-
Name
IMIPRAMINE
- EINECS
- CAS No. 50-49-7
- Article Data30
- CAS DataBase
- Density 1.041g/cm3
- Solubility 18.23mg/L(24 oC)
- Melting Point 174°C
- Formula C19H24 N2
- Boiling Point 403.1°Cat760mmHg
- Molecular Weight 280.413
- Flash Point 179.7°C
- Transport Information
- Appearance
- Safety A human poison by ingestion. An experimental poison by ingestion, subcutaneous, intravenous, and intraperitoneal routes. Human systemic effects by ingestion: somnolence, hallucinations, distorted perceptions, changes in motor activity, ataxia (loss of muscle coordination), coma, nausea and vomiting, irritative dermatitis. An experimental teratogen by ingestion. Other experimental reproductive effects. Mutation data reported. When heated to decomposition it emits toxic fumes of NOx.
- Risk Codes
-
Molecular Structure
- Hazard Symbols
- Synonyms 5H-Dibenz[b,f]azepine,5-[3-(dimethylamino)propyl]-10,11-dihydro- (7CI,8CI); 1-(3-Dimethylaminopropyl)-4,5-dihydro-2,3,6,7-dibenzazepine;5,6-Dihydro-N-[3-(dimethylamino)propyl]-11H-dibenz[b,e]azepine;5-(3-Dimethylaminopropyl)-10,11-dihydro-5H-dibenz[b,f]azepine; Antideprin;Berkomine; Cristalia; Imipramine; Melipramine; N-(3-Dimethylaminopropyl)-o-iminodibenzyl;N-(g-Dimethylaminopropyl)iminodibenzyl;NSC 169866; Org 2463; Prazepine; Sermonil
- PSA 6.48000
- LogP 3.94000
Synthetic route

| Conditions | Yield |
|---|---|
| With sodium tetrahydroborate In water at 50 - 60℃; chemoselective reaction; | 96% |

| Conditions | Yield |
|---|---|
| With hydrogen In octane at 150℃; for 30h; Autoclave; | 89% |
| With hydrogen; tris(acetylacetonato)ruthenium(III); lithium chloride; [2-((diphenylphospino)methyl)-2-methyl-1,3-propanediyl]bis[diphenylphosphine] In tetrahydrofuran at 140℃; for 24h; Autoclave; Inert atmosphere; | 83 %Chromat. |
| With proazaphosphatrane; 9-bora-bicyclo[3.3.1]nonane In tetrahydrofuran at 90℃; under 750.075 Torr; for 1h; Inert atmosphere; Schlenk technique; chemoselective reaction; | 99 %Spectr. |
-
-
876124-39-9
N'-(4-carbomethoxy)desimipramine

-
-
50-49-7
impramine

| Conditions | Yield |
|---|---|
| With lithium aluminium tetrahydride In tetrahydrofuran for 0.5h; Heating; | 79% |

| Conditions | Yield |
|---|---|
| With formic acid In water at 100℃; for 1h; Eschweiler-Clark Amine Methylation; Microwave irradiation; | 61% |

| Conditions | Yield |
|---|---|
| With tris-(dibenzylideneacetone)dipalladium(0); sodium t-butanolate; XPhos In toluene at 110℃; Buchwald-Hartwig Coupling; | 60% |

| Conditions | Yield |
|---|---|
| With rhodium(III) chloride hydrate; potassium tert-butylate at 150℃; for 60h; Sealed tube; High pressure; | 49% |

| Conditions | Yield |
|---|---|
| With oxygen In methanol; phosphate buffer at 25 - 28℃; pH=7.4; Product distribution; Further Variations:; Reagents; Photolysis; UV-irradiation; | A 15% B 15% C 40% D 20% |


| Conditions | Yield |
|---|---|
| With sodium amide; benzene und Erwaermen der Reaktionsloesung mit <3-Chlor-propyl>-dimethyl-amin; |

| Conditions | Yield |
|---|---|
| With sodium hydroxide; potassium carbonate DMSO 1.) room temp., 2 h, 2.) 110 deg C, 18 h; Yield given. Multistep reaction; | |
| With potassium hydroxide; ammonium acetate In toluene at 20℃; Heating / reflux; |

| Conditions | Yield |
|---|---|
| With lithium aluminium tetrahydride 1.) THF, -45 deg C - 60 deg C, 2.) 60 deg C, 10 min; Yield given. Multistep reaction; |

| Conditions | Yield |
|---|---|
| Mechanism; multistep reaction; labelled with 11C; |


| Conditions | Yield |
|---|---|
| Multi-step reaction with 2 steps 1: (NH4)2SO4 / 110 °C 2: 2.) LiAlH4 / 1.) THF, -45 deg C - 60 deg C, 2.) 60 deg C, 10 min View Scheme |

| Conditions | Yield |
|---|---|
| Multi-step reaction with 2 steps 2: 79 percent / LiAlH4 / tetrahydrofuran / 0.5 h / Heating View Scheme |

| Conditions | Yield |
|---|---|
| With platinum(0)-1,3-divinyl-1,1,3,3-tetramethyldisiloxane complex; 1,3-bis-(diphenylphosphino)propane; phenylsilane In dibutyl ether at 60℃; for 18h; Schlenk technique; Inert atmosphere; | 92 %Chromat. |

| Conditions | Yield |
|---|---|
| With sodium hydroxide In water |
-
-
165602-94-8
Imipramine N-glucuronide

-
-
50-49-7
impramine

| Conditions | Yield |
|---|---|
| With hydrogenchloride In water at 90℃; |

| Conditions | Yield |
|---|---|
| In diethyl ether at 20℃; for 20h; | 98% |

| Conditions | Yield |
|---|---|
| In ethyl acetate at 20 - 50℃; Solvent; Temperature; | 96% |
-
-
50-49-7
impramine

| Conditions | Yield |
|---|---|
| In acetonitrile Heating; | 90% |

| Conditions | Yield |
|---|---|
| at 20℃; | 86% |
-
-
50-49-7
impramine

| Conditions | Yield |
|---|---|
| With deuterium In tetrahydrofuran for 36h; Glovebox; Inert atmosphere; regioselective reaction; | 84% |
-
-
50-49-7
impramine

| Conditions | Yield |
|---|---|
| With (1,5-cyclooctadiene)(methoxy)iridium(I) dimer; deuterium In tetrahydrofuran at 55℃; under 750.075 Torr; for 22h; Inert atmosphere; | 80% |

| Conditions | Yield |
|---|---|
| In butanone for 1h; Ambient temperature; | 77% |

| Conditions | Yield |
|---|---|
| With dipotassium hydrogenphosphate In dichloromethane at 20℃; for 48h; | 73% |

| Conditions | Yield |
|---|---|
| With (4s,6s)-2,4,5,6-tetra(9H-carbazol-9-yl)isophthalonitrile; sodium acetate In N,N-dimethyl-formamide at 20℃; for 16h; Irradiation; Inert atmosphere; diastereoselective reaction; | 64% |
-
-
50-49-7
impramine

-
-
21085-72-3
1-bromo-2,3,4-tri-O-acetyl-α-D-glucuronic acid methyl ester

-
-
86492-48-0
((2R,3R,4S,5S,6S)-6-Carboxy-3,4,5-trihydroxy-tetrahydro-pyran-2-yl)-[3-(10,11-dihydro-dibenzo[b,f]azepin-5-yl)-propyl]-dimethyl-ammonium; chloride

| Conditions | Yield |
|---|---|
| With XAD-2 ion-exchange resin; sodium hydrogencarbonate In water; benzene for 72h; Ambient temperature; | 55% |

| Conditions | Yield |
|---|---|
| With [4,4’-bis(1,1-dimethylethyl)-2,2’-bipyridine-N1,N1‘]bis [3,5-difluoro-2-[5-(trifluoromethyl)-2-pyridinyl-N]phenyl-C]iridium(III) hexafluorophosphate; acetic acid In acetonitrile at 20℃; for 24h; Irradiation; Sealed tube; | A 50% B 22% C 23% |


| Conditions | Yield |
|---|---|
| With 2-Picolinic acid; iron(III) chloride; tert-Butyl peroxybenzoate; 18-crown-6 ether In acetonitrile at 50℃; for 48h; | A 6% B 35% C 15% |


| Conditions | Yield |
|---|---|
| In acetonitrile Heating; | 24% |
-
-
50-49-7
impramine

-
A
-
796-28-1
10-Hydroxyimipramine

-
B
-
303-70-8
2-hydroxyimipramine

-
C
-
94436-87-0
4-hydroxyimipramine

| Conditions | Yield |
|---|---|
| Stage #1: impramine With ethylenediaminetetraacetic acid trisodium salt; oxygen; manganese (II) acetate tetrahydrate; ascorbic acid In water pH=4; Udenfriend reaction; Stage #2: With ferrous(II) sulfate heptahydrate; ethylenediaminetetraacetic acid trisodium salt; oxygen In water at 45℃; for 2h; Udenfriend reaction; | A 2.56% B 1.46% C 3.47% |


| Conditions | Yield |
|---|---|
| With oxygen; palladium on activated charcoal for 24h; Ambient temperature; |

| Conditions | Yield |
|---|---|
| With D-glucose; corn steep liquor; S. aureus; peptone; yeast extract In water at 37℃; Product distribution; other reagents; | |
| With oxygen; palladium on activated charcoal In methanol for 24h; Ambient temperature; | |
| With pooled human liver microsomes; Tris buffer; NADPH; magnesium chloride at 37℃; pH=7.4; Enzyme kinetics; | |
| With human hepatic CYP1A2; human hepatic CYP2C9; human hepatic CYP3A4 Enzymatic reaction; | |
| Multi-step reaction with 2 steps 1: N,N,N',N'-tetramethyl-1,8-diaminonaphthalene / 1,2-dichloro-ethane / 1 h / Reflux 2: 2 h / Reflux View Scheme |
-
-
50-49-7
impramine

-
-
144434-72-0
2,8-dibromoimipramine

| Conditions | Yield |
|---|---|
| With N-Bromosuccinimide; silica gel In dichloromethane at 18℃; |
Imipramine Chemical Properties
Molecular Structure of Imipramine (CAS NO.50-49-7):
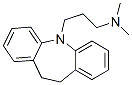
Molecular Formula: C19H24N2
Molecular Weight: 280.41
IUPAC Name: 3-(5,6-dihydrobenzo[b][1]benzazepin-11-yl)-N,N-Dimethylpropan-1-amine
Synonyms of Imipramine (CAS NO.50-49-7): Imipramine ; Aurora ka-7708 ; 1-(3-Dimethylaminopropyl)-4,5-dihydro-2,3,6,7-dibenzazepine ; 10,11-Dihydro-5-(3-(dimethylamino)propyl)-5H-dibenz[b,f]azepine ; 2,2'-(3-Dimethylaminopropylimino)bibenzyl ; 2,2'-(3-Dimethylaminopropylimino)dibenzyl ; 3-(10,11-Dihydro-5H-dibenzo[b,f]azepin-5-yl)-N,N-dimethyl-1-propanamine ; 5-(3-(Dimethylamino)propyl)-10,11-dihydro-5H-dibenz(b,f)azepine
CAS NO: 50-49-7
EINECS: 200-042-1
Mol File: 50-49-7.mol
Index of Refraction: 1.574
Surface Tension: 40.1 dyne/cm
Density: 1.041 g/cm3
Flash Point: 179.7 °C
Enthalpy of Vaporization: 65.43 kJ/mol
Boiling Point: 403.1 °C at 760 mmHg
Vapour Pressure: 1.04E-06 mmHg at 25°C
Properties: white or light yellow crystalline powder, no smell, taste and burning hemp.
Solubility: soluble in water, ethanol; almost insoluble in ether.
Imipramine Uses
Imipramine (CAS NO.50-49-7) is commonly used as antidepressants of tricyclic .
Imipramine Toxicity Data With Reference
| Organism | Test Type | Route | Reported Dose (Normalized Dose) | Effect | Source |
|---|---|---|---|---|---|
| cat | LDLo | intravenous | 20mg/kg (20mg/kg) | Bollettino Chimico Farmaceutico. Vol. 112, Pg. 601, 1973. | |
| cat | LDLo | oral | 100mg/kg (100mg/kg) | Zentralblatt fuer Pharmazie, Pharmakotherapie und Laboratoriums-diagnostik. Vol. 114, Pg. 787, 1975. | |
| child | LD50 | oral | 40mg/kg (40mg/kg) | BEHAVIORAL: "HALLUCINATIONS, DISTORTED PERCEPTIONS" BEHAVIORAL: CHANGES IN MOTOR ACTIVITY (SPECIFIC ASSAY) BEHAVIORAL: COMA | Handbook of Experimental Pharmacology. Vol. 55, Pg. 527, 1980. |
| child | LDLo | oral | 35mg/kg (35mg/kg) | BEHAVIORAL: CONVULSIONS OR EFFECT ON SEIZURE THRESHOLD LUNGS, THORAX, OR RESPIRATION: DYSPNEA CARDIAC: OTHER CHANGES | Schweizerische Medizinische Wochenschrift. Vol. 99, Pg. 1157, 1969. |
| child | TDLo | oral | 30mg/kg (30mg/kg) | BEHAVIORAL: SOMNOLENCE (GENERAL DEPRESSED ACTIVITY) BEHAVIORAL: CHANGES IN MOTOR ACTIVITY (SPECIFIC ASSAY) BEHAVIORAL: ATAXIA | American Journal of Diseases of Children. Vol. 130, Pg. 507, 1976. |
| dog | LDLo | intravenous | 45mg/kg (45mg/kg) | PERIPHERAL NERVE AND SENSATION: FLACCID PARALYSIS WITHOUT ANESTHESIA (USUALLY NEUROMUSCULAR BLOCKAGE) BEHAVIORAL: CONVULSIONS OR EFFECT ON SEIZURE THRESHOLD LUNGS, THORAX, OR RESPIRATION: RESPIRATORY DEPRESSION | Bollettino Chimico Farmaceutico. Vol. 112, Pg. 601, 1973. |
| dog | LDLo | oral | 100mg/kg (100mg/kg) | "Psychotropic Drugs and Related Compounds," 2nd ed., Usdin, E., and D.H. Efron, Washington, DC, 1972Vol. -, Pg. 78, 1972. | |
| human | LD50 | oral | 40mg/kg (40mg/kg) | Proceedings of the European Society for the Study of Drug Toxicity. Vol. 6, Pg. 171, 1965. | |
| human | LDLo | oral | 450mg/kg (450mg/kg) | SKIN AND APPENDAGES (SKIN): "DERMATITIS, IRRITATIVE: AFTER SYSTEMIC EXPOSURE" | British Medical Journal. Vol. 1, Pg. 722, 1979. |
| mammal (species unspecified) | LD50 | oral | 350mg/kg (350mg/kg) | BEHAVIORAL: ANTICONVULSANT | Journal of Medicinal Chemistry. Vol. 8, Pg. 836, 1965. |
| mammal (species unspecified) | LD50 | unreported | 455mg/kg (455mg/kg) | Khimiko-Farmatsevticheskii Zhurnal. Chemical Pharmaceutical Journal. For English translation, see PCJOAU. Vol. 24(7), Pg. 27, 1990. | |
| man | LD50 | oral | 30mg/kg (30mg/kg) | BEHAVIORAL: "HALLUCINATIONS, DISTORTED PERCEPTIONS" BEHAVIORAL: CHANGES IN MOTOR ACTIVITY (SPECIFIC ASSAY) BEHAVIORAL: COMA | Handbook of Experimental Pharmacology. Vol. 55, Pg. 527, 1980. |
| man | LDLo | oral | 71mg/kg (71mg/kg) | BEHAVIORAL: CONVULSIONS OR EFFECT ON SEIZURE THRESHOLD CARDIAC: CHANGE IN RATE ENDOCRINE: CHANGE IN LH | Journal of Toxicology, Clinical Toxicology. Vol. 19, Pg. 239, 1982. |
| man | TDLo | oral | 5357ug/kg/5D- (5.357mg/kg) | BEHAVIORAL: ALTERED SLEEP TIME (INCLUDING CHANGE IN RIGHTING REFLEX) BEHAVIORAL: CHANGE IN REM SLEEP (HUMAN) | Human Psychopharmacology. Vol. 11, Pg. 211, 1996. |
| man | TDLo | oral | 8mg/kg/3D-I (8mg/kg) | BEHAVIORAL: EXCITEMENT | Lancet. Vol. 2, Pg. 568, 1959. |
| man | TDLo | oral | 71428ug/kg/10 (71.428mg/kg) | BEHAVIORAL: REGIDITY SKIN AND APPENDAGES (SKIN): SWEATING: OTHER | Intensive Care Medicine. Vol. 23, Pg. 480, 1997. |
| mouse | LD50 | intraperitoneal | 51600ug/kg (51.6mg/kg) | British Patent Document. Vol. #1460700, | |
| mouse | LD50 | intravenous | 21mg/kg (21mg/kg) | AUTONOMIC NERVOUS SYSTEM: PARASYMPATHOLYTIC BEHAVIORAL: CHANGES IN MOTOR ACTIVITY (SPECIFIC ASSAY) BEHAVIORAL: WAKEFULNESS | Archives Internationales de Pharmacodynamie et de Therapie. Vol. 245, Pg. 283, 1980. |
| mouse | LD50 | oral | 188mg/kg (188mg/kg) | SENSE ORGANS AND SPECIAL SENSES: PTOSIS: EYE BEHAVIORAL: CHANGES IN MOTOR ACTIVITY (SPECIFIC ASSAY) | Pharmaceutical Chemistry Journal Vol. 14, Pg. 773, 1980. |
| mouse | LD50 | subcutaneous | 195ug/kg (0.195mg/kg) | BEHAVIORAL: WAKEFULNESS | Pharmaceutical Chemistry Journal Vol. 15, Pg. 412, 1981. |
| mouse | LD50 | unreported | 107mg/kg (107mg/kg) | AUTONOMIC NERVOUS SYSTEM: "SMOOTH MUSCLE RELAXANT (MECHANISM UNDEFINED, SPASMOLYTIC)" | Journal of Medicinal Chemistry. Vol. 10, Pg. 418, 1967. |
| rabbit | LD50 | intravenous | 18mg/kg (18mg/kg) | Archives Internationales de Pharmacodynamie et de Therapie. Vol. 120, Pg. 450, 1959. | |
| rabbit | LD50 | oral | 385mg/kg (385mg/kg) | Arzneimittel-Forschung. Drug Research. Vol. 19, Pg. 1617, 1969. | |
| rat | LD50 | intraperitoneal | 79mg/kg (79mg/kg) | Archives Internationales de Pharmacodynamie et de Therapie. Vol. 148, Pg. 560, 1964. | |
| rat | LD50 | intravenous | 9300ug/kg (9.3mg/kg) | United States Patent Document. Vol. #4141993, | |
| rat | LD50 | oral | 250mg/kg (250mg/kg) | SENSE ORGANS AND SPECIAL SENSES: PTOSIS: EYE BEHAVIORAL: CHANGES IN MOTOR ACTIVITY (SPECIFIC ASSAY) | Pharmaceutical Chemistry Journal Vol. 14, Pg. 773, 1980. |
| rat | LD50 | subcutaneous | 250mg/kg (250mg/kg) | Archives Internationales de Pharmacodynamie et de Therapie. Vol. 148, Pg. 560, 1964. | |
| women | LDLo | oral | 2mg/kg/1D (2mg/kg) | LUNGS, THORAX, OR RESPIRATION: OTHER CHANGES | Journal of Pharmacy and Pharmacology. Vol. 16, Pg. 265, 1964. |
| women | TDLo | oral | 3mg/kg/32H-I (3mg/kg) | SKIN AND APPENDAGES (SKIN): PHOTOSENSITIVITY: AFTER SYSTEMIC EXPOSURE | JAMA, Journal of the American Medical Association. Vol. 254, Pg. 357, 1985. |
| women | TDLo | oral | 150mg/kg (150mg/kg) | BEHAVIORAL: COMA CARDIAC: OTHER CHANGES VASCULAR: BP LOWERING NOT CHARACTERIZED IN AUTONOMIC SECTION | Journal of Toxicology, Clinical Toxicology. Vol. 33, Pg. 51, 1995. |
Imipramine Consensus Reports
EPA Genetic Toxicology Program.
Imipramine Safety Profile
A human poison by ingestion. An experimental poison by ingestion, subcutaneous, intravenous, and intraperitoneal routes. Human systemic effects by ingestion: somnolence, hallucinations, distorted perceptions, changes in motor activity, ataxia (loss of muscle coordination), coma, nausea and vomiting, irritative dermatitis. An experimental teratogen by ingestion. Other experimental reproductive effects. Mutation data reported. When heated to decomposition it emits toxic fumes of NOx.
Related Products
- Imipramine
- Imipraminehydrochloride
- Imipramine-N-oxide hydrochloride
- 504-97-2
- 504-99-4
- 50499-60-0
- 50-50-0
- 50502-17-5
- 5050-41-9
- 50505-80-1
- 505082-81-5
- 505083-04-5
- 505084-55-9
Hot Products
About|Contact|Cas|Product Name|Molecular|Country|Encyclopedia
Message|New Cas|MSDS|Service|Advertisement|CAS DataBase|Article Data|Manufacturers | Chemical Catalog
©2008 LookChem.com,License: ICP
NO.:Zhejiang16009103
complaints:service@lookchem.com Desktop View