-
Name
Jasmone
- EINECS 207-668-4
- CAS No. 488-10-8
- Article Data97
- CAS DataBase
- Density 0.937 g/cm3
- Solubility 1.48g/L at 20℃
- Melting Point 203 - 205oC
- Formula C11H16O
- Boiling Point 264.2 °C at 760 mmHg
- Molecular Weight 164.247
- Flash Point 107.2 °C
- Transport Information
- Appearance COA
- Safety 26-37/39
- Risk Codes 36/37/38
-
Molecular Structure
-
Hazard Symbols
 Xi
Xi
- Synonyms 2-Cyclopenten-1-one,3-methyl-2-(2-pentenyl)-, (Z)- (8CI);2-Cyclopenten-1-one,3-methyl-2-(2Z)-2-pentenyl- (9CI);Jasmone (6CI);(Z)-Jasmone;3-Methyl-2-(2-cis-pentenyl)-2-cyclopenten-1-one;3-Methyl-2-(cis-2-pentenyl)-2-cyclopenten-1-one;cis-Jasmone;
- PSA 17.07000
- LogP 3.02210
Synthetic route

| Conditions | Yield |
|---|---|
| With hydrogen; Lindlar's catalyst In hexane | 97% |
| With hydrogen; Lindlar's catalyst In ethyl acetate under 760 Torr; | 95% |
| With hydrogen; Lindlar's catalyst In hexane | 90% |
| With hydrogen; Lindlar's catalyst In ethanol | 65% |
| Yield given; |
-
-
82909-46-4
3-bromo-2-<(Z)-2-pentenyl>-2-cyclopenten-1-one

-
-
488-10-8
cis-jasmone

| Conditions | Yield |
|---|---|
| In diethyl ether 1.) -78 degC, 10 min., 2.) 0 degC, 1 h; | 93% |

| Conditions | Yield |
|---|---|
| With sodium hydroxide In ethanol for 4h; Heating; | 92% |
| With potassium hydroxide In ethanol for 2h; Heating; | 91% |
| With sodium hydroxide; water | 88% |
-
-
128192-01-8
2-(2-cis-pentenyl)-3-methyl-5-(phenylthio)-2-cyclopenten-1-one

-
-
488-10-8
cis-jasmone

| Conditions | Yield |
|---|---|
| With nickel In acetone | 88% |
| With nickel |
-
-
106763-43-3
methyl (Z)-2-<4-<(diethoxyphosphoryl)oxy>-1-oxo-4-pentenyl>-4-heptenoate

-
-
488-10-8
cis-jasmone

| Conditions | Yield |
|---|---|
| With sodium hydroxide In tetrahydrofuran at 80℃; for 20h; | 87% |
-
-
32556-66-4, 32556-68-6, 87392-23-2, 131321-83-0, 135029-97-9, 135029-98-0
4-methyl-5-(2-pentenyl)cyclopent-2-en-1-one

-
-
488-10-8
cis-jasmone

| Conditions | Yield |
|---|---|
| With sodium methylate In methanol Ambient temperature; | 83% |

-
-
488-10-8
cis-jasmone

| Conditions | Yield |
|---|---|
| With potassium hydroxide In ethanol at 95℃; for 0.5h; | 76% |

-
-
488-10-8
cis-jasmone

| Conditions | Yield |
|---|---|
| In acetic anhydride for 6h; Heating; | 75% |
-
-
207798-52-5
1-methyl-2-pent-2-enyl-cyclopent-2-enol

-
-
488-10-8
cis-jasmone

| Conditions | Yield |
|---|---|
| With dipyridinium dichromate In dichloromethane at 0℃; for 1h; | 74% |
-
-
132604-79-6
2-(cis-2-Pentenyl)-3-<(phenylsulfonyl)methyl>-2-cyclopenten-1-one

-
-
488-10-8
cis-jasmone

| Conditions | Yield |
|---|---|
| With 2,2'-azobis(isobutyronitrile); tri-n-butyl-tin hydride In benzene for 1h; Heating; | 71% |
| With 2,2'-azobis(isobutyronitrile); tri-n-butyl-tin hydride In benzene Heating; Yield given; |
-
-
165590-85-2
(Z)-3-methyl-2-(pent-2-enyl)cyclopent-2-enol

-
-
488-10-8
cis-jasmone

| Conditions | Yield |
|---|---|
| With jones reagent In acetone | 62% |

| Conditions | Yield |
|---|---|
| With aluminum oxide In benzene for 3h; Heating; | A 60% B n/a |

| Conditions | Yield |
|---|---|
| In toluene under 22800 Torr; for 36h; Heating; | 22% |

-
-
488-10-8
cis-jasmone

| Conditions | Yield |
|---|---|
| With decane; TEA In acetonitrile for 120h; Irradiation; | 15% |
-
-
81431-81-4
3-chloro-2-<(diethoxyphosphoryl)oxy>-1-propene

-
-
68776-91-0
2-acetyl-(Z)-hept-4-enoic acid methyl ester

-
-
488-10-8
cis-jasmone

| Conditions | Yield |
|---|---|
| With n-butyllithium; sodium hydride 1.) hexane, from 0 to 23 deg C, 20 min, 2.) hexane, from 0 to 23 deg C, 1 h 10 min; Yield given. Multistep reaction; |
-
-
21676-03-9
(Z)-3-hexenyl iodide

-
-
69567-06-2
(N-methylanilino)prop-2-enenitrile

-
-
6407-36-9
N-isopropyliden-cyclohexyl amine

-
-
488-10-8
cis-jasmone

| Conditions | Yield |
|---|---|
| With hydrogenchloride; natronlauge; lithium diisopropyl amide Yield given. Multistep reaction; |


| Conditions | Yield |
|---|---|
| With chromium(VI) oxide |
-
-
58282-77-2
2-Formylmethyl-3-methyl-2-cyclopenten-1-one

-
-
6228-47-3
n-propyltriphenylphosphonium bromide

-
-
488-10-8
cis-jasmone

| Conditions | Yield |
|---|---|
| With n-butyllithium 1.) THF-hexane, RT, 1 h, 2.) THF, -10 deg C, 1.5 h; Yield given. Multistep reaction; |
-
-
53376-48-0
ethyl 2-methyl-1-(2-pentyn-1-yl)-3-cyclopenten-5-one-1-carboxylate

-
-
488-10-8
cis-jasmone

| Conditions | Yield |
|---|---|
| With sodium chloride In dimethyl sulfoxide at 160℃; |
-
-
32556-66-4, 32556-68-6, 87392-23-2, 131321-83-0, 135029-97-9, 135029-98-0
(4R,5S)-4-Methyl-5-((E)-pent-2-enyl)-cyclopent-2-enone

-
A
-
488-10-8
cis-jasmone

-
B
-
6261-18-3
trans-jasmone

| Conditions | Yield |
|---|---|
| With Amberlite IRA-400 In methanol for 24h; Ambient temperature; Yield given. Yields of byproduct given. Title compound not separated from byproducts; |
-
-
79155-17-2
N-[1-ethoxycarbonyl-3-(3-indolyl)propyl]-L-alanyl-L-proline

-
-
488-10-8
cis-jasmone

| Conditions | Yield |
|---|---|
| With diethylamine In chloroform Heating; |
-
-
73959-78-1
4-Methyl-3-((Z)-pent-2-enyl)-1,2-bis-trimethylsilanyloxy-cyclopentene

-
-
488-10-8
cis-jasmone

| Conditions | Yield |
|---|---|
| With phosphoric acid; toluene-4-sulfonic acid 1.) 60 deg C, 3 h, 2.) DMF, 60 deg C, 20 h; Yield given. Multistep reaction; |
-
-
925-90-6
ethylmagnesium bromide

-
-
79858-12-1
2-methylene-3-methyl-3-methoxy-methyloxycyclopentanone

-
-
74-86-2
acetylene

-
-
488-10-8
cis-jasmone

| Conditions | Yield |
|---|---|
| With copper(l) iodide Yield given. Multistep reaction; |


-
-
488-10-8
cis-jasmone

| Conditions | Yield |
|---|---|
| With sodium hydroxide |

-
-
488-10-8
cis-jasmone

| Conditions | Yield |
|---|---|
| With hydrogen bromide; acetic acid anschliessend Erwaermen mit Zink-Pulver; |

-
-
488-10-8
cis-jasmone

| Conditions | Yield |
|---|---|
| With hydrogenchloride at 0℃; Behandeln des Reaktionsprodukts mit Natrium in Xylol und anschliessendem Erhitzen mit wss. H2SO4; |

-
-
488-10-8
cis-jasmone

| Conditions | Yield |
|---|---|
| With diethyl ether; aluminium amalgam; water |
-
-
892145-35-6
tert-butyl-dimethyl-(3-methyl-2-pent-2-enyl-cyclopent-2-enyloxy)-silane

-
-
488-10-8
cis-jasmone
-
-
488-10-8
cis-jasmone

-
-
165590-85-2
(Z)-3-methyl-2-(pent-2-enyl)cyclopent-2-enol

| Conditions | Yield |
|---|---|
| With lithium pyrrolidinoborohydride at 25℃; | 98% |
| With LiPyrrBH3 In tetrahydrofuran at 25℃; for 3h; | 98% |
| With sodium tetrahydroborate; calcium chloride In methanol 1a) 30 min, 25 deg C, 1b) 0 deg C, 1 h; | 93% |

| Conditions | Yield |
|---|---|
| With hydrogen; SC-1 Ni2B In methanol at 25℃; under 760 Torr; for 24h; | 97% |
| With hydrogen; palladium In methanol at 20℃; for 19h; | 60% |
| With hydrogen; [(C6Me6)2Ru2(PPh2)H2][BF4] In ethanol at 60℃; under 37503 Torr; for 24h; | 47% |

| Conditions | Yield |
|---|---|
| With oxygen; copper diacetate; palladium diacetate; p-benzoquinone at 90℃; under 7600.51 Torr; for 2h; Kinetics; Temperature; | 87% |
-
-
488-10-8
cis-jasmone

-
-
75-24-1
trimethylaluminum

-
-
157562-89-5
cis-2-(2-penten-1-yl)-3,3-dimethylcyclopentanone

| Conditions | Yield |
|---|---|
| With bis(acetylacetonate)nickel(II) In tetrahydrofuran; hexane for 4h; Ambient temperature; | 85% |
-
-
488-10-8
cis-jasmone

-
-
7051-39-0, 82768-42-1, 82768-43-2
3-methyl-2-(2-cis-pentenyl)cyclopentanone

| Conditions | Yield |
|---|---|
| With sodium t-butanolate; poly(methylhydrosiloxane); 1,3-bis(2,6-diisopropylphenyl)imidazolium copper(I) chloride In toluene at 20℃; for 1h; | 85% |
-
-
488-10-8
cis-jasmone

| Conditions | Yield |
|---|---|
| With Fe(bpmen)(OTf)2; dihydrogen peroxide; acetic acid In acetonitrile at 20℃; Concentration; | 80% |
-
-
488-10-8
cis-jasmone

-
-
107046-58-2, 107046-59-3, 148694-04-6
2-((2S,3S)-2,3-Dihydroxy-pentyl)-3-methyl-cyclopent-2-enone

| Conditions | Yield |
|---|---|
| With N,N'-dimethyl-N,N'-bis(2-pyridylmethyl)ethane-1,2-diamineiron(II) bis(triflate); dihydrogen peroxide In acetonitrile at 20℃; for 0.166667h; Catalytic behavior; stereoselective reaction; | 80% |

| Conditions | Yield |
|---|---|
| With magnesium sulfate; trifluoroacetic acid; diisopropylamine 2,2,2-trifluoroacetic acid salt In tetrahydrofuran at 60℃; for 24h; | 80% |
-
-
488-10-8
cis-jasmone

-
-
917-54-4
methyllithium

-
-
157562-89-5
cis-2-(2-penten-1-yl)-3,3-dimethylcyclopentanone

| Conditions | Yield |
|---|---|
| With copper(l) iodide; boron trifluoride diethyl etherate In diethyl ether at -78 - 20℃; for 4h; | 63% |

| Conditions | Yield |
|---|---|
| With sodium hydride In dimethyl sulfoxide for 4h; Ambient temperature; | 54% |
-
-
488-10-8
cis-jasmone

-
-
51615-66-8
2-(2,3-dibromobutyl)-3-methylcyclopent-2-enone

| Conditions | Yield |
|---|---|
| With hydrogen bromide; dimethyl sulfoxide In chloroform; water at 20℃; for 24h; | 50% |
-
-
488-10-8
cis-jasmone

| Conditions | Yield |
|---|---|
| With sodium hydroxide; dihydrogen peroxide In methanol for 3h; Ambient temperature; | 47% |
-
-
488-10-8
cis-jasmone

| Conditions | Yield |
|---|---|
| In ethyl acetate for 20h; Irradiation; | A 42% B 29% |
-
-
488-10-8
cis-jasmone

-
-
107046-58-2, 107046-59-3, 148694-04-6
2-[(2SR,3RS)-2,3-dihydroxypentyl]-3-methylcyclopent-2-enone

-
-
107046-58-2, 107046-59-3, 148694-04-6
2-((2S,3S)-2,3-Dihydroxy-pentyl)-3-methyl-cyclopent-2-enone

| Conditions | Yield |
|---|---|
| With [Fe(CF3SO3)2(1-[2′-(6'-methylpyridyl)methyl]-4,7-dimethyl-1,4,7-triazacyclononane)]; water; dihydrogen peroxide In acetonitrile at 0℃; for 0.5h; Green chemistry; chemoselective reaction; | A 34% B 9% |
-
-
488-10-8
cis-jasmone

| Conditions | Yield |
|---|---|
| With oxygen; rose bengal In methanol for 20h; Irradiation; | A 5% B 5% C 30% D 18% |

-
-
488-10-8
cis-jasmone

-
-
107046-58-2, 107046-59-3, 148694-04-6
2-((2S,3S)-2,3-Dihydroxy-pentyl)-3-methyl-cyclopent-2-enone

| Conditions | Yield |
|---|---|
| With oxone; [Fe(tris(2-pyridyl)amine)(triflate)2] In water; acetonitrile at 8℃; for 6h; stereoselective reaction; | A 30% B 30% |
Jasmone Chemical Properties
Molecule structure of Jasmone (CAS NO.488-10-8):
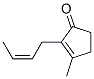
IUPAC Name: 3-Methyl-2-[(Z)-pent-2-enyl]cyclopent-2-en-1-one
Molecular Weight: 164.24414 g/mol
Molecular Formula: C11H16O
Density: 0.937 g/cm3
Boiling Point: 264.2 °C at 760 mmHg
Flash Point: 107.2 °C
Index of Refraction: 1.491
Molar Refractivity: 50.77 cm3
Molar Volume: 175.1 cm3
Polarizability: 20.12×10-24 cm3
Surface Tension: 31.1 dyne/cm
Enthalpy of Vaporization: 50.21 kJ/mol
Vapour Pressure: 0.00982 mmHg at 25 °C
XLogP3-AA: 2.2
H-Bond Acceptor: 1
Rotatable Bond Count: 3
Tautomer Count: 10
Exact Mass: 164.120115
MonoIsotopic Mass: 164.120115
Topological Polar Surface Area: 17.1
Heavy Atom Count: 12
Complexity: 233
Canonical SMILES: CCC=CCC1=C(CCC1=O)C
Isomeric SMILES: CC/C=C\CC1=C(CCC1=O)C
InChI: InChI=1S/C11H16O/c1-3-4-5-6-10-9(2)7-8-11(10)12/h4-5H,3,6-8H2,1-2H3/b5-4-
InChIKey: XMLSXPIVAXONDL-PLNGDYQASA-N
EINECS: 207-668-4
Product Categories: ketone Flavor
Jasmone Uses
Jasmone (CAS NO.488-10-8) can act as an attractant or a repellent for various insects. However it is used primarily in perfumes and cosmetics. It also can be used in jasmine and mint flavor.
Jasmone Production
Jasmone is manufactured within plants by the metabolism of jasmonate, from linolenic acid by the octadecanoid pathway.
Jasmone Toxicity Data With Reference
| 1. | skn-rbt 500 mg/24H MOD | FCTXAV Food and Cosmetics Toxicology. 17 (1979),845. | ||
| 2. | orl-rat LD50:5000 mg/kg | FCTXAV Food and Cosmetics Toxicology. 17 (1979),845. |
Jasmone Consensus Reports
Reported in EPA TSCA Inventory.
Jasmone Safety Profile
Hazard Codes:  Xi
Xi
Risk Statements: 36/37/38
R36/37/38:Irritating to eyes, respiratory system and skin.
Safety Statements: 26-37/39
S26: In case of contact with eyes, rinse immediately with plenty of water and seek medical advice.
S37/39:Wear suitable gloves and eye/face protection.
WGK Germany: 2
RTECS: GY7301000
Jasmone Specification
Jasmone (CAS NO.488-10-8) is also named as (Z)-Jasmone ; 2-Cyclopenten-1-one, 3-methyl-2-(2-pentenyl)-, (Z)- ; 3-Methyl-2-(2-pentenyl)-2-cyclopenten-1-one, (Z)- ; 3-Methyl-2-(cis-2-penten-1-yl)-2-cyclopenten-1-one ; 4-07-00-00337 (Beilstein Handbook Reference) ; BRN 1907713 ; FEMA No. 3196 . It is a colorless to pale yellow liquid that has the odor of jasmine. It slightly soluble in water, soluble in ethanol, ether and carbon tetrachloride and oil.
About|Contact|Cas|Product Name|Molecular|Country|Encyclopedia
Message|New Cas|MSDS|Service|Advertisement|CAS DataBase|Article Data|Manufacturers | Chemical Catalog
©2008 LookChem.com,License: ICP
NO.:Zhejiang16009103
complaints:service@lookchem.com Desktop View