sulfuric acid Ambient temperature; | 79% |
-
-
68579-60-2
2-(methylamino)-1-phenylethanol

-
-
306-08-1
Homovanillic acid

-
-
749247-36-7
2-(4-hydroxy-3-methoxy-phenyl)-N-(2-hydroxy-2-phenyl-ethyl)-N-methyl-acetamide

| Conditions | Yield |
|---|---|
| With benzotriazol-1-ol; N-(3-dimethylaminopropyl)-N-ethylcarbodiimide In dichloromethane | 97% |

| Conditions | Yield |
|---|---|
| Stage #1: 4-Methylbenzyl alcohol; Homovanillic acid With dmap; dicyclohexyl-carbodiimide In 1,2-dichloro-ethane at 55℃; for 4h; Stage #2: With sodium tetrahydroborate In tetrahydrofuran for 2h; Temperature; Inert atmosphere; Cooling with ice; | 96.8% |
-
-
306-08-1
Homovanillic acid

-
-
100-39-0
benzyl bromide

-
-
65340-85-4
benzyl 2-(4-(benzyloxy)-3-methoxyphenyl)acetate

| Conditions | Yield |
|---|---|
| With potassium carbonate In acetonitrile for 4h; Reflux; | 95% |
| With potassium carbonate In acetonitrile at 80℃; for 8h; | 88% |
| With sodium In methanol |
-
-
358-23-6
trifluoromethylsulfonic anhydride

-
-
306-08-1
Homovanillic acid

-
-
1243245-99-9
2-(3-methoxy-4-(trifluoromethylsulfonyloxy)phenyl)acetic acid

| Conditions | Yield |
|---|---|
| With triethylamine In dichloromethane at 0℃; | 95% |
-
-
306-08-1
Homovanillic acid

-
-
55-81-2
4-Methoxyphenethylamine

-
-
1395084-66-8
2-(4-hydroxy-3-methoxyphenyl)-N-[2-(4-methoxyphenyl)ethyl]acetamide

| Conditions | Yield |
|---|---|
| In 5,5-dimethyl-1,3-cyclohexadiene for 16h; Reflux; Inert atmosphere; | 95% |
-
-
306-08-1
Homovanillic acid

-
-
122149-21-7
N-(benzyloxy)octan-1-amine

-
-
1400943-51-2
N-benzyloxy-N-octyl-2-(4-hydroxy-3-methoxyphenyl)acetamide

| Conditions | Yield |
|---|---|
| With benzotriazol-1-ol; 1-ethyl-(3-(3-dimethylamino)propyl)-carbodiimide hydrochloride In dichloromethane for 4.5h; Inert atmosphere; | 94% |
-
-
306-08-1
Homovanillic acid

-
-
112151-61-8
O-benzyl-N-heptylhydroxylamine

-
-
1400943-49-8
N-benzyloxy-N-heptyl-2-(4-hydroxy-3-methoxyphenyl)acetamide

| Conditions | Yield |
|---|---|
| With benzotriazol-1-ol; 1-ethyl-(3-(3-dimethylamino)propyl)-carbodiimide hydrochloride In dichloromethane | 93% |
-
-
306-08-1
Homovanillic acid

-
-
850163-67-6
Phenyl-acetic acid (1aR,1bS,4aS,7aS,7bS,8R,9R,9aS)-9a-acetoxy-4a,7b-dihydroxy-3-hydroxymethyl-1,1,6,8-tetramethyl-5-oxo-1a,1b,4,4a,5,7a,7b,8,9,9a-decahydro-1H-cyclopropa[3,4]benzo[1,2-e]azulen-9-yl ester

-
-
777859-96-8
13-acetyl-20-homovanillyl-12-phenylacetyl-4α-phorbol

| Conditions | Yield |
|---|---|
| With di-tert-butyl-diazodicarboxylate; triphenylphosphine In tetrahydrofuran at 20℃; for 1h; | 91% |

| Conditions | Yield |
|---|---|
| In dichloromethane | 90% |


| Conditions | Yield |
|---|---|
| molecular sieve In dichloromethane | 90% |
| With dmap; 1-ethyl-(3-(3-dimethylamino)propyl)-carbodiimide hydrochloride In N,N-dimethyl-formamide at 20℃; for 15h; |
-
-
306-08-1
Homovanillic acid

-
-
100-46-9
benzylamine

-
-
391609-30-6
N-benzyl-4-hydroxy-3-methoxyphenylacetamide

| Conditions | Yield |
|---|---|
| With 4 A molecular sieve at 140 - 150℃; for 3h; | 89% |
-
-
306-08-1
Homovanillic acid

-
-
3718-88-5
3-iodobenzylamine hydrochloride

-
-
873099-30-0
2-(4-hydroxy-3-methoxyphenyl)-N-(3-iodobenzyl)acetamide

| Conditions | Yield |
|---|---|
| With 1-hydroxy-7-aza-benzotriazole; triethylamine; dicyclohexyl-carbodiimide In tetrahydrofuran at 20℃; | 88% |

| Conditions | Yield |
|---|---|
| With benzotriazol-1-ol; 1-ethyl-(3-(3-dimethylamino)propyl)-carbodiimide hydrochloride; triethylamine In N,N-dimethyl-formamide at 70℃; for 4h; | 88% |
-
-
67-56-1
methanol

-
-
306-08-1
Homovanillic acid

-
-
149-73-5
trimethyl orthoformate

-
-
15964-80-4
2-(4-hydroxy-3-methoxyphenyl)acetic acid methyl ester

| Conditions | Yield |
|---|---|
| With sulfuric acid for 18h; Heating / reflux; | 85% |

-
-
844901-56-0
3,3-dimethyl-5-[4-(trifluoromethyl)phenyl]-2,3-dihydro-1H-indole

-
-
306-08-1
Homovanillic acid

-
-
847064-35-1
4-(2-{3,3-dimethyl-5-[4-(trifluoromethyl)phenyl]-2,3-dihydro-1H-indol-1-yl}-2-oxoethyl)-2-methoxyphenol

| Conditions | Yield |
|---|---|
| With 1-ethyl-(3-(3-dimethylamino)propyl)-carbodiimide hydrochloride In 1,2-dichloro-ethane at 0 - 20℃; | 82% |

| Conditions | Yield |
|---|---|
| With magnesium sulfate; novozyme 435 In toluene at 50℃; for 16h; Enzymatic reaction; | 81.5% |
-
-
306-08-1
Homovanillic acid

-
-
120-20-7
2-(3,4-dimethoxyphenyl)-ethylamine

-
-
361389-65-3
N-[2-(3,4-dimethoxyphenyl)ethyl]-2-(3-hydroxy-4-methoxyphenyl)acetamide

| Conditions | Yield |
|---|---|
| With O-(1H-benzotriazol-1-yl)-N,N,N',N'-tetramethyluronium hexafluorophosphate; N-ethyl-N,N-diisopropylamine In N,N-dimethyl-formamide at 20℃; Inert atmosphere; | 79% |
| With O-(1H-benzotriazol-1-yl)-N,N,N',N'-tetramethyluronium hexafluorophosphate; N-ethyl-N,N-diisopropylamine In N,N-dimethyl-formamide at 20℃; Inert atmosphere; | 79% |
| With 1-ethyl-(3-(3-dimethylamino)propyl)-carbodiimide hydrochloride; triethylamine In dichloromethane at 20℃; |
-
-
71-23-8
propan-1-ol

-
-
306-08-1
Homovanillic acid

-
-
52744-26-0
Propyl-2-(4-hydroxy-3-methoxy-phenyl)acetate

| Conditions | Yield |
|---|---|
| With acetyl chloride at 60℃; for 3h; | 78.2% |
| With sulfuric acid at 90℃; for 7h; |

| Conditions | Yield |
|---|---|
| With di-isopropyl azodicarboxylate; thiamine diphosphate In tetrahydrofuran at 20℃; Mitsunobu esterification; | 78% |

| Conditions | Yield |
|---|---|
| With sulfuric acid In toluene for 2h; Dean-Stark; Reflux; | 77.3% |
-
-
110-89-4
piperidine

-
-
306-08-1
Homovanillic acid

-
-
53283-49-1
2-(4-Hydroxy-3-methoxy-phenyl)-1-piperidin-1-yl-ethanone

| Conditions | Yield |
|---|---|
| With dmap; 1-ethyl-(3-(3-dimethylamino)propyl)-carbodiimide hydrochloride In N,N-dimethyl-formamide at 20℃; for 3h; | 75% |
Homovanillic acid Chemical Properties
IUPAC Name: 2-(4-Hydroxy-3-methoxyphenyl)acetic acid
Molecular Structure:
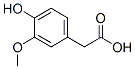
Molecular Formula: C9H10O4
Molecular Weight: 182.1733
Synonyms of Homovanillic acid (CAS NO.306-08-1): Acetic acid, (4-hydroxy-3-methoxyphenyl)-; Vanilacetic acid; 4-hydroxy-3-methoxy-benzeneaceticaci; 4-hydroxy-3-methoxy-Benzeneaceticacid; Vanillacetic Acid
Melting Point: 142-145 °C(lit.)
Flash Point: 151.9 °C
Boiling Point: 368.7 °C at 760 mmHg
Density: 1.307 g/cm3
Vapour Pressure: 4.36E-06 mmHg at 25°C
Index of Refraction: 1.573
Storage temp.: Store at RT.
Water Solubility: soluble
Solvent Solubility: soluble in benzene, slightly soluble in alcohol, ether, insoluble in cyclohexane
Appearance: almost a white to brown-gray powder.
Homovanillic acid Uses
Homovanillic acid (CAS NO.306-08-1) is a major metabolite of catecholamine metabolism. It is used as a reagent for the determination of oxidative enzymes.
Homovanillic acid Safety Profile
Hazard Codes of Homovanillic acid (CAS NO.306-08-1):  Xi
Xi
Risk Statements: 36/37/38
R36/37/38: Irritating to eyes, respiratory system and skin.
Safety Statements: 26-36
S26: In case of contact with eyes, rinse immediately with plenty of water and seek medical advice.
S36: Wear suitable protective clothing.
WGK Germany: 3
F: 10-23
TSCA: T
HazardClas:s IRRITANT
Homovanillic acid Standards and Recommendations
ASSAY: 98.0% min
Homovanillic acid Specification
Storage: Keep container in a cool, well-ventilated area. Keep container tightly closed.
About|Contact|Cas|Product Name|Molecular|Country|Encyclopedia
Message|New Cas|MSDS|Service|Advertisement|CAS DataBase|Article Data|Manufacturers | Chemical Catalog
©2008 LookChem.com,License: ICP
NO.:Zhejiang16009103
complaints:service@lookchem.com Desktop View