-
Name
ALPHA-METHYL-L-P-TYROSINE
- EINECS 211-599-5
- CAS No. 672-87-7
- Article Data7
- CAS DataBase
- Density 1.283g/cm3
- Solubility
- Melting Point 320-340°C dec.
- Formula C10H13 N O3
- Boiling Point 383.7oC at 760 mmHg
- Molecular Weight 195.218
- Flash Point 185.9oC
- Transport Information
- Appearance White Solid
- Safety 2368400
- Risk Codes 2368400
-
Molecular Structure
- Hazard Symbols
- Synonyms Tyrosine,a-methyl-, L- (8CI);(S)-2-(4-Hydroxybenzyl)-2-aminopropanoic acid; (S)-a-Methyltyrosine; Demser; L(-)-Metyrosine;L-Metyrosine; L-a-MT;L-a-Methyl-p-tyrosine; L-a-Methyltyrosine; MK 781;Metirosine; Metyrosine; a-Methyl-L-tyrosine
- PSA 83.55000
- LogP 1.43700
Synthetic route
-
-
1299492-19-5
(2S)-2-amino-3-(4-methoxyphenyl)-2-methyl-propionamide hydrogen bromide salt

-
-
672-87-7
L-methyltyrosine

| Conditions | Yield |
|---|---|
| With water; hydrogen bromide at 105℃; for 17h; Product distribution / selectivity; | 86% |
-
-
1299492-09-3
2-(S)-amino-3-(4-methoxyphenyl)-2-methylpropionamide

-
-
672-87-7
L-methyltyrosine

| Conditions | Yield |
|---|---|
| With water; hydrogen bromide at 120℃; for 5h; Product distribution / selectivity; | 84% |
| Multi-step reaction with 2 steps 1: water; hydrogen bromide / methanol / 100 °C / 15 - 22.5 Torr 2: water; hydrogen bromide / 17 h / 105 °C View Scheme |
-
-
1299492-14-0
(2S)-2-amino-3-(4-methoxyphenyl)-2-methyl-propionamide hydrochloride

-
-
672-87-7
L-methyltyrosine

| Conditions | Yield |
|---|---|
| With hydrogen bromide In water at 120℃; for 4h; Product distribution / selectivity; | 83% |
-
-
1299492-20-8
(2S)-2-amino-3-(4-methoxyphenyl)-2-methylpropionamide formic acid salt

-
-
672-87-7
L-methyltyrosine

| Conditions | Yield |
|---|---|
| With water; hydrogen bromide at 105℃; for 17h; Product distribution / selectivity; | 82% |

-
-
672-87-7
L-methyltyrosine

| Conditions | Yield |
|---|---|
| With hydrogenchloride In 1,4-dioxane at 100℃; for 4h; | 75.4% |

-
-
672-87-7
L-methyltyrosine

| Conditions | Yield |
|---|---|
| Stage #1: tert-butyl (2R)-2-amino-3-(4-methoxyphenyl)-2-methylpropanoate With boron tribromide In dichloromethane at 20℃; for 1.5h; Stage #2: With sodium hydrogencarbonate In dichloromethane; water Product distribution / selectivity; | 45.4% |
-
-
121703-96-6
N-Chloroacetyl-α-methyltyrosine

-
A
-
672-87-7
L-methyltyrosine

-
B
-
672-86-6
α-methyl-D-tyrosine

-
C
-
121704-37-8
N-Chloroacetyl-D-α-methyltyrosine

-
D
-
121703-96-6, 121704-37-8
N-Chloroacetyl-L-α-methyltyrosine

| Conditions | Yield |
|---|---|
| With potassium hydroxide; porcine kidney acylase I pH 7.5-8.0; Yields of byproduct given; | A 30% B n/a C n/a D n/a |
| With potassium hydroxide; porcine kidney acylase I pH 7.5-8.0; Yields of byproduct given; | A n/a B 30% C n/a D n/a |
| With potassium hydroxide; porcine kidney acylase I pH 7.5-8.0; Yield given. Yields of byproduct given; |

-
-
65555-88-6
α-methyl-β-p-methoxyphenyl-alanine

-
-
672-87-7
L-methyltyrosine

| Conditions | Yield |
|---|---|
| With hydrogen bromide |
-
-
121703-96-6
N-Chloroacetyl-α-methyltyrosine

-
A
-
672-87-7
L-methyltyrosine

-
B
-
121704-37-8
N-Chloroacetyl-D-α-methyltyrosine

| Conditions | Yield |
|---|---|
| With potassium hydroxide; potassium phosphate buffer; porcine kidney acylase I at 40℃; relative initial rate of hydrolysis, also with Aspergillus acylase I as a catalyst; with or without CoCl2; |
-
-
16109-65-2
2-Nitro-2-(4-hydroxybenzyl)propionsaeureethylester

-
-
672-87-7
L-methyltyrosine

| Conditions | Yield |
|---|---|
| With hydrogen; platinum(IV) oxide; nickel 1.) MeOH, reflux, 1 h, 2.) MeOH, 28 h; Yield given. Multistep reaction; |

| Conditions | Yield |
|---|---|
| Multi-step reaction with 2 steps 1: 56.1 percent / tetrabutylammonium chloride, potassium fluoride dihydrate / toluene / 48 h / Heating 2: 2.) H2 / 1.) Raney-Ni, 2.) PtO2 / 1.) MeOH, reflux, 1 h, 2.) MeOH, 28 h View Scheme |

| Conditions | Yield |
|---|---|
| Multi-step reaction with 4 steps 1: NaH / tetrahydrofuran / 0 - 25 °C 2: α-chymotrypsin / dimethylsulfoxide; H2O / 22 - 25 °C / potassium phosphate ( pH 7.0); with pig liver esterase the reaction rate is higher but the e.e. is lower 3: 1.) acyl azide formation, 2.) Curtius rearrangement 4: aq. HBr (48 percent) View Scheme |
-
-
21118-89-8
2-<(4-methoxyphenyl)methyl>-2-methylpropanedioic acid, dimethyl ester

-
-
672-87-7
L-methyltyrosine

| Conditions | Yield |
|---|---|
| Multi-step reaction with 3 steps 1: α-chymotrypsin / dimethylsulfoxide; H2O / 22 - 25 °C / potassium phosphate ( pH 7.0); with pig liver esterase the reaction rate is higher but the e.e. is lower 2: 1.) acyl azide formation, 2.) Curtius rearrangement 3: aq. HBr (48 percent) View Scheme |
-
-
21186-54-9
(R)-2-(4-Methoxy-benzyl)-2-methyl-malonic acid monomethyl ester

-
-
672-87-7
L-methyltyrosine

| Conditions | Yield |
|---|---|
| Multi-step reaction with 2 steps 1: 1.) acyl azide formation, 2.) Curtius rearrangement 2: aq. HBr (48 percent) View Scheme |

| Conditions | Yield |
|---|---|
| Multi-step reaction with 5 steps 1.1: water; methanol / 20 h / 40 °C / Sealed tube 2.1: sulfuric acid / dichloromethane; water / 3.25 h / -10 - 0 °C / Cooling with ice 2.2: pH 8 - 9 3.1: ammonium formate / palladium on carbon / isopropyl alcohol / 1 h / Inert atmosphere; Reflux 4.1: water; hydrogen bromide / methanol / 100 °C / 15 - 22.5 Torr 5.1: water; hydrogen bromide / 17 h / 105 °C View Scheme | |
| Multi-step reaction with 4 steps 1.1: water; methanol / 20 h / 40 °C / Sealed tube 2.1: sulfuric acid / dichloromethane; water / 3.25 h / -10 - 0 °C / Cooling with ice 2.2: pH 8 - 9 3.1: palladium on carbon / water; methanol / 5.75 h / 20 - 60 °C 4.1: water; hydrogen bromide / 17 h / 105 °C View Scheme | |
| Multi-step reaction with 4 steps 1.1: water; methanol / 20 h / 40 °C / Sealed tube 2.1: sulfuric acid / dichloromethane; water / 3.25 h / -10 - 0 °C / Cooling with ice 2.2: pH 8 - 9 3.1: ammonium formate / palladium on carbon / isopropyl alcohol / 1 h / Inert atmosphere; Reflux 4.1: water; hydrogen bromide / 5 h / 120 °C View Scheme |
-
-
1299492-07-1
2-[1-(S)-cyano-2-(4-methoxyphenyl)-1-methylethylamino]-2-(R)-phenylacetamide

-
-
672-87-7
L-methyltyrosine

| Conditions | Yield |
|---|---|
| Multi-step reaction with 4 steps 1.1: sulfuric acid / dichloromethane; water / 3.25 h / -10 - 0 °C / Cooling with ice 1.2: pH 8 - 9 2.1: ammonium formate / palladium on carbon / isopropyl alcohol / 1 h / Inert atmosphere; Reflux 3.1: water; hydrogen bromide / methanol / 100 °C / 15 - 22.5 Torr 4.1: water; hydrogen bromide / 17 h / 105 °C View Scheme | |
| Multi-step reaction with 3 steps 1.1: sulfuric acid / dichloromethane; water / 3.25 h / -10 - 0 °C / Cooling with ice 1.2: pH 8 - 9 2.1: palladium on carbon / water; methanol / 5.75 h / 20 - 60 °C 3.1: water; hydrogen bromide / 17 h / 105 °C View Scheme | |
| Multi-step reaction with 3 steps 1.1: sulfuric acid / dichloromethane; water / 3.25 h / -10 - 0 °C / Cooling with ice 1.2: pH 8 - 9 2.1: ammonium formate / palladium on carbon / isopropyl alcohol / 1 h / Inert atmosphere; Reflux 3.1: water; hydrogen bromide / 5 h / 120 °C View Scheme |
-
-
1299492-08-2
2-[(R)-(carbamoylphenylmethyl)-amino]-3-(4-methoxyphenyl)-2-(S)-methylpropionamide

-
-
672-87-7
L-methyltyrosine

| Conditions | Yield |
|---|---|
| Multi-step reaction with 3 steps 1: ammonium formate / palladium on carbon / isopropyl alcohol / 1 h / Inert atmosphere; Reflux 2: water; hydrogen bromide / methanol / 100 °C / 15 - 22.5 Torr 3: water; hydrogen bromide / 17 h / 105 °C View Scheme | |
| Multi-step reaction with 2 steps 1: ammonium formate / palladium on carbon / isopropyl alcohol / 1 h / Inert atmosphere; Reflux 2: water; hydrogen bromide / 5 h / 120 °C View Scheme | |
| Multi-step reaction with 2 steps 1: palladium on carbon / water; methanol / 5.75 h / 20 - 60 °C 2: water; hydrogen bromide / 17 h / 105 °C View Scheme |
-
-
1299492-11-7
3-(4-methoxyphenyl)-2-methyl-2-((S)-1-phenyl-ethylamino)-propionamide

-
-
672-87-7
L-methyltyrosine

| Conditions | Yield |
|---|---|
| Multi-step reaction with 3 steps 1: hydrogenchloride / isopropyl alcohol; water 2: hydrogen / palladium on carbon / methanol / 1.33 h / 50 °C / 2250.23 Torr 3: hydrogen bromide / water / 4 h / 120 °C View Scheme |
-
-
1299492-12-8
3-(4-methoxyphenyl)-2-methyl-2-((1S)-1-phenyl-ethylamino)-propionamide hydrochloride

-
-
672-87-7
L-methyltyrosine

| Conditions | Yield |
|---|---|
| Multi-step reaction with 3 steps 1: 2-methyl-propan-1-ol / Heating; Reflux 2: hydrogen / palladium on carbon / methanol / 1.33 h / 50 °C / 2250.23 Torr 3: hydrogen bromide / water / 4 h / 120 °C View Scheme |

-
-
672-87-7
L-methyltyrosine

| Conditions | Yield |
|---|---|
| Multi-step reaction with 2 steps 1: hydrogen / palladium on carbon / methanol / 1.33 h / 50 °C / 2250.23 Torr 2: hydrogen bromide / water / 4 h / 120 °C View Scheme |
-
-
1299492-17-3
C22H26ClNO3

-
-
672-87-7
L-methyltyrosine

| Conditions | Yield |
|---|---|
| Multi-step reaction with 2 steps 1: hydrogenchloride / isopropyl alcohol; water / 1 h / 20 °C 2: boron tribromide / dichloromethane / 1.5 h / 20 °C View Scheme |
-
-
658-48-0
2-amino-3-(4-hydroxy-phenyl)-2-methyl-propionic acid

-
A
-
672-87-7
L-methyltyrosine

-
B
-
672-86-6
α-methyl-D-tyrosine

| Conditions | Yield |
|---|---|
| With Merck RP-18 WF254S plates coated with Nτ-n-decyl-L-spinacine and Cu acetate In ethanol; water Resolution of racemate; |

| Conditions | Yield |
|---|---|
| Stage #1: N-(benzylidene)-O-methoxymethyl tyrosine tert-butyl ester; methyl iodide With ((11bS)-(+)-4,4-dibutyl-4,5-dihydro-2,6-bis(3,4,5-triflourophenyl)-3H-dinaphth[2,1-c:1’,2’-e]azepinium bromide); cesium hydroxide In toluene at 0℃; for 0.5h; Inert atmosphere; Stage #2: With hydrogenchloride In water; toluene at 60℃; for 0.5h; Inert atmosphere; stereoselective reaction; | n/a |
-
-
28920-43-6
(fluorenylmethoxy)carbonyl chloride

-
-
672-87-7
L-methyltyrosine

-
-
246539-83-3
Fmoc-L-(α-Me)Tyr-OH

| Conditions | Yield |
|---|---|
| With sodium hydroxide In acetonitrile at 20℃; for 3h; Acylation; | 100% |

| Conditions | Yield |
|---|---|
| With thionyl chloride at 0 - 20℃; Heating / reflux; | 96% |
| With thionyl chloride at 0 - 20℃; | |
| With thionyl chloride at 20℃; for 24h; Inert atmosphere; Reflux; | 1.48 g |

| Conditions | Yield |
|---|---|
| In N,N-dimethyl-formamide at 20℃; for 16h; | 89% |
| In N,N-dimethyl-formamide at 20℃; for 16h; | 89% |

| Conditions | Yield |
|---|---|
| Stage #1: L-methyltyrosine With chloro-trimethyl-silane; N-ethyl-N,N-diisopropylamine In dichloromethane Inert atmosphere; Stage #2: di-tert-butyl dicarbonate In dichloromethane Inert atmosphere; Stage #3: With methanol for 0.5h; Inert atmosphere; | 67% |
| With sodium hydroxide; sodium hydrogencarbonate In 1,4-dioxane at 20℃; pH=8 - 9; | 60% |
| With sodium hydroxide; tert-butyl alcohol | 35% |

-
-
672-87-7
L-methyltyrosine

| Conditions | Yield |
|---|---|
| With sodium hydroxide In ethanol; water Ru-complex was refluxed in mixt. of H2O and EtOH, to this soln. was added ligand followed by aq. NaOH, mixt. was heated at 70°C for 30 min, cooled to room temp., filtered, aq. soln. of NaClO4 was added, mixt.was stored in the dark for 48 h; solid was collected at the pump, washed with ice-cold H2O, dried in vac. over silica gel at room temp.; elem. anal.; | 41% |


-
-
672-87-7
L-methyltyrosine

| Conditions | Yield |
|---|---|
| With sodium hydroxide In ethanol; water Ru-complex was refluxed in mixt. of H2O and EtOH, to this soln. was added ligand followed by aq. NaOH, mixt. was heated at 70°C for 30 min, cooled to room temp., filtered, aq. soln. of NaClO4 was added, mixt.was stored in the dark for 48 h; solid was collected at the pump, washed with ice-cold H2O, dried in vac. over silica gel at room temp.; elem. anal.; | 39% |


| Conditions | Yield |
|---|---|
| With 18F-fluoride In formic acid at 4℃; Product distribution; Further Variations:; Solvents; Temperatures; | A 13% B 2% |
| With 18F-fluoride; hydrogen fluoride; boron trifluoride |
-
-
672-87-7
L-methyltyrosine

| Conditions | Yield |
|---|---|
| With ammonium hydroxide; iodine; potassium iodide |

| Conditions | Yield |
|---|---|
| With chloro-trimethyl-silane; N-ethyl-N,N-diisopropylamine 1.) CHCl3, 70 deg C, 1 h; 2.) CHCl3, 70 deg C, 1 h; 3.) CHCl3, 80 deg C, 17 h; Yield given. Multistep reaction; |
-
-
672-87-7
L-methyltyrosine

| Conditions | Yield |
|---|---|
| With 18F-fluoride; hydrogen fluoride |

| Conditions | Yield |
|---|---|
| With thionyl chloride Heating; |
-
-
672-87-7
L-methyltyrosine

-
-
675106-56-6
Boc-(L)(α-Me)Tyr-OMe

| Conditions | Yield |
|---|---|
| Multi-step reaction with 2 steps 1: SOCl2 / Heating 2: aq. NaHCO3; NaOH / dioxane / 20 °C / pH 8 - 9 View Scheme |
-
-
672-87-7
L-methyltyrosine

-
-
675106-57-7
Boc-(L)(α-Me)Tyr(Tf)-OMe

| Conditions | Yield |
|---|---|
| Multi-step reaction with 3 steps 1: SOCl2 / Heating 2: aq. NaHCO3; NaOH / dioxane / 20 °C / pH 8 - 9 3: 80 percent / Et3N / CH2Cl2 / 3 h / 20 °C View Scheme |
-
-
672-87-7
L-methyltyrosine

-
-
675106-58-8
Boc-(L)(α-Me)Phe(4-PO3Et2)-OMe

| Conditions | Yield |
|---|---|
| Multi-step reaction with 4 steps 1: SOCl2 / Heating 2: aq. NaHCO3; NaOH / dioxane / 20 °C / pH 8 - 9 3: 80 percent / Et3N / CH2Cl2 / 3 h / 20 °C 4: 77 percent / Pd(PPh3)4; N-methylmorpholine / acetonitrile / Heating View Scheme |
-
-
672-87-7
L-methyltyrosine

-
-
675106-63-5
Boc-(α-Me)Tyr-Asn-NH2

| Conditions | Yield |
|---|---|
| Multi-step reaction with 2 steps 1: 60 percent / aq. NaHCO3; NaOH / dioxane / 20 °C / pH 8 - 9 2: 86 percent / DIEA; HOAt; HATU / dimethylformamide / 72 h / 20 °C View Scheme |
-
-
672-87-7
L-methyltyrosine

| Conditions | Yield |
|---|---|
| Multi-step reaction with 5 steps 1: SOCl2 / Heating 2: aq. NaHCO3; NaOH / dioxane / 20 °C / pH 8 - 9 3: 80 percent / Et3N / CH2Cl2 / 3 h / 20 °C 4: 77 percent / Pd(PPh3)4; N-methylmorpholine / acetonitrile / Heating 5: 100 percent / aq. HCl / Heating View Scheme |
-
-
672-87-7
L-methyltyrosine

-
-
675106-65-7
Boc-(α-Me)pTyr(Bzl2)-Asn-NH2

| Conditions | Yield |
|---|---|
| Multi-step reaction with 3 steps 1: 60 percent / aq. NaHCO3; NaOH / dioxane / 20 °C / pH 8 - 9 2: 86 percent / DIEA; HOAt; HATU / dimethylformamide / 72 h / 20 °C View Scheme |
-
-
672-87-7
L-methyltyrosine

-
-
675106-64-6
Boc-(α-Me)pTyr(MeSATE)2-Asn-NH2

| Conditions | Yield |
|---|---|
| Multi-step reaction with 3 steps 1: 60 percent / aq. NaHCO3; NaOH / dioxane / 20 °C / pH 8 - 9 2: 86 percent / DIEA; HOAt; HATU / dimethylformamide / 72 h / 20 °C View Scheme |
-
-
672-87-7
L-methyltyrosine

| Conditions | Yield |
|---|---|
| Multi-step reaction with 4 steps 1: 60 percent / aq. NaHCO3; NaOH / dioxane / 20 °C / pH 8 - 9 2: 86 percent / DIEA; HOAt; HATU / dimethylformamide / 72 h / 20 °C 3: 93 percent / CH2Cl2 / 3 h / 20 °C View Scheme |
-
-
672-87-7
L-methyltyrosine

| Conditions | Yield |
|---|---|
| Multi-step reaction with 4 steps 1: 60 percent / aq. NaHCO3; NaOH / dioxane / 20 °C / pH 8 - 9 2: 86 percent / DIEA; HOAt; HATU / dimethylformamide / 72 h / 20 °C 3: CH2Cl2 / 1 h / 0 °C View Scheme |
L-alpha-Methyltyrosine Chemical Properties
Molecule structure of L-Tyrosine, a-methyl- (CAS NO.672-87-7):
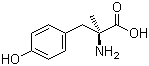
IUPAC Name: (2S)-2-amino-3-(4-hydroxyphenyl)-2-methylpropanoic acid
Molecular Weight: 195.21512 [g/mol]
Molecular Formula: C10H13NO3
Index of Refraction: 1.599
Molar Refractivity: 52.01 cm3
Molar Volume: 152 cm3
Surface Tension: 60.6 dyne/cm
Density: 1.283 g/cm3
Melting Point: 320-340 °C
Storage temp.: −20 °C
Flash Point: 185.9 °C
Enthalpy of Vaporization: 66.7 kJ/mol
Boiling Point: 383.7 °C at 760 mmHg
Vapour Pressure: 1.42E-06 mmHg at 25 °C
XLogP3-AA: -1.6
H-Bond Donor: 3
H-Bond Acceptor: 4
Rotatable Bond Count: 3
Tautomer Count: 2
Exact Mass: 195.089543
MonoIsotopic Mass: 195.089543
Topological Polar Surface Area: 83.6
Heavy Atom Count: 14
Canonical SMILES: CC(CC1=CC=C(C=C1)O)(C(=O)O)N
Isomeric SMILES: C[C@](CC1=CC=C(C=C1)O)(C(=O)O)N
InChI: InChI=1S/C10H13NO3/c1-10(11,9(13)14)6-7-2-4-8(12)5-3-7/h2-5,12H,6,11H2,1H3,(H,13,14)/t10-/m0/s1
InChIKey: NHTGHBARYWONDQ-JTQLQIEISA-N
EINECS: 211-599-5
Product Categories of L-Tyrosine, a-methyl- (CAS NO.672-87-7): Amino acids methyl、ethyl、t-butyl series;Amino Acids & Derivatives
L-alpha-Methyltyrosine Uses
L-Tyrosine, a-methyl- (CAS NO.672-87-7) is a tyrosine hydroxylase inhibitor. It is also an antihypertensive in pheochromocytoma.
L-alpha-Methyltyrosine Safety Profile
Safety Statements: 22-24/25
S22:Do not breathe dust.
S24/25:Avoid contact with skin and eyes.
WGK Germany: 3
L-alpha-Methyltyrosine Specification
L-Tyrosine, a-methyl- (CAS NO.672-87-7) is also called Alpha-Methyltyrosine ; Metyrosine [USAN] ;
(-)-(S)-2-Amino-3-(4-hydroxyphenyl)-2-methylpropionsaeure ; (-)-alpha-Methyl-L-tyrosine (S)-alpha-Methyltyrosine ; Demser ; L-alpha-Methyltyrosine ; Metirosina ; Metirosina [INN-Spanish] ; Metirosinum ; Metirosinum [INN-Latin] ; Metyrosine ; L-Tyrosine, alpha-methyl-, (-)- ; Metirosine . It is white Solid.
Related Products
- L-alpha-Methyltyrosine
- 672883-68-0
- 672883-95-3
- 67289-05-8
- 672-89-9
- 672906-68-2
- 672906-71-7
- 672906-72-8
- 67292-34-6
- 67292-36-8
- 6729-30-2
Hot Products
About|Contact|Cas|Product Name|Molecular|Country|Encyclopedia
Message|New Cas|MSDS|Service|Advertisement|CAS DataBase|Article Data|Manufacturers | Chemical Catalog
©2008 LookChem.com,License: ICP
NO.:Zhejiang16009103
complaints:service@lookchem.com Desktop View