-
Name
Menadione
- EINECS 200-372-6
- CAS No. 58-27-5
- Article Data233
- CAS DataBase
- Density 1.225 g/cm3
- Solubility insoluble in water
- Melting Point 105-107 °C(lit.)
- Formula C11H8O2
- Boiling Point 304.5 °C at 760 mmHg
- Molecular Weight 172.183
- Flash Point 113.8 °C
- Transport Information
- Appearance bright yellow crystals
- Safety 26-36-37/39-24
- Risk Codes 22-36/37/38-43
-
Molecular Structure
-
Hazard Symbols
 Xn,
Xn, Xi
Xi
- Synonyms 1,4-Naphthalenedione,2-methyl-;2-Methyl-1,4-naphthalenedione;Kipca;VK3;Karcon;Kayklot;Kanone;Kayquinone;Memodol;Menaquinone 0;Kappaxin;Aquakay;2-Methylnaphthoquinone;Menadionum;Klottone;Kappaxin (TN);3-Methyl-1,4-naphthoquinone;K-Thrombyl;2-Methyl-1,4-naphthochinon [German];Synkay;Methyl-1,4-naphthoquinone;Methyl-1,4-naphthalenedione;Usaf ek-5185;Kappaxan (VAN);Mitenone;2-Methyl-1,4-naftochinon [Czech];Prestwick_313;Menadion;2-Methyl-1,4-naphthochinon;Vitamin K3;1,4-Naphthoquinone, 2-methyl-;Juva-K;1,4-Naphthalenedione, 2-methyl-;Riboflavin (Vitamin B2);Kolklot;Vitamin K2 (0);Hemodal;1, 4-Naphthoquinone, 2-methyl-;Koaxin;Menaphthone;Kaykot;Menaphthon;MNQ;Kaergona;2-methyl-1,4-dihydronaphthalene-1,4-dione;
- PSA 34.14000
- LogP 2.01190
Synthetic route

| Conditions | Yield |
|---|---|
| With iodate form of Amberlyst A26 In dichloromethane at 20℃; for 2h; further reagent; | 100% |
| With sulfuric acid; sodium bromide In dichloromethane; water at 15℃; for 2.98h; Electrolysis; | 99% |
| With carbon dioxide; oxygen In water at 35 - 60℃; under 57005.7 - 62256.2 Torr; for 0.0833333h; Autoclave; | 96% |

| Conditions | Yield |
|---|---|
| With Co(salN-Medpt); oxygen In acetonitrile for 0.5h; | 100% |
| Stage #1: 2-methylnaphthalen-1-ol In acetonitrile at 74.84℃; Stage #2: With dihydrogen peroxide In acetonitrile for 0.75h; Reflux; chemoselective reaction; | 97.3% |
| With sulfuric acid; dihydrogen peroxide In water at 36 - 40℃; for 6h; Temperature; Solvent; Green chemistry; | 94.7% |

| Conditions | Yield |
|---|---|
| With dihydrogen peroxide; acetic acid In water at 75℃; for 5h; Product distribution / selectivity; | 100% |
| With C69H63Cl6N3O9(3-)*1.5Cu(2+); acetic acid In water at 40 - 100℃; for 14h; Catalytic behavior; Reagent/catalyst; Temperature; Concentration; | 93% |
| With dihydrogen peroxide; acetic acid In acetonitrile Catalytic behavior; Solvent; Reflux; | 93% |

-
-
58-27-5
menadione

| Conditions | Yield |
|---|---|
| With palladium 10% on activated carbon In acetone for 24h; Reflux; Inert atmosphere; | 100% |
| With sodium dichromate; sulfuric acid; acetic acid a) 80-90 deg C, 1 h, b) RT, 12 h; | 93% |
| With manganese(II) bromide; copper(I) bromide In dimethyl sulfoxide at 85℃; under 600.06 Torr; for 26h; Temperature; | 91.3% |
-
-
92071-59-5
C11H10O2

-
-
58-27-5
menadione

| Conditions | Yield |
|---|---|
| With palladium 10% on activated carbon In acetone Reflux; | 100% |

| Conditions | Yield |
|---|---|
| With sodium hydroxide In chloroform; water at 20℃; Product distribution / selectivity; | 100% |
| With water; sodium carbonate at 25℃; for 120h; Time; |

| Conditions | Yield |
|---|---|
| With carbon dioxide; oxygen In water at 37 - 80℃; under 60756.1 - 69757 Torr; for 0.125h; Pressure; Temperature; Autoclave; | 98.4% |
| With lithium perchlorate In acetonitrile; tert-butyl alcohol at 16 - 18℃; for 8.6h; electrolysis on Pt electrodes; | 94% |
| With chromium(VI) oxide; acetic acid at 20℃; |
-
-
16368-80-2
2-methyl-5,6,7,8-tetrahydronaphthalene-1,4-diol

-
-
58-27-5
menadione

| Conditions | Yield |
|---|---|
| With carbon dioxide; oxygen In water at 37 - 80℃; under 58505.9 - 72007.2 Torr; for 0.111111h; Autoclave; | 95.7% |
-
-
53772-19-3
1,4-dimethoxy-2-methylnaphthalene

-
-
58-27-5
menadione

| Conditions | Yield |
|---|---|
| With ammonium cerium(IV) nitrate In acetonitrile at 20℃; for 0.166667h; Oxidation; Demethylation; | 94% |
| With bis-[(trifluoroacetoxy)iodo]benzene In methanol; water at 20℃; for 0.5h; | 92% |
| With tert.-butylhydroperoxide; poly(bis-1,2-phenylene) diselenide In tert-butyl alcohol at 80℃; for 12h; | 90% |
| With ammonium persulfate; silver nitrate In water; acetonitrile for 18h; Ambient temperature; | 56% |

| Conditions | Yield |
|---|---|
| Stage #1: phthalic acid dimethyl ester; tert-butyl 3-cyanobutanoate With potassium tert-butylate In toluene at 100 - 105℃; for 3h; Green chemistry; Stage #2: With sodium hydroxide In water; toluene at 20 - 30℃; for 3h; Green chemistry; Stage #3: With hydrogenchloride In water; toluene at 60℃; for 3h; pH=2 - 2.5; Green chemistry; | 92.3% |

| Conditions | Yield |
|---|---|
| 92% |

| Conditions | Yield |
|---|---|
| Stage #1: phthalic acid di(tert-butyl)ester; tert-butyl 3-cyanobutanoate With potassium tert-butylate In toluene at 100 - 105℃; for 3h; Green chemistry; Stage #2: With sodium hydroxide In water; toluene at 20 - 30℃; for 3h; Green chemistry; Stage #3: With hydrogenchloride In water; toluene at 60℃; for 3h; pH=2 - 2.5; Green chemistry; | 91.9% |

| Conditions | Yield |
|---|---|
| Stage #1: phthalic acid di(tert-butyl)ester; C8H13NO2 With potassium tert-butylate In 5,5-dimethyl-1,3-cyclohexadiene at 100 - 105℃; for 3h; Green chemistry; Stage #2: With sodium hydroxide In 5,5-dimethyl-1,3-cyclohexadiene; water at 20 - 30℃; for 3h; Green chemistry; Stage #3: With hydrogenchloride In 5,5-dimethyl-1,3-cyclohexadiene; water at 60℃; for 3h; pH=2 - 2.5; Green chemistry; | 91.9% |

| Conditions | Yield |
|---|---|
| Stage #1: phthalic acid dimethyl ester; methyl 3-cyanobutanoate With sodium methylate In methanol; toluene at 90 - 100℃; for 3h; Green chemistry; Stage #2: With sodium hydroxide In water; toluene at 20 - 30℃; for 3h; Green chemistry; Stage #3: With hydrogenchloride In water; toluene at 60℃; for 3h; pH=2 - 2.5; Green chemistry; | 90.7% |
-
-
82654-73-7
2-methyl-5,8-dihydro-1,4-naphthoquinone

-
-
58-27-5
menadione

| Conditions | Yield |
|---|---|
| With lithium perchlorate In acetonitrile at 16 - 17℃; for 3.2h; electrolysis on Pt electrodes; | 90% |

| Conditions | Yield |
|---|---|
| In tert-butyl alcohol for 12h; Methylation; UV-irradiation; | 90% |

| Conditions | Yield |
|---|---|
| Stage #1: rac-ethyl 3-cyanobutanoate; phthalic acid dimethyl ester With sodium ethanolate In methanol; toluene at 90 - 100℃; for 3h; Green chemistry; Stage #2: With sodium hydroxide In water; toluene at 20 - 30℃; for 3h; Green chemistry; Stage #3: With hydrogenchloride In water; toluene at 60℃; for 3h; pH=2 - 2.5; Green chemistry; | 89% |

| Conditions | Yield |
|---|---|
| Stage #1: phthalic acid dimethyl ester; C8H13NO2 With sodium methylate In methanol; chlorobenzene at 90 - 100℃; for 3h; Green chemistry; Stage #2: With sodium hydroxide In water; chlorobenzene at 20 - 30℃; for 3h; Green chemistry; Stage #3: With hydrogenchloride In water; chlorobenzene at 60℃; for 3h; pH=2 - 2.5; Green chemistry; | 88.3% |
-
-
7469-77-4
2-methylnaphthalen-1-ol

-
-
546-67-8
lead(IV) tetraacetate

-
A
-
58-27-5
menadione

-
B
-
76274-21-0
2-acetoxy-2-methylnaphthalen-1(2H)-one

| Conditions | Yield |
|---|---|
| In benzene Ambient temperature; | A 3% B 88% |


| Conditions | Yield |
|---|---|
| Stage #1: Diethyl phthalate; methyl 3-cyanobutanoate With sodium ethanolate In methanol; toluene at 90 - 100℃; for 3h; Green chemistry; Stage #2: With sodium hydroxide In water; toluene at 20 - 30℃; for 3h; Green chemistry; Stage #3: With hydrogenchloride In water; toluene at 60℃; for 3h; pH=2 - 2.5; Green chemistry; | 87.8% |

| Conditions | Yield |
|---|---|
| With oxygen In dimethyl sulfoxide at 55℃; for 24h; pH=4.5; Reagent/catalyst; Enzymatic reaction; | 85.8% |

-
-
58-27-5
menadione

| Conditions | Yield |
|---|---|
| With pyridinium chlorochromate In dichloromethane at 20℃; for 5h; | 81% |

| Conditions | Yield |
|---|---|
| With sulfuric acid; dihydrogen peroxide; acetic anhydride; acetic acid at 60℃; for 6.33333h; Product distribution / selectivity; | A n/a B 80% |
| With perchloric acid; dihydrogen peroxide; acetic anhydride; acetic acid at 60℃; for 6.33333h; Product distribution / selectivity; | A n/a B 80% |
| With phosphoric acid; dihydrogen peroxide; acetic anhydride; acetic acid at 60℃; for 6.33333h; Product distribution / selectivity; | A n/a B 75% |
-
-
83-72-7
2-Hydroxy-1,4-naphthoquinone

-
-
67-68-5
dimethyl sulfoxide

-
A
-
58-27-5
menadione

-
B
-
80596-51-6
2,3-dimethyl-5-hydroxy-1,4-naphthoquinone

| Conditions | Yield |
|---|---|
| With copper(II) sulfate at 50℃; for 2h; | A 60% B 80% |

-
-
1515-76-0, 35694-19-0, 35694-20-3
1-acetoxybuta-1,3-diene

-
-
95-71-6
2-methylbenzene-1,4-diol

-
-
58-27-5
menadione

| Conditions | Yield |
|---|---|
| With laccase In acetate buffer at 55℃; for 24h; pH=4.5; Diels-Alder reaction; | 75% |
-
-
7469-77-4
2-methylnaphthalen-1-ol

-
A
-
58-27-5
menadione

-
B
-
76274-21-0
2-acetoxy-2-methylnaphthalen-1(2H)-one

| Conditions | Yield |
|---|---|
| With lead(IV) acetate In acetonitrile Ambient temperature; | A 16% B 74% |

| Conditions | Yield |
|---|---|
| With dihydrogen peroxide; methyltrioxorhenium(VII) In acetic acid at 20℃; for 2h; | A 6% B 74% |
-
-
13615-40-2
3-methylnaphthalen-1-ol

-
-
58-27-5
menadione

| Conditions | Yield |
|---|---|
| With salcomine; oxygen In N,N-dimethyl-formamide for 3h; Ambient temperature; | 70% |
| With salcomine; oxygen In N,N-dimethyl-formamide for 3h; | 64% |

-
A
-
58-27-5
menadione

-
B
-
107866-11-5
3,3′-dimethyl-1,1′-binaphthalenyl-4,4′-diol

| Conditions | Yield |
|---|---|
| With Jones reagent (excess) In acetone at 0℃; | A n/a B 68% |
-
-
27613-27-0
3-oxo-1,3-dihydroisobenzofuran-1-carbonitrile

-
-
80-62-6
methacrylic acid methyl ester

-
-
58-27-5
menadione

| Conditions | Yield |
|---|---|
| Stage #1: 3-oxo-1,3-dihydroisobenzofuran-1-carbonitrile With lithium tert-butoxide In tetrahydrofuran at -78 - -60℃; for 0.416667h; Inert atmosphere; Stage #2: methacrylic acid methyl ester In tetrahydrofuran at -78 - 20℃; Inert atmosphere; | 64% |
-
-
58-27-5
menadione

-
-
15448-59-6, 61840-91-3, 82864-14-0, 105016-62-4
2,3-epoxy-2-methyl-2,3-dihydro-1,4-naphthoquinone

| Conditions | Yield |
|---|---|
| With dihydrogen peroxide; β‐cyclodextrin In water at 25℃; for 96h; pH 9; other reagents (t-BuOOH, NaOCl, PhC(CH3)2OOH), MCPBA), other solvents (DMF, DMSO), addition of base (NaOH, NaHCO3, Na2CO3); | 100% |
| With dihydrogen peroxide; β‐cyclodextrin; alpha cyclodextrin In water at 25℃; for 96h; Product distribution; pH 9; other oxidizing agents (t-BuOOH, NaOCl, PhC(CH3)2OOH), MCPBA); other solvents (DMF, DMSO), addition of base (NaOH, Na2CO3, NaHCO3); enantioselectivity (up to 40percent ee by NMR); same reaction with further naphthoquinones; | 100% |
| With lithium hydroxide; tetrabutylammomium bromide; dihydrogen peroxide In toluene at 15 - 25℃; for 0.416667h; Epoxidation; ultrasonic irradiation; | 100% |

| Conditions | Yield |
|---|---|
| With sodium dithionite In diethyl ether; water | 100% |
| With sodium dithionite In diethyl ether; water at 20℃; | 100% |
| With sodium dithionite In diethyl ether; water | 100% |
-
-
108-22-5
Isopropenyl acetate

-
-
58-27-5
menadione

-
-
75858-24-1
Acetic acid (1S,2aR,8aR)-1,8a-dimethyl-3,8-dioxo-1,2,2a,3,8,8a-hexahydro-cyclobuta[b]naphthalen-1-yl ester

| Conditions | Yield |
|---|---|
| In benzene for 1h; Irradiation; | 100% |
-
-
58-27-5
menadione

-
-
74785-15-2
2,3-dihydro-2-methyl-2-(3-methyl-but-2-enyl)-1,4-naphthoquinone

| Conditions | Yield |
|---|---|
| In N,N-dimethyl-formamide at -23℃; for 3h; | 100% |

| Conditions | Yield |
|---|---|
| In tetrahydrofuran (Ar); stirring (1 h, -78°C); soln. filtn. off, drying (vac.); elem. anal.; | 100% |

| Conditions | Yield |
|---|---|
| With dibenzoyl peroxide In acetic acid for 4h; Heating; | 99% |


-
-
58-27-5
menadione

-
-
1342800-33-2
N'-(3-methyl-4-oxo-1,4-dihydronaphthalen-1-ylidene)-1-(4-bromophenyl)-5-oxopyrrolidine-3-carbohydrazide

| Conditions | Yield |
|---|---|
| With hydrogenchloride In water; isopropyl alcohol for 19h; Reflux; | 99% |

| Conditions | Yield |
|---|---|
| With tetradecafluorohexane; lithium perfluorooctane sulfonate In water at 25℃; for 72h; Diels-Alder reaction; | 98% |

| Conditions | Yield |
|---|---|
| With dibenzoyl peroxide In acetic acid for 4h; Heating; | 98% |
-
-
58-27-5
menadione

| Conditions | Yield |
|---|---|
| With tert.-butylhydroperoxide; C49H63Cl6N7O2*ClH; potassium hydroxide In tert-butyl methyl ether; water at 0℃; for 0.5h; enantioselective reaction; | 98% |
| With sodium hypochlorite; diphenylether; Cinchona alkaloid phase-transfer catalyst In chlorobenzene at -10℃; |
-
-
956593-14-9
C12H15N3O2

-
-
58-27-5
menadione

-
-
1342800-28-5
N'-(3-methyl-4-oxo-1,4-dihydronaphthalen-1-ylidene)-1-(3-methylphenyl)-5-oxopyrrolidine-3-carbohydrazide

| Conditions | Yield |
|---|---|
| With hydrogenchloride In water; isopropyl alcohol for 19h; Reflux; | 98% |
-
-
58-27-5
menadione

-
-
872-35-5
1,3-dihydro-imidazole-2-thione

-
-
1449761-93-6
2-(1H-imidazole-2-ylthio)-3-methylnaphthalene-1,4-dione

| Conditions | Yield |
|---|---|
| In methanol at 20℃; for 8h; | 98% |

| Conditions | Yield |
|---|---|
| With (2S)-2-{diphenyl[(trimethylsilyl)oxy]methyl}pyrrolidine; benzoic acid In chloroform at 20℃; for 20h; | 98% |

| Conditions | Yield |
|---|---|
| Stage #1: menadione With palladium on activated charcoal; hydrogen In methanol for 6h; Stage #2: dimethyl sulfate With sodium methylate In methanol at 50℃; for 0.333333h; Inert atmosphere; | 97.1% |
| Stage #1: menadione With hydrogenchloride; tin(ll) chloride In ethanol at 20℃; for 0.5h; Stage #2: dimethyl sulfate With potassium hydroxide In ethanol; acetone at 60℃; for 2h; | 73% |
| Stage #1: menadione With hydrogenchloride; tin(ll) chloride In ethanol at 20℃; Stage #2: dimethyl sulfate With potassium hydroxide In acetone at 60℃; | |
| Stage #1: menadione With hydrogenchloride; tin(ll) chloride In ethanol at 40℃; for 0.166667h; Stage #2: dimethyl sulfate With potassium hydroxide In acetone at 20℃; for 2.5h; Further stages.; | |
| Stage #1: menadione With sodium dithionite In ethyl acetate at 20℃; for 0.5h; Stage #2: dimethyl sulfate With sodium methylate In isopropyl alcohol at 63℃; for 0.5h; |
-
-
58-27-5
menadione

| Conditions | Yield |
|---|---|
| 2,4,6-tri(4-pyridyl)-1,3,5-triazine In water-d2 at 20℃; for 3h; Irradiation; | 96% |

-
-
58-27-5
menadione

-
-
1342800-34-3
N'-(3-methyl-4-oxo-1,4-dihydronaphthalen-1-ylidene)-1-(4-chlorophenyl)-5-oxopyrrolidine-3-carbohydrazide

| Conditions | Yield |
|---|---|
| With hydrogenchloride In water; isopropyl alcohol for 19h; Reflux; | 96% |
-
-
58-27-5
menadione

| Conditions | Yield |
|---|---|
| With (1,2-dimethoxyethane)dichloronickel(II); bathophenanthroline In acetonitrile at 100℃; for 6h; Sealed tube; Inert atmosphere; | 96% |

| Conditions | Yield |
|---|---|
| With aluminum oxide; bromine for 2h; Ambient temperature; other reagents: iodine monobromide, acetic acid; | 95% |
| With N-chloro-succinimide; copper(II) chloride monohydrate In acetonitrile at 82℃; for 4h; regioselective reaction; | 95.9% |
| Stage #1: menadione With bromine In dichloromethane at 25℃; for 1h; Stage #2: With pyridine In dichloromethane at 25℃; for 12h; | 93% |

| Conditions | Yield |
|---|---|
| With hydrogenchloride; acetic acid at 20℃; | 95% |
| With hydrogenchloride; acetic acid | 87% |
| With hydrogenchloride In water; acetic acid at 60 - 65℃; for 0.5h; | 86% |
Menadione Chemical Properties
IUPAC Name: 2-methylnaphthalene-1,4-dione
Empirical Formula: C11H8O2
Molecular Weight: 172.18g/mol
EINECS: 200-372-6
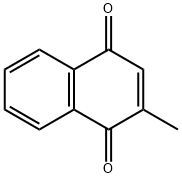
Structure of Menadione (CAS NO.58-27-5):
Index of Refraction: 1.592
Molar Refractivity: 47.6 cm3
Molar Volume: 140.5 cm3
Polarizability: 18.87×10-24cm3
Surface Tension: 46.4 dyne/cm
Density: 1.225 g/cm3
Flash Point: 113.8 °C
Enthalpy of Vaporization: 54.49 kJ/mol
Melting Point: 105-107 °C(lit.)
Boiling Point: 304.5 °C at 760 mmHg
Vapour Pressure: 0.000871 mmHg at 25°C
Solubility: oil: soluble
Form: crystalline
Color: yellow
Water Solubility: INSOLUBLE
Sensitive: Light Sensitive
Stability: Stable. May be light sensitive. Incompatible with strong oxidizing agents.
Product Categories: Biochemistry;Vitamins;Nutritional fortification substances;Vitamins and derivatives;Vitamin Ingredients;Miscellaneous Compounds
Canonical SMILES: CC1=CC(=O)C2=CC=CC=C2C1=O
InChI: InChI=1S/C11H8O2/c1-7-6-10(12)8-4-2-3-5-9(8)11(7)13/h2-6H,1H3
InChIKey: MJVAVZPDRWSRRC-UHFFFAOYSA-N
Menadione Uses
Menadione (CAS NO.58-27-5) is used as a source of vitamin K in the treatment of hypoprothrombinemia against vitamin K deficiency.
Menadione Toxicity Data With Reference
| Organism | Test Type | Route | Reported Dose (Normalized Dose) | Effect | Source |
|---|---|---|---|---|---|
| chicken | LDLo | intraperitoneal | 100mg/kg (100mg/kg) | Proceedings of the Society for Experimental Biology and Medicine. Vol. 43, Pg. 125, 1940. | |
| mouse | LD50 | intraperitoneal | 50mg/kg (50mg/kg) | National Technical Information Service. Vol. AD691-490, | |
| mouse | LD50 | oral | 500mg/kg (500mg/kg) | Proceedings of the Society for Experimental Biology and Medicine. Vol. 43, Pg. 125, 1940. | |
| mouse | LD50 | subcutaneous | 138mg/kg (138mg/kg) | Journal of Pharmacology and Experimental Therapeutics. Vol. 75, Pg. 111, 1942. | |
| rabbit | LDLo | oral | 230mg/kg (230mg/kg) | VASCULAR: OTHER CHANGES | Journal of Pharmacology and Experimental Therapeutics. Vol. 75, Pg. 111, 1942. |
| rat | LD50 | intraperitoneal | 75mg/kg (75mg/kg) | Toxicology and Applied Pharmacology. Vol. 18, Pg. 185, 1971. |
Menadione Consensus Reports
Reported in EPA TSCA Inventory.
Menadione Safety Profile
Hazard Codes:  Xn,
Xn, Xi
Xi
Risk Statements: 22-36/37/38-43
R22:Harmful if swallowed.
R36/37/38:Irritating to eyes, respiratory system and skin.
R43:May cause sensitization by skin contact.
Safety Statements: 26-36-37/39-24
S26: In case of contact with eyes, rinse immediately with plenty of water and seek medical advice.
S36:Wear suitable protective clothing.
S37/39:Wear suitable gloves and eye/face protection.
S24:Avoid contact with skin.
WGK Germany: 3
RTECS: QL9100000
F: 8
Poison by ingestion, intraperitoneal, and subcutaneous routes. Experimental teratogenic effects. Questionable carcinogen with experimental tumorigenic data. Human mutation data reported. When heated to decomposition it emits acrid smoke and irritating fumes.
Menadione Standards and Recommendations
IDENTIFICATION: Conforms
ASSAY: 98.5 - 101.5%
RESIDUE ON IGNITION: 0.1% max
LOSS ON DRYING: 0.5% max
HEAVY METALS: 20ppm max
Menadione Specification
Menadione , its cas register number is 58-27-5. It also can be called 1,4-Naphthalenedione, 2-methyl- ; 2-Methyl-1,4-naphthochinon ; 2-Methylnaphthoquinone ; 3-Methyl-1,4-naphthoquinone ; Aquakay ; Aquinone ; Kayquinone ; Kipca ;
Kipca-oil soluble ; Memodol ; Menaquinone ; Mitenon ; Mitenone ; Panosine ; Thyloquinone ; Vitamin K3 . Menadione (CAS NO.58-27-5) is a bright yellow crystals.
Related Products
- Menadione
- Menadione nicotinamide bisulfite
- Menadione sodium bisulfite
- 58278-06-1
- 58278-19-6
- 58280-31-2
- 582-83-2
- 58-28-6
- 58290-35-0
- 58293-56-4
- 58296-58-5
- 5830-30-8
- 583-03-9
Hot Products
About|Contact|Cas|Product Name|Molecular|Country|Encyclopedia
Message|New Cas|MSDS|Service|Advertisement|CAS DataBase|Article Data|Manufacturers | Chemical Catalog
©2008 LookChem.com,License: ICP
NO.:Zhejiang16009103
complaints:service@lookchem.com Desktop View