-
Name
Hydroxocobalamin
- EINECS 236-533-2
- CAS No. 13422-51-0
- Article Data10
- CAS DataBase
- Density
- Solubility Methanol: 10 mg/mL at 20 °C, clear, dark red
- Melting Point 200oC (decomposes)
- Formula C62H89CoN13O15P
- Boiling Point
- Molecular Weight 1347.44
- Flash Point
- Transport Information
- Appearance dark red crystal or crystalline powder
- Safety 26-36
- Risk Codes 36/37/38
-
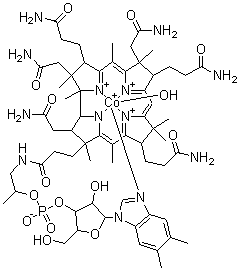
Molecular Structure
-
Hazard Symbols
 Xi
Xi
- Synonyms Cobinamide,dihydroxide, dihydrogen phosphate (ester), mono(inner salt), 3'-ester with5,6-dimethyl-1-a-D-ribofuranosyl-1H-benzimidazole;AlphaRedisol;Axlon;Ciplamin H;Cobalamin, hydroxo-;Cobalex;Cobalin H;Docclan;Docevita;Droxomin;Ducobee Hy;Hydrocobalamin;Hydrovit;Hydroxy vitamin B12;Hyxobamine;Neo-Betalin 12;Neo-Cytamen;Neo-Rojamin;OH-Duphar;Primabalt RP;Redisol H;Vibeden;Vitadurin;Vitamin B12a;
- PSA 484.10000
- LogP 6.43870
Synthetic route

| Conditions | Yield |
|---|---|
| In water vitamin B12a reacted with NaOH in H2O; |

| Conditions | Yield |
|---|---|
| With titanium (III) citrate In water-d2 Schlenk technique; C2H2 was allowed to equilibrate for 4 h under Tris buffer in D2O (pD 9.4) and soln. of Ti(III) citrate in D2O; Co complex wasreacted with Ti(III) citrate (60 equiv.) in Tris buffer in D2O and inje cted; soln. was stirred for 1 h; extd. (phenol/CH2Cl2, 1/1); phenol extracts washed (distd. H2O); dild. with 1-butanol/CH2Cl2 (1/1); extd. (distd. H2O); lyophilized; dissolved in ammonium acetate buffer (pH 4); chromd. (buffer/MeCN, 95/5 to 70/30); | 85% |


| Conditions | Yield |
|---|---|
| With acetate or citrate buffer or aq. HClO4 In not given (N2); dissolving hydroxocobalamin in buffer or aq. HClO4; |
-
-
13422-51-0
hydroxocob(III)alamin

-
-
19255-48-2
9-(2-chloroethyl)adenine

-
-
59209-78-8
(adenylethyl)cobalamine

| Conditions | Yield |
|---|---|
| With NaBH4 In ethanol; water Soln. of NaBH4 in water was added to a soln. of hydroxocobalamine in water and 9-(2-chloroethyl)adenine in ethanol, mixt. was stirred for 30 min under N2;; excess NaBH4 was quenched with acetone, excess of the ligand was filtered; soln. was desalted on an Amberlite XAD-2 column; product was purified with a SP-Sephadex column, eluent - H2O; eluate was concd., product was pptd. by addn. of acetone;; |
-
-
13422-51-0
hydroxocob(III)alamin

-
-
19255-49-3
9-(3-chloropropyl)-9H-purin-6-amine

-
-
34502-77-7
(adenylpropyl)cobalamine

| Conditions | Yield |
|---|---|
| With NaBH4 In ethanol; water Soln. of NaBH4 in water was added to a soln. of hydroxocobalamine in water and 9-(3-chloropropyl)adenine in ethanol, mixt. was stirred for 30 min under N2;; excess NaBH4 was quenched with acetone, excess of the ligand was filtered; soln. was desalted on an Amberlite XAD-2 column; product was purified with a SP-Sephadex column, eluent - H2O; eluate was concd., product was pptd. by addn. of acetone;; |
-
-
13422-51-0
hydroxocob(III)alamin

-
-
69293-19-2
N9-(4-chlorobutyl)adenine

-
-
21806-90-6
(adehylbutyl)cobalamine

| Conditions | Yield |
|---|---|
| With NaBH4 In ethanol; water Soln. of NaBH4 in water was added to a soln. of hydroxocobalamine in water and 9-(4-chlorobutyl)adenine in ethanol, mixt. was stirred for 30 min under N2;; excess NaBH4 was quenched with acetone, excess of the ligand was filtered; soln. was desalted on an Amberlite XAD-2 column; product was purified with a SP-Sephadex column, eluent - H2O; eluate was concd., product was pptd. by addn. of acetone;; |
-
-
13422-51-0
hydroxocob(III)alamin

-
-
53359-09-4
N9-(5-chloropentyl)adenine

-
-
56226-23-4
(adenylpentyl)cobalamine

| Conditions | Yield |
|---|---|
| With NaBH4 In ethanol; water Soln. of NaBH4 in water was added to a soln. of hydroxocobalamine in water and 9-(5-chloropentyl)adenine in ethanol, mixt. was stirred for 30 min under N2;; excess NaBH4 was quenched with acetone, excess of the ligand was filtered; soln. was desalted on an Amberlite XAD-2 column; product was purified with a SP-Sephadex column, eluent - H2O; eluate was concd., product was pptd. by addn. of acetone;; |
-
-
13422-51-0
hydroxocob(III)alamin

-
-
56575-56-5
4-bromo-but-1-ene-2,3-dicarboxylic acid

| Conditions | Yield |
|---|---|
| With zinc In methanol complex reduced with Zn dust in anaerobic MeOH contg. 10% NH4I in a centrifuge tube, injection of soln. of organic compd.; |
-
-
13422-51-0
hydroxocob(III)alamin

-
-
56575-59-8
dimethyl β-bromomethylitaconate

| Conditions | Yield |
|---|---|
| With zinc In methanol complex reduced with Zn dust in anaerobic MeOH contg. 10% NH4I in a centrifuge tube, injection of organic compd.; |

| Conditions | Yield |
|---|---|
| With NaBH4; Co nitrate In water Schlenk technique; aq. soln. of NaBH4 was added to aq. soln. of hydroxocobalamin, Co nitrate under Ar; ClCCH was distd. at 25°C for 2 h; extd. (phenol/CH2Cl2, 1/1); phenol extracts washed (distd. H2O); dild. with 1-butanol/CH2Cl2 (1/1); extd. (distd. H2O); lyophilized; dissolved in ammonium acetate buffer (pH 4); chromd. (Vydec C18 protein and peptidecolumn; buffer/MeCN, 95/5 to 70/30); |

| Conditions | Yield |
|---|---|
| With NaBH4; Co nitrate In water Schlenk technique; aq. soln. of NaBH4 was added to aq. soln. of hydroxocobalamin, Co nitrate under Ar; HCCH was added at 25°C for 2 h; extd. (phenol/CH2Cl2, 1/1); phenol extracts washed (distd. H2O); dild. with 1-butanol/CH2Cl2 (1/1); extd. (distd. H2O); lyophilized; dissolved in ammonium acetate buffer (pH 4); chromd. (Vydec C18 protein and peptidecolumn; buffer/MeCN, 95/5 to 70/30); |
-
-
13422-51-0
hydroxocob(III)alamin

| Conditions | Yield |
|---|---|
| In H2O Co complex react. with (CH3NH2C6H12)N(CH3)N(O)NO(1+) at 25°C. (pH=10.8); |

| Conditions | Yield |
|---|---|
| In H2O byproducts: N-nitrosodiethylamine; Co complex react. with 1.2 equiv. of (C2H5)2NN(O)NO(1-) at 25°C. in alkaline soln.; |

| Conditions | Yield |
|---|---|
| With sodium dithionite; ascorbic acid In water at 25℃; Kinetics; Acidic conditions; |
-
-
13422-51-0
hydroxocob(III)alamin

| Conditions | Yield |
|---|---|
| With sodium dithionite; sodium hydroxide In water at 25℃; Kinetics; |
Hydroxocobalamin Chemical Properties
EINECS: 236-534-8
Empirical Formula: C62H89CoN13O15P
Molecular Weight: 1346.3551
Nominal Mass: 1345 Da
Average Mass: 1346.3551 Da
Monoisotopic Mass: 1345.56707 Da
Storage tempreture: 2-8 °C
Solubility: Methanol: 10 mg/mL at 20 °C, clear, dark red
Structure of Hydroxocobalamin (CAS NO.13422-51-0):
InChI
InChI=1/C62H90N13O14P.Co.H2O/c1-29-20-39-40(21-30(29)2)75(28-70-39)57-52(84)53(41(27-76)87-57)89-90(85,86)88-31(3)26-69-49(83)18-19-59(8)37(22-46(66)80)56-62(11)61(10,25-48(68)82)36(14-17-45(65)79)51(74-62)33(5)55-60(9,24-47(67)81)34(12-15-43(63)77)38(71-55)23-42-58(6,7)35(13-16-44(64)78)50(72-42)32(4)54(59)73-56;;/h20-21,23,28,31,34-37,41,52-53,56-57,76,84H,12-19,22,24-27H2,1-11H3,(H15,63,64,65,66,67,68,69,71,72,73,74,77,78,79,80,81,82,83,85,86);;1H2/q;+3;/p-3/t31-,34+,35+,36+,37-,41+,52+,53+,56?,57-,59+,60-,61-,62-;;/m0../s1/rC62H90CoN13O15P/c1-29-20-39-40(21-30(29)2)75(28-71-39)57-52(85)53(41(27-77)89-57)91-92(87,88)90-31(3)26-70-49(84)18-19-59(8)37(22-46(67)81)56-62(11)61(10,25-48(69)83)36(14-17-45(66)80)51(74-62)32(4)54-60(9,24-47(68)82)34(12-15-43(64)78)38(72-54)23-42-58(6,7)35(13-16-44(65)79)50(73-42)33(5)55(59)76(56)63-86/h20-21,23,28,31,34-37,41,52-53,56-57,77,85-86H,12-19,22,24-27H2,1-11H3,(H2,64,78)(H2,65,79)(H2,66,80)(H2,67,81)(H2,68,82)(H2,69,83)(H,70,84)(H,87,88)/q+1/p-1/b42-23-,54-32-,55-33-/t31-,34+,35+,36+,37-,41+,52+,53+,56?,57-,59+,60-,61-,62-/m0/s1
Smiles
N1([C@@H]2[C@@]3([C@]([C@H](CCC(N)=O)C(=N3)C(=C3[C@]([C@H](CCC(N)=O)C(=N3)C=C3N=C(C(=C1[C@@]([C@H]2CC(N)=O)(CCC(NC[C@@H](OP(O[C@@H]1[C@H](O[C@@H]([C@@H]1O)n1c2c(cc(C)c(c2)C)nc1)CO)(=O)[O-])C)=O)C)C)[C@H](C3(C)C)CCC(N)=O)(CC(N)=O)C)C)(CC(N)=O)C)C)[Co+]O
Hydroxocobalamin Uses
Hydroxocobalamin (CAS NO.13422-51-0) is used in many pharmaceuticals, supplements and as food additive; and it has also been used in the treatment of cyanide poisoning. Hydroxocobalamin Injection USP, are used to rectify the following causes of B12 deficiency.
Hydroxocobalamin Toxicity Data With Reference
| Organism | Test Type | Route | Reported Dose (Normalized Dose) | Effect | Source |
|---|---|---|---|---|---|
| mouse | LD50 | intravenous | > 50mg/kg (50mg/kg) | Drugs in Japan Vol. -, Pg. 867, 1990. |
Hydroxocobalamin Safety Profile
Hazard Codes:  Xi
Xi
Risk Statements: 36/37/38
R36/37/38:Irritating to eyes, respiratory system and skin.
Safety Statements: 26-36
S26: In case of contact with eyes, rinse immediately with plenty of water and seek medical advice.
S36:Wear suitable protective clothing.
Hydroxocobalamin Specification
Hydroxocobalamin , its cas register number is 13422-51-0. It also can be called Vitamin B12b ; Ciplamin H ; and AlphaRedisol . It is a basic member of the cobalamin family of compounds, and is not a form normally found in the human body, but is easily converted in the body to usable coenzyme forms of B12. It is a term that refers to a group of compounds called cobalamins that are available in the human body in a variety of mostly interconvertible forms. Hydroxocobalamin (CAS NO.13422-51-0) is the form of B12 produced by many bacteria which are used to produce the vitamin commercially.
About|Contact|Cas|Product Name|Molecular|Country|Encyclopedia
Message|New Cas|MSDS|Service|Advertisement|CAS DataBase|Article Data|Manufacturers | Chemical Catalog
©2008 LookChem.com,License: ICP
NO.:Zhejiang16009103
complaints:service@lookchem.com Desktop View