-
Name
Isoxazole
- EINECS 206-018-7
- CAS No. 288-14-2
- Article Data61
- CAS DataBase
- Density 1.055 g/cm3
- Solubility
- Melting Point -67.1°C
- Formula C3H3NO
- Boiling Point 95.5 °C at 760 mmHg
- Molecular Weight 69.0629
- Flash Point 8.9 °C
- Transport Information UN 1993 3/PG 2
- Appearance colorless liquid
- Safety 16-23/33-33-29-7/9
- Risk Codes 11
-
Molecular Structure
-
Hazard Symbols
 F,
F,  Xi
Xi
- Synonyms 1,2-Oxazole;1-Oxa-2-azacyclopentadiene;2-Azafuran;NSC 137774;
- PSA 26.03000
- LogP 0.67460
Synthetic route

| Conditions | Yield |
|---|---|
| With hydroxylamine hydrochloride at 110 - 120℃; Green chemistry; | 85.5% |

| Conditions | Yield |
|---|---|
| With hydroxylamine hydrochloride In water at 70℃; for 3.5h; | 43% |
| With hydrogenchloride; water; hydroxylamine | |
| With hydrogenchloride; hydroxylamine | |
| With hydroxylamine hydrochloride |

| Conditions | Yield |
|---|---|
| In tetrahydrofuran; water under 760 Torr; for 1.5h; Ambient temperature; | 28% |
| With water In tetrahydrofuran Ambient temperature; Yield given; |

| Conditions | Yield |
|---|---|
| With hydroxylamine | |
| With hydrogenchloride; hydroxylamine |

| Conditions | Yield |
|---|---|
| With hydrogenchloride; water; hydroxylamine |

| Conditions | Yield |
|---|---|
| With sulfuric acid Erhitzen des Reaktionsprodukts mit wss. H2SO4; |

| Conditions | Yield |
|---|---|
| With hydrogenchloride; water; hydroxylamine |
-
-
98021-69-3
acetic acid-(1-bromo-3,3-dichloro-propyl ester)

-
-
288-14-2
ISOXAZOLE

| Conditions | Yield |
|---|---|
| With hydrogenchloride; ethanol; hydroxylamine | |
| With hydrogenchloride; ethanol; hydroxylamine; sodium acetate |
-
-
86046-91-5
1-acetoxy-1,3,3-tribromopropane

-
-
288-14-2
ISOXAZOLE

| Conditions | Yield |
|---|---|
| With hydrogenchloride; ethanol; hydroxylamine | |
| With hydrogenchloride; ethanol; hydroxylamine; sodium acetate |

| Conditions | Yield |
|---|---|
| With sulfuric acid |

-
-
288-14-2
ISOXAZOLE

-
-
25186-34-9
3-aminoprop-2-enal

| Conditions | Yield |
|---|---|
| With hydrogen; nickel In methanol at 20℃; under 2068.65 Torr; | 100% |
| With hydrogen; Raney nickel In methanol under 2068.65 Torr; for 24h; Parr apparatus; | 94% |
| With hydrogen; nickel In methanol; chloroform |
-
-
288-14-2
ISOXAZOLE

-
-
219679-74-0
3-methyl-4-(4-methylthiophenyl)-5-phenylisoxazole

-
-
181695-93-2
3-methyl-5-(4-methylsulfonylphenyl)-4-phenylisoxazole

| Conditions | Yield |
|---|---|
| In tetrahydrofuran; methanol; water | 95% |

-
-
288-14-2
ISOXAZOLE

-
-
15814-32-1
1,5-diphenylpenta-1,4-diyn-3-ol

| Conditions | Yield |
|---|---|
| With chloro(1,3-bis(2,6-di-i-propylphenyl)imidazol-2-ylidene)gold(I); silver trifluoromethanesulfonate In 1,2-dichloro-ethane at 80℃; for 4h; Inert atmosphere; chemoselective reaction; | 94% |
-
-
288-14-2
ISOXAZOLE

-
-
1613231-71-2
1,5-di(thiophen-3-yl)penta-1,4-diyn-3-ol

| Conditions | Yield |
|---|---|
| With chloro(1,3-bis(2,6-di-i-propylphenyl)imidazol-2-ylidene)gold(I); silver trifluoromethanesulfonate In 1,2-dichloro-ethane at 80℃; for 5h; Inert atmosphere; chemoselective reaction; | 93% |

| Conditions | Yield |
|---|---|
| Stage #1: carbon dioxide With AuOH(1,3-bis(2,6-diisopropylphenyl)imidazol-2-ylidene); potassium hydroxide In tetrahydrofuran at 20℃; under 1125.11 Torr; for 0.25h; Stage #2: ISOXAZOLE In tetrahydrofuran at 20℃; under 1125.11 Torr; for 12h; Stage #3: With hydrogenchloride In tetrahydrofuran; water regioselective reaction; | 91% |
-
-
288-14-2
ISOXAZOLE

| Conditions | Yield |
|---|---|
| With chloro(1,3-bis(2,6-di-i-propylphenyl)imidazol-2-ylidene)gold(I); silver trifluoromethanesulfonate In 1,2-dichloro-ethane at 80℃; for 3h; Inert atmosphere; chemoselective reaction; | 91% |

| Conditions | Yield |
|---|---|
| With chloro(1,3-bis(2,6-di-i-propylphenyl)imidazol-2-ylidene)gold(I); silver(I) triflimide In 1,2-dichloro-ethane at 65℃; for 14h; Reagent/catalyst; | 88% |

| Conditions | Yield |
|---|---|
| With chloro(1,3-bis(2,6-di-i-propylphenyl)imidazol-2-ylidene)gold(I); silver trifluoromethanesulfonate In 1,2-dichloro-ethane at 80℃; for 7h; Inert atmosphere; chemoselective reaction; | 87% |

| Conditions | Yield |
|---|---|
| With chloro(1,3-bis(2,6-di-i-propylphenyl)imidazol-2-ylidene)gold(I); silver(I) triflimide In 1,2-dichloro-ethane at 65℃; for 20h; | 87% |

| Conditions | Yield |
|---|---|
| With copper diacetate In toluene at 110℃; for 5h; regioselective reaction; | A 87% B 7% |


| Conditions | Yield |
|---|---|
| With chloro(1,3-bis(2,6-di-i-propylphenyl)imidazol-2-ylidene)gold(I); silver(I) triflimide In 1,2-dichloro-ethane at 65℃; for 25h; | 85% |

| Conditions | Yield |
|---|---|
| With chloro(1,3-bis(2,6-di-i-propylphenyl)imidazol-2-ylidene)gold(I); silver(I) triflimide In 1,2-dichloro-ethane at 65℃; for 23h; | 85% |
-
-
288-14-2
ISOXAZOLE

-
-
59046-72-9
o-(phenylethynyl)benzaldehyde

| Conditions | Yield |
|---|---|
| With copper diacetate In toluene at 110℃; for 4h; regioselective reaction; | A 85% B 8% |


| Conditions | Yield |
|---|---|
| With copper diacetate In toluene at 110℃; for 3.5h; regioselective reaction; | A 85% B 8% |

-
-
288-14-2
ISOXAZOLE

| Conditions | Yield |
|---|---|
| With sodium hydroxide In ethanol at 20℃; for 5h; | 85% |
-
-
288-14-2
ISOXAZOLE

| Conditions | Yield |
|---|---|
| With [1,3-bis(2,6-diisopropylphenyl)-1H-imidazol-2-(3H)-ylidene]gold(I)chloride; silver(I) triflimide In 1,2-dichloro-ethane at 70℃; for 4h; | A 84% B 8% |


| Conditions | Yield |
|---|---|
| With chloro(1,3-bis(2,6-di-i-propylphenyl)imidazol-2-ylidene)gold(I); silver(I) triflimide In 1,2-dichloro-ethane at 65℃; for 24h; | 84% |

| Conditions | Yield |
|---|---|
| With chloro(1,3-bis(2,6-di-i-propylphenyl)imidazol-2-ylidene)gold(I); silver(I) triflimide In 1,2-dichloro-ethane at 65℃; for 12h; | 83% |

| Conditions | Yield |
|---|---|
| With chloro(1,3-bis(2,6-di-i-propylphenyl)imidazol-2-ylidene)gold(I); silver(I) triflimide In 1,2-dichloro-ethane at 65℃; for 24h; | 83% |
-
-
288-14-2
ISOXAZOLE

-
-
3717-23-5
(Z)-p-chlorobenzaldehyde oxime

-
-
70-55-3
toluene-4-sulfonamide

-
-
536-74-3
phenylacetylene

-
-
130966-62-0
3-(4-chlorophenyl)-4-phenylisoxazole

| Conditions | Yield |
|---|---|
| With chloroamine-T In methanol; water | 82% |

-
-
288-14-2
ISOXAZOLE

-
-
1222139-46-9
(S)-tert-butyl 2-((5-ethynylpyridin-3-yloxy)methyl)azetidine-1-carboxylate

-
-
75233-61-3
2-(2-nitroethoxy)tetrahydro-2H-pyran

-
-
1222139-58-3
3-[[1-(tert-butoxycarbonyl)-2(S)-azetidinyl]methoxy]-5-[3-[(tetrahydro-2H-pyran-2-yloxy)methyl]-5-isoxazolyl]pyridine

| Conditions | Yield |
|---|---|
| With phenyl isocyanate; triethylamine In hexane; benzene at 60℃; for 24h; | 82% |


| Conditions | Yield |
|---|---|
| With chloro(1,3-bis(2,6-di-i-propylphenyl)imidazol-2-ylidene)gold(I); silver(I) triflimide In 1,2-dichloro-ethane at 65℃; for 23h; | 82% |
-
-
288-14-2
ISOXAZOLE

-
-
124-38-9
carbon dioxide

-
-
74-88-4
methyl iodide

-
-
15055-81-9
methyl 1,2-oxazole-5-carboxylate

| Conditions | Yield |
|---|---|
| Stage #1: carbon dioxide With AuOH(1,3-bis(2,6-diisopropylphenyl)imidazol-2-ylidene); potassium hydroxide In tetrahydrofuran at 20℃; under 1125.11 Torr; for 0.25h; Stage #2: ISOXAZOLE In tetrahydrofuran at 20℃; under 1125.11 Torr; for 12h; Stage #3: methyl iodide In tetrahydrofuran regioselective reaction; | 81% |


| Conditions | Yield |
|---|---|
| With chloro(1,3-bis(2,6-di-i-propylphenyl)imidazol-2-ylidene)gold(I); silver(I) triflimide In 1,2-dichloro-ethane at 65℃; for 33h; | 81% |

| Conditions | Yield |
|---|---|
| In benzene (N2); stirring; filtn., washing (benzene), drying (vac.); elem. anal.; | 80% |
Isoxazole Chemical Properties
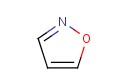
Molecular structure of Isoxazole (CAS NO.288-14-2) is:
Product Name: Isoxazole
CAS Registry Number: 288-14-2
IUPAC Name: 1,2-oxazole
Molecular Weight: 69.06202 [g/mol]
Molecular Formula: C3H3NO
XLogP3: 0.1
H-Bond Donor: 0
H-Bond Acceptor: 2
EINECS: 206-018-7
Surface Tension: 31.7 dyne/cm
Density: 1.055 g/cm3
Flash Point: 8.9 °C
Refractive index: n20/D 1.427(lit.)
Enthalpy of Vaporization: 32.12 kJ/mol
Boiling Point: 95.5 °C at 760 mmHg
Vapour Pressure: 51.7 mmHg at 25 °C
Product Categories: Isoxazoles, Oxadiazoles, Oxazoles;pharmacetical;Boronic ester;Isoxazole;Organoborons;Thiophens;Building Blocks;Heterocyclic Building Blocks;Isoxazoles
Isoxazole Uses
Isoxazole (CAS NO.288-14-2) is used in organic synthesis.
Isoxazole Safety Profile
Hazard Codes:  F,
F, Xi
Xi
Risk Statements: 11
R11:Highly flammable.
Safety Statements: 16-23/33-33-29-7/9
S16:Keep away from sources of ignition.
S23:Do not breathe vapour.
S33:Take precautionary measures against static discharges.
S29:Do not empty into drains.
S7/9:Keep container tightly closed and in a well-ventilated place.
RIDADR: UN 1993 3/PG 2
WGK Germany: 3
F: 10
Hazard Note: Highly Flammable
HazardClass: 3
PackingGroup: II
HS Code: 29349990
Isoxazole Specification
Isoxazole , its cas register number is 288-14-2. It also can be called 1,2-Oxazole ; 1-Oxa-2-azacyclopentadiene .It is a colorless liquid and very soluble in water.It can be found in some natural products, such as ibotenic acid ,and it also form the basis for a number of drugs, including the COX-2 inhibitor valdecoxib (Bextra).
Related Products
- Isoxazole
- Isoxazole, 3-(2,4-dichlorophenyl)-
- Isoxazole, 3-(2-thienyl)-5-(trifluoromethyl)-
- Isoxazole, 3-(trifluoromethyl)-
- Isoxazole, 3-chloro-
- Isoxazole, 4-iodo-5-methyl-3-phenyl-
- Isoxazole, 4-nitro-
- Isoxazole, 5-(4-fluorophenyl)-
- Isoxazole, 5-methoxy-
- Isoxazole, 5-phenyl-
- 288150-92-5
- 288151-30-4
- 288151-43-9
- 288154-16-5
- 288158-29-2
- 288159-40-0
- 28816-02-6
- 2881-63-2
- 288-16-4
- 2881-83-6
Hot Products
About|Contact|Cas|Product Name|Molecular|Country|Encyclopedia
Message|New Cas|MSDS|Service|Advertisement|CAS DataBase|Article Data|Manufacturers | Chemical Catalog
©2008 LookChem.com,License: ICP
NO.:Zhejiang16009103
complaints:service@lookchem.com Desktop View