-
Name
methyl 3-phenyl-L-alaninate
- EINECS 219-934-7
- CAS No. 2577-90-4
- Article Data198
- CAS DataBase
- Density 1.1 g/cm3
- Solubility
- Melting Point 131-133 °C
- Formula C10H13NO2
- Boiling Point 264.2 °C at 760 mmHg
- Molecular Weight 179.219
- Flash Point 126 °C
- Transport Information
- Appearance
- Safety
- Risk Codes
-
Molecular Structure
- Hazard Symbols
- Synonyms Methyl 3-phenyl-L-alaninate;
- PSA 52.32000
- LogP 1.42970
Synthetic route

| Conditions | Yield |
|---|---|
| With thionyl chloride | 100% |
| With thionyl chloride at -10 - 20℃; Inert atmosphere; | 100% |
| With thionyl chloride at 0 - 20℃; | 99% |
-
-
129397-81-5
methyl (((9H-fluoren-9-yl)methoxy)carbonyl)-L-phenylalaninate

-
-
100-53-8
phenylmethanethiol

-
A
-
2577-90-4
methyl (2S)-2-amino-3-phenylpropanoate

| Conditions | Yield |
|---|---|
| With tetrabutyl ammonium fluoride In N,N-dimethyl-formamide for 0.0333333h; Product distribution; other protected peptides, other thiol, var. TBAF conc., var. time, var. solvent, with or without ultrasound mixing; | A n/a B 100% |
| With tetrabutyl ammonium fluoride In N,N-dimethyl-formamide for 0.05h; | A n/a B 100% |

-
-
64590-81-4
(E)-α-(N-acylamino)cinnamic acid methyl ester

-
-
2577-90-4
methyl (2S)-2-amino-3-phenylpropanoate

| Conditions | Yield |
|---|---|
| With hydrogen; (S)Fc-α-S(C5H5)Fe(C5H3(PPh2)CH(OH)C6H4(PPh2)); [Rh(norbornadiene)2]BF4 In methanol at 25℃; under 750.075 Torr; for 1h; Product distribution / selectivity; | 100% |
| With hydrogen; (S)-1-diphenylphosphino-2-[α-(S)-methoxy(o-diphenylphosphinophenyl)methyl]ferrocene; [Rh(norbornadiene)2]BF4 In methanol at 25℃; under 750.075 Torr; for 1h; Product distribution / selectivity; | 100% |
-
-
7524-50-7
methyl (2S)-2-amino-3-phenylpropanoate hydrochloride

-
-
2577-90-4
methyl (2S)-2-amino-3-phenylpropanoate

| Conditions | Yield |
|---|---|
| With Amberlyst A21 In dichloromethane at 0 - 20℃; for 2h; stereoselective reaction; | 99% |
| With Amberlyst A21 resin In dichloromethane at 0 - 20℃; for 2h; Reagent/catalyst; | 99% |
| With potassium carbonate In dichloromethane; water at 20℃; | 97% |
-
-
35909-92-3
N-carbobenzoxy-L-phenylalanine methyl ester

-
-
2577-90-4
methyl (2S)-2-amino-3-phenylpropanoate

| Conditions | Yield |
|---|---|
| With hydrogen In methanol at 27℃; under 750.075 Torr; for 2h; Reagent/catalyst; Green chemistry; | 99% |
-
-
129397-81-5
methyl (((9H-fluoren-9-yl)methoxy)carbonyl)-L-phenylalaninate

-
-
2577-90-4
methyl (2S)-2-amino-3-phenylpropanoate

| Conditions | Yield |
|---|---|
| With piperazine; diethyl {[(1-methoxy-1-oxo-3-phenylpropan-2-yl)amino]methylidene}propanedioate In ethanol at 20℃; for 0.5h; | 98% |
| With sodium azide In N,N-dimethyl-formamide at 50℃; for 3h; | 97% |
| With triethylamine; 1-butyl-3-methylimidazolium Tetrafluoroborate In neat (no solvent) at 25℃; Green chemistry; | 82% |
| With aluminium trichloride; N,N-dimethyl-aniline In dichloromethane for 4h; Heating; | |
| With Octanethiol; tetrabutyl ammonium fluoride In N,N-dimethyl-formamide for 0.166667h; Inert atmosphere; |
-
-
1152-61-0
N-Cbz-L-Asp

-
A
-
2577-90-4
methyl (2S)-2-amino-3-phenylpropanoate

-
B
-
33605-72-0
N-carbobenzyloxy-L-aspartyl-L-phenylalanine methyl ester

| Conditions | Yield |
|---|---|
| In N-methyl-acetamide; water | A 95.5% B n/a |

| Conditions | Yield |
|---|---|
| With sodium hydroxide; sulfuric acid In methanol; water | 94.5% |
-
-
107897-41-6
N-(((tert-butyldimethylsilyl)oxy)carbonyl)phenylalanine methyl ester

-
-
2577-90-4
methyl (2S)-2-amino-3-phenylpropanoate

| Conditions | Yield |
|---|---|
| With tetrabutyl ammonium fluoride In tetrahydrofuran for 1h; Ambient temperature; | 93% |
-
-
211567-40-7
(S)-2-(2-Aza-bicyclo[2.2.1]hept-5-en-2-yl)-3-phenyl-propionic acid methyl ester

-
-
2577-90-4
methyl (2S)-2-amino-3-phenylpropanoate

| Conditions | Yield |
|---|---|
| Bio-Rad AG 50W-X2 In ethanol at 45℃; for 1.5h; | 93% |

-
-
2577-90-4
methyl (2S)-2-amino-3-phenylpropanoate

| Conditions | Yield |
|---|---|
| With mushroom tyrosinase E.C. 1.14.18.1; oxygen In phosphate buffer; acetonitrile at 20℃; | 93% |
-
-
228704-07-2
N-Tsoc-phenylalanine methyl ester

-
-
2577-90-4
methyl (2S)-2-amino-3-phenylpropanoate

| Conditions | Yield |
|---|---|
| With tetrabutyl ammonium fluoride In tetrahydrofuran at 0℃; | 92% |
| With tetrabutyl ammonium fluoride In tetrahydrofuran for 0.166667h; Inert atmosphere; Schlenk technique; | 89% |

-
-
2577-90-4
methyl (2S)-2-amino-3-phenylpropanoate

| Conditions | Yield |
|---|---|
| With Amberlite IRA-400 bound triethylammonium tetrathiomolybdate In methanol at 28℃; for 1h; ultrasonication; | 92% |
-
-
128369-71-1
(S)-methyl-(N-allyloxycarbonyl)phenylalaninate

-
-
2577-90-4
methyl (2S)-2-amino-3-phenylpropanoate

| Conditions | Yield |
|---|---|
| With sodium tetrahydroborate; tetrakis(triphenylphosphine) palladium(0) In tetrahydrofuran | 91% |
-
-
34143-74-3
1H,1H,2H,2H-Perfluorodecanethiol

-
-
203873-67-0
(S)-methyl 2-(2-nitrophenylsulfonamido)-3-phenylpropanoate

-
A
-
2577-90-4
methyl (2S)-2-amino-3-phenylpropanoate

| Conditions | Yield |
|---|---|
| With potassium carbonate In acetonitrile at 50℃; | A 91% B n/a |

-
-
1363553-45-0
(S)-methyl 2-(4,5-dimethoxy-2-methylbenzylamino)-3-phenylpropanoate

-
-
2577-90-4
methyl (2S)-2-amino-3-phenylpropanoate

| Conditions | Yield |
|---|---|
| With ammonium cerium (IV) nitrate; water In acetonitrile at 20℃; for 48h; Inert atmosphere; | 91% |
-
-
51987-73-6
(S)-2-tert-butoxycarbonylamino-3-phenyl-propionic acid methyl ester

-
-
2577-90-4
methyl (2S)-2-amino-3-phenylpropanoate

| Conditions | Yield |
|---|---|
| With phosphoric acid In dichloromethane at 20℃; for 3h; | 90% |
| With 3-butyl-l-methyl-1H-imidazol-3-iumtrifloroacetate In 1,4-dioxane; water at 80 - 82℃; for 3h; | 70% |
| With molecular sieve; boron trifluoride diethyl etherate In dichloromethane for 20h; Ambient temperature; |
-
-
1222062-82-9
diethyl {[(1-methoxy-1-oxo-3-phenylpropan-2-yl)amino]methylidene}propanedioate

-
-
2577-90-4
methyl (2S)-2-amino-3-phenylpropanoate

| Conditions | Yield |
|---|---|
| With ethylenediamine In ethanol at 20℃; for 2.3h; | 90% |
-
-
344765-03-3
2-(4-methoxybenzylamino)-3-phenypropionic acid methyl ester

-
-
2577-90-4
methyl (2S)-2-amino-3-phenylpropanoate

| Conditions | Yield |
|---|---|
| With hydrogenchloride; riboflavin tetraacetate; water In acetonitrile for 1h; pH=3; Irradiation; In air; | 90% |

-
-
2577-90-4
methyl (2S)-2-amino-3-phenylpropanoate

| Conditions | Yield |
|---|---|
| With mushroom tyrosinase E.C. 1.14.18.1; oxygen In phosphate buffer; acetonitrile at 20℃; | 87% |
-
-
116911-32-1
(S)-2-azido-3-phenylpropionic acid methyl ester

-
-
2577-90-4
methyl (2S)-2-amino-3-phenylpropanoate

| Conditions | Yield |
|---|---|
| With palladium 10% on activated carbon; hydrogen In ethanol at 20℃; for 12h; | 85% |
| With hydrogen; palladium on activated charcoal |

| Conditions | Yield |
|---|---|
| With sulfuric acid In methanol | 83.2% |
| With sulfuric acid In methanol | |
| Multi-step reaction with 2 steps 1.1: acetyl chloride / 0.17 h / 10 °C 1.2: 8 h / 10 - 20 °C 2.1: sodium hydroxide / chloroform View Scheme |

| Conditions | Yield |
|---|---|
| With hydroxylamine hydrochloride; sodium acetate In methanol | 80% |
-
-
67-56-1
methanol

-
-
81917-69-3
N-(1-Benzotriazolylcarbonyl)-L-phenylalanin

-
-
2577-90-4
methyl (2S)-2-amino-3-phenylpropanoate

| Conditions | Yield |
|---|---|
| With TEA at 20℃; for 4h; | 78% |
-
-
79561-72-1
N,N-bis-allyl-L-phenylalanine methyl ester

-
-
2577-90-4
methyl (2S)-2-amino-3-phenylpropanoate

| Conditions | Yield |
|---|---|
| With Wilkinson's catalyst In water; acetonitrile for 2h; Heating; | 76% |
-
-
87900-19-4
(2R,5S)-2-Benzyl-5-isopropyl-3,6-dimethoxy-2,5-dihydro-pyrazine

-
A
-
4070-48-8
L-Valine methyl ester

-
B
-
2577-90-4
methyl (2S)-2-amino-3-phenylpropanoate

-
C
-
21685-51-8
D-phenylalanine methyl ester

| Conditions | Yield |
|---|---|
| With hydrogenchloride for 10h; Ambient temperature; | A n/a B n/a C 73% |


-
-
2577-90-4
methyl (2S)-2-amino-3-phenylpropanoate

| Conditions | Yield |
|---|---|
| Stage #1: methyl (2-methoxybenzoyl)phenylalaninate With Schwartz's reagent In tetrahydrofuran at -5 - 0℃; for 2h; Inert atmosphere; Stage #2: With ammonium chloride In tetrahydrofuran at 20℃; for 12h; Inert atmosphere; | 68% |
-
-
106018-94-4
methyl N-{[2-(trimethylsilyl)ethyl]sulfonyl}-L-phenylalaninate

-
-
2577-90-4
methyl (2S)-2-amino-3-phenylpropanoate

| Conditions | Yield |
|---|---|
| With hydrogen fluoride; methoxybenzene In dichloromethane at 0℃; for 1h; desulfonylation; | 65% |
-
-
2577-90-4
methyl (2S)-2-amino-3-phenylpropanoate

-
-
13734-34-4
N-tert-butoxycarbonyl-L-phenylalanine

-
-
13122-89-9
(S)-methyl 2-((S)-2-(tert-butoxycarbonylamino)-3-phenylpropanamido)-3-phenylpropanoate

| Conditions | Yield |
|---|---|
| With N-ethyl-N,N-diisopropylamine; N-[(dimethylamino)-3-oxo-1H-1,2,3-triazolo[4,5-b]pyridin-1-yl-methylene]-N-methylmethanaminium hexafluorophosphate In various solvent(s) at 65℃; for 3h; | 100% |
| With 6-chloro-3-((dimethylamino)(dimethyliminio)methyl)-1H-benzo[d][1,2,3]triazol-3-ium-1-olatehexafluorophosphate(V); N-ethyl-N,N-diisopropylamine In dichloromethane | 98% |
| With 4-(4,6-dimethoxy-1,3,5-triazin-2-yl)-4-methylmorpholinium chloride In Dichlorodifluoromethane; water at 20℃; for 3h; | 96% |

| Conditions | Yield |
|---|---|
| With boron trifluoride diethyl etherate; diborane In tetrahydrofuran at 20℃; for 1h; | 100% |
| With hydrogen In ethanol at 110℃; under 30003 Torr; Reagent/catalyst; Autoclave; chemoselective reaction; | 91.1% |
| With lithium aluminium tetrahydride In tetrahydrofuran for 4h; Heating; | 86% |
-
-
2577-90-4
methyl (2S)-2-amino-3-phenylpropanoate

-
-
1152-61-0
N-Cbz-L-Asp

-
-
33605-72-0
N-carbobenzyloxy-L-aspartyl-L-phenylalanine methyl ester

| Conditions | Yield |
|---|---|
| thermolysin-immobilized capsule membrane In chloroform at 45℃; for 24h; | 100% |
| thermolysin-immobilized capsule membrane In chloroform at 45℃; for 24h; Mechanism; KM, kcat; other catalyst, rection time, temperature; | 100% |
| at 55℃; cross-linked enzyme crystal (T-CLEC); | 95% |

| Conditions | Yield |
|---|---|
| With ammonium bicarbonate; water In dichloromethane for 48h; α-chymotrypsin; | 100% |
| With sodium hydroxide In water; ethyl acetate pH=9 - 10; | 93% |
| at 25℃; for 0.833333h; enzyme alcalase from Bacillus licheniforms; pH 8.2; |
-
-
2577-90-4
methyl (2S)-2-amino-3-phenylpropanoate

-
-
108-24-7
acetic anhydride

-
-
3618-96-0
(S)-N-acetylphenylalanine

| Conditions | Yield |
|---|---|
| In dichloromethane at 20℃; for 4h; | 100% |
| With sodium hydrogencarbonate In dichloromethane; water at 25℃; for 4h; Schotten-Baumann Reaction; | 100% |
| With triethylamine In dichloromethane at 20℃; for 1h; | 94% |
-
-
24424-99-5
di-tert-butyl dicarbonate

-
-
2577-90-4
methyl (2S)-2-amino-3-phenylpropanoate

-
-
51987-73-6
(S)-2-tert-butoxycarbonylamino-3-phenyl-propionic acid methyl ester

| Conditions | Yield |
|---|---|
| With N-ethyl-N,N-diisopropylamine | 100% |
| copper(II) bis(tetrafluoroborate) at 30 - 35℃; for 0.166667h; | 99% |
| With sodium hydrogencarbonate In 1,4-dioxane; water at 0℃; for 1h; | 99% |
-
-
2577-90-4
methyl (2S)-2-amino-3-phenylpropanoate

| Conditions | Yield |
|---|---|
| In dichloromethane | 100% |
-
-
24424-99-5
di-tert-butyl dicarbonate

-
-
2577-90-4
methyl (2S)-2-amino-3-phenylpropanoate

-
-
154409-62-8
methyl (S)-2-[bis(tert-butoxycarbonyl) amino]-3-phenylpropanoate

| Conditions | Yield |
|---|---|
| With dmap | 100% |
| Stage #1: di-tert-butyl dicarbonate; methyl (2S)-2-amino-3-phenylpropanoate With sodium carbonate In tetrahydrofuran; water at 0 - 20℃; Stage #2: With dmap In acetonitrile at 20℃; | 92% |
-
-
2577-90-4
methyl (2S)-2-amino-3-phenylpropanoate

-
-
47173-80-8
N-Boc-D-serine(Bzl)-OH

-
-
192723-29-8
(S)-2-((R)-3-Benzyloxy-2-tert-butoxycarbonylamino-propionylamino)-3-phenyl-propionic acid methyl ester

| Conditions | Yield |
|---|---|
| With O-(benzotriazol-1-yl)-N,N,N',N'-tetramethyluronium tetrafluoroborate; N-ethyl-N,N-diisopropylamine In dichloromethane at 20℃; | 100% |
| With O-(benzotriazol-1-yl)-N,N,N',N'-tetramethyluronium tetrafluoroborate; N-ethyl-N,N-diisopropylamine In dichloromethane |
-
-
61357-36-6
1-boraadamantane tetrahydrofuranate

-
-
2577-90-4
methyl (2S)-2-amino-3-phenylpropanoate

| Conditions | Yield |
|---|---|
| 100% |
-
-
2577-90-4
methyl (2S)-2-amino-3-phenylpropanoate

-
-
112695-98-4, 79777-82-5
N-(tert-butoxycarbonyl)-L-tert-leucine

| Conditions | Yield |
|---|---|
| With 6-chloro-3-((dimethylamino)(dimethyliminio)methyl)-1H-benzo[d][1,2,3]triazol-3-ium-1-olatehexafluorophosphate(V); N-ethyl-N,N-diisopropylamine In dichloromethane | 100% |
-
-
2577-90-4
methyl (2S)-2-amino-3-phenylpropanoate

-
-
13122-89-9
(S)-methyl 2-((S)-2-(tert-butoxycarbonylamino)-3-phenylpropanamido)-3-phenylpropanoate

| Conditions | Yield |
|---|---|
| With zinc diacetate; sodium acetate In tetrahydrofuran at 60℃; for 24h; Reagent/catalyst; Temperature; | 100% |

| Conditions | Yield |
|---|---|
| Stage #1: N-Fmoc L-Phe With N,N'-dimethylbenzylamine; isopropyl chloroformate; N-ethyl-N,N-diisopropylamine In 1,4-dioxane; acetonitrile at 60℃; for 0.00139167h; Flow reactor; Stage #2: methyl (2S)-2-amino-3-phenylpropanoate With 1-methyl-1H-imidazole; hydrogenchloride In 1,4-dioxane; water; acetonitrile at 60℃; for 0.0333333h; Flow reactor; | 100% |

| Conditions | Yield |
|---|---|
| With triethylamine In chloroform at 20℃; for 0.0222222h; Flow reactor; | 99.9% |
| In diethyl ether for 3h; Heating; | 57% |
-
-
2577-90-4
methyl (2S)-2-amino-3-phenylpropanoate

-
-
616-34-2
methoxycarbonylmethylamine

-
-
721-90-4
(S)-PheGly

| Conditions | Yield |
|---|---|
| Stage #1: methyl (2S)-2-amino-3-phenylpropanoate at 50℃; Ionic liquid; Inert atmosphere; Stage #2: With trifluoroacetic acid Stage #3: methoxycarbonylmethylamine Temperature; Further stages; | 99.9% |
-
-
13139-15-6
N-tert-butoxycarbonyl-L-leucine

-
-
2577-90-4
methyl (2S)-2-amino-3-phenylpropanoate

-
-
15136-32-0, 87976-66-7, 120342-25-8, 5874-73-7
(N-(tert-butoxycarbonyl)-L-leucinyl)-L-phenylalanine methyl ester

| Conditions | Yield |
|---|---|
| With O-(benzotriazol-1-yl)-N,N,N',N'-tetramethyluronium tetrafluoroborate; N-ethyl-N,N-diisopropylamine In dichloromethane at 20℃; Inert atmosphere; | 99% |
| With O-(benzotriazol-1-yl)-N,N,N',N'-tetramethyluronium tetrafluoroborate; N-ethyl-N,N-diisopropylamine In dichloromethane; acetonitrile at 20℃; Inert atmosphere; | 99% |
| With O-(benzotriazol-1-yl)-N,N,N',N'-tetramethyluronium tetrafluoroborate; N-ethyl-N,N-diisopropylamine In dichloromethane at 20℃; for 1h; Inert atmosphere; | 99% |
-
-
2577-90-4
methyl (2S)-2-amino-3-phenylpropanoate

-
-
88744-38-1
benzotriazol-1-yl p-aminobenzoate

| Conditions | Yield |
|---|---|
| With triethylamine In N,N-dimethyl-formamide Ambient temperature; | 99% |
-
-
2577-90-4
methyl (2S)-2-amino-3-phenylpropanoate

-
-
100-39-0
benzyl bromide

-
-
184774-09-2
N,N-dibenzyl-L-phenylalanine methyl ester

| Conditions | Yield |
|---|---|
| With sodium hydrogencarbonate In tetrahydrofuran; dimethyl sulfoxide for 12h; Reflux; | 99% |
| With sodium hydrogencarbonate In tetrahydrofuran; dimethyl sulfoxide at 85℃; for 16h; |
-
-
2577-90-4
methyl (2S)-2-amino-3-phenylpropanoate

-
-
37553-65-4, 64263-83-8, 85466-66-6
N-tert-butoxycarbonyl-N-methyl-D-phenylalanine

-
-
934715-26-1
Boc-D-Phe(N-Me)-Phe-OMe

| Conditions | Yield |
|---|---|
| With O-(benzotriazol-1-yl)-N,N,N',N'-tetramethyluronium tetrafluoroborate; N-ethyl-N,N-diisopropylamine In dichloromethane at 20℃; Inert atmosphere; | 99% |
| With O-(benzotriazol-1-yl)-N,N,N',N'-tetramethyluronium tetrafluoroborate; N-ethyl-N,N-diisopropylamine In dichloromethane; acetonitrile at 20℃; Inert atmosphere; | 97% |
| With O-(benzotriazol-1-yl)-N,N,N',N'-tetramethyluronium tetrafluoroborate; N-ethyl-N,N-diisopropylamine In dichloromethane at 20℃; | 90% |
| With O-(benzotriazol-1-yl)-N,N,N',N'-tetramethyluronium tetrafluoroborate; N-ethyl-N,N-diisopropylamine In dichloromethane |
-
-
2577-90-4
methyl (2S)-2-amino-3-phenylpropanoate

-
-
18942-49-9
Boc-D-Phe-OH

-
-
94202-58-1
t-Boc-D-phenylalanyl-L-phenylalanine methyl ester

| Conditions | Yield |
|---|---|
| With O-(benzotriazol-1-yl)-N,N,N',N'-tetramethyluronium tetrafluoroborate; N-ethyl-N,N-diisopropylamine In dichloromethane; acetonitrile at 20℃; Inert atmosphere; | 99% |
| With O-(benzotriazol-1-yl)-N,N,N',N'-tetramethyluronium tetrafluoroborate; N-ethyl-N,N-diisopropylamine In dichloromethane at 20℃; | 98% |
| With 4-(4,6-dimethoxy-1,3,5-triazin-2-yl)-4-methylmorpholinium chloride | 95% |
-
-
2577-90-4
methyl (2S)-2-amino-3-phenylpropanoate

-
-
66863-43-2
(3S)-2-tert-butoxycarbonyl-1,2,3,4-tetrahydro-beta-carboline-3-carboxylic acid

-
-
900865-72-7
(S)-tert-butyl 3-((S)-1-methoxy-1-oxo-3-phenylpropan-2-ylcarbamoyl)-3,4-dihydro-1H-pyrido[3,4-b]indole-2(9H)-carboxylate

| Conditions | Yield |
|---|---|
| Stage #1: (3S)-2-tert-butoxycarbonyl-1,2,3,4-tetrahydro-beta-carboline-3-carboxylic acid With benzotriazol-1-ol In tetrahydrofuran at 0℃; for 0.166667h; Stage #2: methyl (2S)-2-amino-3-phenylpropanoate With 4-methyl-morpholine; dicyclohexyl-carbodiimide In tetrahydrofuran at 0 - 20℃; for 18h; | 99% |
| With benzotriazol-1-ol; dicyclohexyl-carbodiimide In tetrahydrofuran at 0 - 20℃; for 18h; | 98% |
-
-
2577-90-4
methyl (2S)-2-amino-3-phenylpropanoate

-
-
173775-54-7
(R)-N-(tert-butoxycarbonyl)-4-fluoro-3-nitrophenylalanine

| Conditions | Yield |
|---|---|
| With benzotriazol-1-ol; N-(3-dimethylaminopropyl)-N-ethylcarbodiimide In dichloromethane at 25℃; for 12h; | 99% |
-
-
2577-90-4
methyl (2S)-2-amino-3-phenylpropanoate

-
-
98-59-9
p-toluenesulfonyl chloride

-
-
51220-86-1, 149703-21-9, 42384-33-8
N-(p-toluenesulfonyl)-L-phenylalanine methyl ester

| Conditions | Yield |
|---|---|
| With triethylamine In dichloromethane at 20℃; for 12h; Inert atmosphere; | 99% |
| In water at 110℃; for 0.0833333h; Microwave irradiation; Green chemistry; chemoselective reaction; | 94% |
| β‐cyclodextrin In various solvent(s) at 20℃; for 0.75h; pH=8; | 90% |
| With erbium(III) triflate In 2-methyltetrahydrofuran for 2h; Green chemistry; |

| Conditions | Yield |
|---|---|
| at 120℃; for 10h; Inert atmosphere; Neat (no solvent); stereoselective reaction; | 99% |
-
-
2577-90-4
methyl (2S)-2-amino-3-phenylpropanoate

-
-
1363906-80-2
1-acetyl-2,3,4,6,7,8-hexahydropyrrolo[1,2-a]pyrimidinium tetraphenylborate

-
-
3618-96-0
(S)-N-acetylphenylalanine

| Conditions | Yield |
|---|---|
| In acetonitrile at 80℃; for 1h; Inert atmosphere; | 99% |
L-Phenylalanine methyl ester Specification
The L-Phenylalanine, methylester, with the CAS registry number 2577-90-4, is also known as Methyl 3-phenyl-L-alaninate. Its EINECS registry number is 219-934-7. This chemical's molecular formula is C10H13NO2 and molecular weight is 179.21572. What's more, its systematic name is Methyl L-phenylalaninate.
Physical properties about L-Phenylalanine, methylester are: (1)ACD/LogP: 1.27; (2)# of Rule of 5 Violations: 0; (3)#H bond acceptors: 3; (4)#H bond donors: 2; (5)#Freely Rotating Bonds: 5; (6)Polar Surface Area: 29.54 Å2; (7)Index of Refraction: 1.53; (8)Molar Refractivity: 50.33 cm3; (9)Molar Volume: 162.8 cm3; (10)Polarizability: 19.95×10-24 cm3; (11)Surface Tension: 42 dyne/cm; (12)Density: 1.1 g/cm3; (13)Flash Point: 126 °C; (14)Enthalpy of Vaporization: 50.2 kJ/mol; (15)Boiling Point: 264.2 °C at 760 mmHg; (16)Vapour Pressure: 0.00986 mmHg at 25 °C.
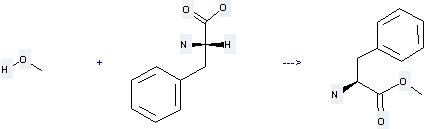
Preparation of L-Phenylalanine, methylester: this chemical is prepared by reaction of L-Phenylalanine with Methanol by heating. The reaction needs reagent Thionyl chloride. The yield is about 95 %.
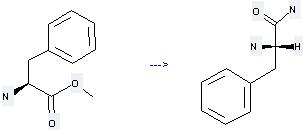
Uses of L-Phenylalanine, methylester: it is used to produce other chemicals. For example, it is used to produce L-Phenylalanine amide at ambient temperature. The reaction needs reagent aq. NH4OH and solvent Toluene. The reaction time is 16 hours. The yield is about 79 %.
You can still convert the following datas into molecular structure:
(1) SMILES: O=C(OC)[C@@H](N)Cc1ccccc1
(2) InChI: InChI=1/C10H13NO2/c1-13-10(12)9(11)7-8-5-3-2-4-6-8/h2-6,9H,7,11H2,1H3/t9-/m0/s1
(3) InChIKey: VSDUZFOSJDMAFZ-VIFPVBQEBN
Related Products
- L-Phenylalanine
- L-Phenylalanine 4-methyl-7-coumarinylamide trifluoroacetate salt
- L-Phenylalanine benzyl ester hydrochloride
- L-Phenylalanine hydrochloride
- L-Phenylalanine methyl ester
- L-Phenylalanine, 4-amino-N-[(1,1-dimethylethoxy)carbonyl]-, methylester
- L-Phenylalanine, L-a-aspartyl-
- L-Phenylalanine, L-b-aspartyl-
- L-Phenylalanine, L-b-aspartyl-, 2-methyl ester
- L-Phenylalanine, L-g-glutamyl-
- 25779-13-9
- 25781-92-4
- 2578-33-8
- 2578-45-2
- 25784-83-2
- 25784-91-2
- 25785-10-8
- 25785-60-8
- 2578-57-6
- 25786-72-5
Hot Products
About|Contact|Cas|Product Name|Molecular|Country|Encyclopedia
Message|New Cas|MSDS|Service|Advertisement|CAS DataBase|Article Data|Manufacturers | Chemical Catalog
©2008 LookChem.com,License: ICP
NO.:Zhejiang16009103
complaints:service@lookchem.com Desktop View