-
Name
MERCURIC ACETATE
- EINECS 216-491-1
- CAS No. 1600-27-7
- Article Data47
- CAS DataBase
- Density 3.29 g/cm3
- Solubility Soluble in water.
- Melting Point 179-182 °C(lit.)
- Formula C2H4O2.1/2Hg
- Boiling Point 117.1 °C at 760 mmHg
- Molecular Weight 318.679
- Flash Point 40 °C
- Transport Information UN 1629 6.1/PG 2
- Appearance white or off-white powder
- Safety 13-28-36-45-60-61
- Risk Codes 26/27/28-33-50/53
-
Molecular Structure
-
Hazard Symbols
 T+,
T+,  N
N
- Synonyms Aceticacid, mercuridi- (7CI);Acetic acid, mercury(2+) salt (8CI,9CI);Bis(acetyloxy)mercury;Diacetoxymercury;Mercuric acetate;Mercuric diacetate;Mercuric(II) acetate;Mercury acetate (Hg(O2C2H3)2);Mercurydiacetate;Mercury(2+) acetate;Mercury(II) acetate;Mercury(II) diacetate;
- PSA 80.26000
- LogP -2.49010
Synthetic route

| Conditions | Yield |
|---|---|
| With mercury(II) oxide |
-
-
108-24-7
acetic anhydride

-
A
-
1600-27-7
mercury(II) diacetate

-
B
-
631-61-8
ammonium acetate

-
C
-
64-19-7
acetic acid


| Conditions | Yield |
|---|---|
| Heating / reflux; | |
| In water at 50℃; | |
| at 50℃; |
-
-
748084-55-1
mercuric carbonate

-
-
1600-27-7
mercury(II) diacetate

| Conditions | Yield |
|---|---|
| With acetic acid In acetic acid | |
| With acetic acid In acetic acid aq. acetic acid; |

-
-
1600-27-7
mercury(II) diacetate

| Conditions | Yield |
|---|---|
| With acetic acid In acetic acid on water bath; filtered, cooled, washed with ethyl acetate, recrystd., drying above CaCl2 in vacuum; | |
| With acetic acid In acetic acid washed with chloroform, drying above caustic soda in vacuum; | |
| With acetic acid In acetic acid aq. acetic acid; on water bath; filtered, cooled, washed with ethyl acetate, recrystd., drying above CaCl2 in vacuum; | |
| With glacial acetic acid In acetic acid washed with chloroform, drying above caustic soda in vacuum; |

| Conditions | Yield |
|---|---|
| In not given byproducts: KNO3; | |
| In not given byproducts: KNO3; |

-
-
1600-27-7
mercury(II) diacetate

| Conditions | Yield |
|---|---|
| With CH3COOH; H2O2; nitric acid In not given refluxed 40°C, mixed with H2O2, 80°C 30 min, evaporated at ambient temp. and vacuum; | |
| With dihydrogen peroxide; acetic acid; nitric acid In not given refluxed 40°C, mixed with H2O2, 80°C 30 min, evaporated at ambient temp. and vacuum; |

| Conditions | Yield |
|---|---|
| In acetic acid | >99 |
| In acetic acid | >99 |

-
-
1600-27-7
mercury(II) diacetate

| Conditions | Yield |
|---|---|
| With acetic anhydride 4-5-fold excess of (CH3CO)2O; | |
| With acetic anhydride 4-5-fold excess of (CH3CO)2O; |

| Conditions | Yield |
|---|---|
| With nitric acid In water Hg(NO3)2 acidified with HNO3; | |
| With HNO3 In water Hg(NO3)2 acidified with HNO3; |

| Conditions | Yield |
|---|---|
| In further solvent(s) all manipulations by Schlenk technique; no reaction even in molar ratio of Hg compd.:org. compd. 1:2, only dismutation; |


| Conditions | Yield |
|---|---|
| In pyridine disproportion in pyridine; | |
| In water Irradiation (UV/VIS); heating; | |
| In water Irradiation (UV/VIS); heating; | |
| In pyridine disproportion in pyridine; |

-
-
1600-27-7
mercury(II) diacetate

| Conditions | Yield |
|---|---|
| With acetic anhydride In acetic anhydride |

| Conditions | Yield |
|---|---|
| In acetic acid Irradiation (UV/VIS); | |
| In acetic acid Irradiation (UV/VIS); |

-
-
37082-85-2
(HgCN)(1+)*(CH3CO2)(1-)={HgCN}CH3CO2

-
A
-
1600-27-7
mercury(II) diacetate

| Conditions | Yield |
|---|---|
| With H2O | |
| With water |

| Conditions | Yield |
|---|---|
| In water Kinetics; equil.; equil. constant given;; | |
| In water Kinetics; equil.; equil. constant given;; |

| Conditions | Yield |
|---|---|
| In not given |

| Conditions | Yield |
|---|---|
| In benzene under N2, kept for several hours at room temp.; |

| Conditions | Yield |
|---|---|
| In dichloromethane Electrolysis; controlled potential oxidative electrolysis at a Hg pool electrode in CH2Cl2 at 20°C in the presence of 0.1 M Bu4NClO4; further unidentified products; not isolated; |
-
-
84839-07-6
[(acetoxymercuriothio)methyl]triethylsilane

-
B
-
1600-27-7
mercury(II) diacetate

| Conditions | Yield |
|---|---|
| In benzene dilute soln.; |
-
-
84839-12-3
C2H3O2(1-)*C4H11O3SSi(1-)*Hg(2+)

-
B
-
1600-27-7
mercury(II) diacetate

| Conditions | Yield |
|---|---|
| In benzene dilute soln.; |
-
-
84839-14-5
C2H3O2(1-)*C6H15O3SSi(1-)*Hg(2+)

-
B
-
1600-27-7
mercury(II) diacetate

| Conditions | Yield |
|---|---|
| In benzene dilute soln.; |
-
-
84839-13-4
C2H3O2(1-)*C5H13O3SSi(1-)*Hg(2+)

-
B
-
1600-27-7
mercury(II) diacetate

| Conditions | Yield |
|---|---|
| In benzene dilute soln.; |

| Conditions | Yield |
|---|---|
| In benzene dilute soln.; |

-
B
-
1600-27-7
mercury(II) diacetate

| Conditions | Yield |
|---|---|
| In not given decomp. in soln.;; |
-
-
1600-27-7
mercury(II) diacetate

-
-
122714-12-9
2,3,6-tri-O-benzoyl-4-deoxy-α-D-xylo-hexopyranosyl chloride

-
-
122714-13-0
1-O-acetyl-2,3,6-tri-O-benzoyl-4-deoxy-β-D-xylo-hexopyranose

| Conditions | Yield |
|---|---|
| In acetic acid for 1h; Ambient temperature; | 100% |

| Conditions | Yield |
|---|---|
| In dichloromethane for 3h; Ambient temperature; | A n/a B 100% |


| Conditions | Yield |
|---|---|
| trifluoroborane diethyl ether In methanol 0.2 Mol catalysts, in 0.2 molar soln., 24h, 50°C; | 100% |
| trifluoroborane diethyl ether In methanol 0.2 Mol catalysts, in 0.2 molar soln., 24h, 50°C; | 100% |
| perchloric acid In methanol 0.34 Mol HClO4, in 0.2 molar soln., 70h, 50°C; | 60% |
-
-
13688-78-3
4-methylbenzenetrifluorosilane

-
-
1600-27-7
mercury(II) diacetate

-
-
2440-35-9
p-CH3-C6H4-Hg-CH3CO2

| Conditions | Yield |
|---|---|
| In benzene addn. of 4-CH3-PhSiF3 to suspn. of HgAc2, mixt. heated (5 min, 80°C); cooled, filtered; | 100% |

-
-
1600-27-7
mercury(II) diacetate

| Conditions | Yield |
|---|---|
| In tetrahydrofuran byproducts: Hg, dme; react. at room temp. for 16 h under N2; suspn. filtered, filter washed (THF), filtrate and washings evapd. (vac.); elem. anal.; | 100% |

| Conditions | Yield |
|---|---|
| In benzene byproducts: F3SiOCOCH3; addn. of PhSiF3 to suspn. of HgAc2, mixt. heated ( 10-15 min, 80°C); cooled, filtered; | 100% |
| In benzene byproducts: F3SiOCOCH3; suspn. of HgAc2 in benzene treated with PhSiF3, after 10-15 min heated to boiling; crystn. upon cooling; | 100% |
| In benzene byproducts: F3SiOCOCH3; react. of starting materials in soln. at 25°C for 2h; | 99% |
-
-
157-33-5
bicyclo[1.1.0]butane

-
-
1600-27-7
mercury(II) diacetate

-
-
57297-71-9
1-acetoxymercuri-3-acetoxycyclobutane

| Conditions | Yield |
|---|---|
| In dichloromethane addn. of bicylobutane to suspn. of Hg(OAc)2 in CH2Cl2 (stirring), stirring (30 min); removal of solvent (evapn.); | 100% |
-
-
62830-22-2
ortho-biphenylenemercury trimer

-
-
1600-27-7
mercury(II) diacetate

-
-
71445-59-5
C12H8(HgO2CCH3)2

| Conditions | Yield |
|---|---|
| In methanol the biphenylenemercury suspensed in boiling methanol; mercuric acetate added until all the trimer had dissolved; a little more trimer was added; filtration; evaporation to a small volume; crystals washed quickly withcold glacial acetic acid and copious amounts of distilled water; dried at 80°C; elem. anal.; | 100% |
| In 1,2,5-trimethyl-benzene refluxed in boiling mesitylene for 30 min; solvent was partially evaporated; cooled; further evaporation of the filtrate; | 0% |

| Conditions | Yield |
|---|---|
| In methanol addn. of soln. of 1 mmol Hg acetate in methanol to soln. of 1 mmol triazene in methanol; stirring mixture at ambient temp. for 12 h;; washing with ether and water;; | 100% |
- HN4CN3(H)C6H4Cl
-
93680-29-6
HN4CN3(H)C6H4Cl

-
-
1600-27-7
mercury(II) diacetate

-
-
108054-29-1
[Hg(N4C)N=NNC6H4Cl](n)

| Conditions | Yield |
|---|---|
| In methanol addn. of soln. of 1 mmol Hg acetate in methanol to soln. of 1 mmol triazene in methanol; stirring mixture at ambient temp. for 12 h;; washing with ether and water;; | 100% |

| Conditions | Yield |
|---|---|
| In methanol addn. of soln. of 1 mmol Hg acetate in methanol to soln. of 1 mmol triazene in methanol; stirring mixture at ambient temp. for 12 h;; washing with ether and water;; | 100% |
-
-
18441-99-1
(triethylsilyl)methanethiol

-
-
1600-27-7
mercury(II) diacetate

-
-
84839-07-6
[(acetoxymercuriothio)methyl]triethylsilane

| Conditions | Yield |
|---|---|
| In tetrahydrofuran byproducts: CH3COOH; stirring (1 h, room temp.); solvent driving off (rotary evaporator), residue diln. (benzene), unchanged Hg-acetate filtration off, benzene and acetic acid evapn. (vac.) from filtrate, residue (thick oil) crystn. during 1 d; elem. anal.; | 100% |

-
-
1600-27-7
mercury(II) diacetate

| Conditions | Yield |
|---|---|
| In chloroform | 100% |

-
-
1600-27-7
mercury(II) diacetate

| Conditions | Yield |
|---|---|
| In chloroform | 100% |
-
-
49834-25-5
3,4,5-trimethoxy(N-tert-butylcarbamoyl)benzene

-
-
1600-27-7
mercury(II) diacetate

| Conditions | Yield |
|---|---|
| With HOAc In ethanol; water air and moisture free atmosphere; refluxing (5 h), stirring (overnight),KCl soln. (H2O) addn., stirring (10 min); filtering, washing (H2O), drying (70°C); elem. anal.; | 100% |


| Conditions | Yield |
|---|---|
| With triethylamine In methanol for 0.75h; | 100% |

-
-
1600-27-7
mercury(II) diacetate

| Conditions | Yield |
|---|---|
| Stage #1: N-(3,5-bis{[ethyl(methyl)amino]methyl}phenyl)hexadecanamide; mercury(II) diacetate In ethanol for 24h; Inert atmosphere; Reflux; Stage #2: lithium chloride In ethanol for 0.25h; Inert atmosphere; Reflux; | 100% |


| Conditions | Yield |
|---|---|
| Stage #1: C37H56N6O7Si; acetic acid In dichloromethane at 20℃; for 6h; Stage #2: mercury(II) diacetate In dichloromethane at 0℃; for 2h; | 100% |

-
-
1600-27-7
mercury(II) diacetate

| Conditions | Yield |
|---|---|
| In methanol; acetonitrile byproducts: acetic acid; soln. of Hg salt in MeOH added to soln. of ligand (2 equiv.) in MeCN; heated to 70°C; stirred for 1 h; cooled to ambient temp.; Et2O layered onto filtrate; crystd. for several d; collected by filtration; washed with Et2O; dried in vac.; elem. anal.; | 99.4% |

| Conditions | Yield |
|---|---|
| In ethanol for 0.166667h; Heating; Yields of byproduct given; | A n/a B 99% |


| Conditions | Yield |
|---|---|
| In ethanol for 0.25h; Heating; Yields of byproduct given; | A n/a B 99% |

-
-
1600-27-7
mercury(II) diacetate

| Conditions | Yield |
|---|---|
| In acetic acid for 0.5h; Ambient temperature; | 99% |
-
-
54905-13-4
isopropyl aldehyde dithiophenylacetal

-
-
1600-27-7
mercury(II) diacetate

-
-
55999-41-2
(1-Acetoxy-isobutyl)-phenyl-sulfid

| Conditions | Yield |
|---|---|
| In acetic acid Ambient temperature; | 99% |

| Conditions | Yield |
|---|---|
| In methanol mixing MeOH (or EtOH) solns. of stoich. amts. of Hg(OAc)2 and carboxylic acid; elem. anal.; | 99% |
-
-
115560-11-7
(η5-pentamethylcyclopentadienyl)(η5-indenyl)ruthenium(II)

-
-
1600-27-7
mercury(II) diacetate

| Conditions | Yield |
|---|---|
| In diethyl ether; ethanol under N2; mixt. Ru-complex, 3 equiv Hg-compd., EtOH and Et2O stirred (ambient temp., 12 h); evapn. (reduced pressure), washed (hexane), solid dissolved in CH2Cl2, filtered (Celite), evapn. (reduced pressure), elem. anal.; | 99% |

| Conditions | Yield |
|---|---|
| In 1,4-dioxane; methanol salen metal complex dissolved in hot 1,4-dioxane; to this soln. added mercury salt dissolved in MeOH with stirring; mixt. heated at 120°Cfor 5 h; cooled; soln. concd.; crystals were obtained; elem. anal.; | 99% |

-
-
1600-27-7
mercury(II) diacetate

-
-
71958-91-3
5,5-dimethyl-2-(3-methyl-3-butenyl)-1,3-cyclohexanedione

-
-
111120-09-3
2-chloromercurymethyl-2,7,7-trimethyl-3,4,5,6,7,8-hexahydro-2H-benzopyran-5-one

| Conditions | Yield |
|---|---|
| With potassium chloride In tetrahydrofuran; water to a soln. of mercuric acetate in THF is added the olefin-compd. at room temp. under stirring for 3-5 h., a soln. of KCl in water is added and the soln. is stirred for 5 min.; the soln. is diluted with water and extracted with CH2Cl2, the extract is dried over anhydrous Na2SO4 and the solvent is evaporated under red. pressure, benzene is added to the residue and then evaporated, crystn. from AcOEt; | 99% |

-
-
1600-27-7
mercury(II) diacetate

| Conditions | Yield |
|---|---|
| With diisopropylethylamine In chloroform-d1; d(4)-methanol titration of porphyrine by Hg(OAc)2; monitored by UV; solvent evapd., solid dissolved in CH2Cl2, excess of mercury salt removed by filtration through celite, precipitated with pentane; elem. anal.; | 99% |
| In pyridine room temp.; 15 min; monitored by UV; solvent evapd., solid dissolved in CH2Cl2, excess of mercury salt removed by filtration through celite, precipitated with pentane; elem. anal.; | 82% |
-
-
1108157-65-8
1-vinyl-4,5,6,7-tetrahydroindole-2-carbonitrile

-
-
1600-27-7
mercury(II) diacetate

-
-
1372648-14-0
2-acetoxy-2-(2-cyano-4,5,6,7-tetrahydroindol-1-yl)ethylmercury acetate

| Conditions | Yield |
|---|---|
| In acetonitrile by a react. in dry CH3CN at 60°C for 4 h; | 99% |
-
-
1372648-02-6
3-vinyl-4,5-dihydro-3H-benzo[e]indole-2-carbaldehyde

-
-
1600-27-7
mercury(II) diacetate

-
-
1372648-17-3
C6H4C2H4C4HNCHO2CCH3CH2HgO2CCH3CHO

| Conditions | Yield |
|---|---|
| In acetonitrile by a react. in dry CH3CN at 35°C for 2 h; | 99% |
Mercury acetate Chemical Properties
IUPAC: Diacetyloxymercury
Mercuric acetate (1600-27-7) is also called for AI3-04458 ; Acetic acid, mercuridi- ; Acetic acid, mercury(2+) salt ; Anthracene, 1,4-dihydro-, compd. with mercury diacetate (1:1) ; Bis(acetyloxy)mercury ; CCRIS 7488 ; Caswell No.543A ; Diacetoxymercury ; EINECS 216-491-1 ; EPA Pesticide Chemical Code 052104 ; HSDB 1244 ; Mercuric diacetate ; Mercury acetate ; Mercury acetate (Hg(CH3CO2)2) ; Mercury acetate (Hg(O2C2H3)2) ; Mercury diacetate ; Mercury(2+) acetate ; Mercury(II) acetate ; Mercuryl acetate ; NSC 215199 ; Acetic acid, mercury(2+) salt and so on.
Molecular Formula: C4H6HgO4
Molecular Weight: 318.68
EINECS: 216-491-1
Melting Point: 179-182 °C(lit.)
Density: 3,29 g/cm3
LogP: ACD/LogP: -0.29
ACD/LogD (pH 5.5): -1.07
ACD/LogD (pH 7.4): -2.86
ACD/BCF (pH 5.5): 1
ACD/BCF (pH 7.4): 1
ACD/KOC (pH 5.5): 2.73
ACD/KOC (pH 7.4): 1
H bond acceptors: 2
H bond donors: 1
Freely Rotating Bonds: 0
Heavy Atom Count: 9
Formal Charge: 0
Complexity: 108
Isotope Atom Count: 0
Defined Atom StereoCenter Count: 0
Undefined Atom StereoCenter Count: 0
Defined Bond StereoCenter Count: 0
Undefined Bond StereoCenter Count: 0
Covalently-Bonded Unit Count: 1
Flash Point: 40 °C
Enthalpy of Vaporization: 23.7 kJ/mol
Boiling Point: 117.1 °C at 760 mmHg
Vapour Pressure: 13.9 mmHg at 25°C
The appearance of Mercuric acetate (1600-27-7) is white or off-white powder.
The molecular structure of Mercuric acetate (1600-27-7) :
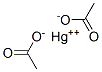
Mercury acetate Uses
Mercuric acetate (1600-27-7) is employed as a reagent to generate organomercury compounds from unsaturated organic precursors.
Mercury acetate Toxicity Data With Reference
| 1. | oth-mus:oth 50 mg/L | MUREAV Mutation Research. 17 (1973),93. | ||
| 2. | orl-rat LD50:40,900 µg/kg | GISAAA Gigiena i Sanitariya. 46 (8)(1981),12. | ||
| 3. | skn-rat LD50:570 mg/kg | GTPZAB Gigiena Truda i Professionalnye Zabolevaniia. Labor Hygiene and Occupational Diseases. 25 (7)(1981),27. | ||
| 4. | orl-mus LD50:23,900 µg/kg | GISAAA Gigiena i Sanitariya. 46 (8)(1981),12. | ||
| 5. | ipr-mus LD50:6500 µg/kg | GTPZAB Gigiena Truda i Professionalnye Zabolevaniia. Labor Hygiene and Occupational Diseases. 25 (7)(1981),27. | ||
| 6. | scu-mus LDLo:20 mg/kg | MOLAAF Monatsschrift fuer Ohrenheilkunde und Laryngo-rhinologie. 73 (1939),751. | ||
| 7. | ivn-mus LD50:4390 µg/kg | NYKZAU Nippon Yakurigaku Zasshi. Japanese Journal of Pharmacology. 57 (1961),219. | ||
| 8. | orl-uns LD50:65 mg/kg | GISAAA Gigiena i Sanitariya. 49 (9)(1984),11. |
Mercury acetate Consensus Reports
EPA Extremely Hazardous Substances List. Mercury and its compounds are on the Community Right-To-Know List. EPA Genetic Toxicology Program. Reported in EPA TSCA Inventory.
Mercury acetate Safety Profile
Poison by ingestion, intravenous, intraperitoneal, and subcutaneous routes. Moderately toxic by skin contact. An experimental teratogen. Experimental reproductive effects. Mutation data reported. When heated to decomposition it emits toxic fumes of Hg. See also MERCURY COMPOUNDS, INORGANIC; MERCURY COMPOUNDS, ORGANIC.
Hazard Codes: T+ ,N
,N 
Risk Statements: 26/27/28-33-50/53
R26/27/28:Very toxic by inhalation, in contact with skin and if swallowed.
R33:Danger of cumulative effects.
R50/53:Very toxic to aquatic organisms, may cause long-term adverse effects in the aquatic environment.
Safety Statements: 13-28-36-45-60-61
S13:Keep away from food, drink and animal foodstuffs.
S28:After contact with skin, wash immediately with plenty of soap-suds.
S36:Wear suitable protective clothing.
S45:In case of accident or if you feel unwell, seek medical advice immediately (show the label whenever possible.)
S60:This material and its container must be disposed of as hazardous waste.
S61:Avoid release to the environment. Refer to special instructions / safety data sheets.
RIDADR: UN 1629 6.1/PG 2
WGK Germany: 3
RTECS: AI8575000
TSCA: Yes
HazardClass: 6.1
PackingGroup: II
Mercury acetate Standards and Recommendations
OSHA PEL: CL 0.1 mg(Hg)/m3 (skin)
ACGIH TLV: TWA 0.1 mg(Hg)/m3 (skin); BEI: 35 µg/g creatinine total inorganic mercury in urine preshift; 15 µg/g creatinine total inorganic mercury in blood at end of shift at end of workweek.
DFG MAK: Confirmed Animal Carcinogen with Unknown Relevance to Humans
NIOSH REL: (Mercury, Aryl and Inorganic) CL 0.1 mg/m3 (skin)
DOT Classification: 6.1; Label: Poison
Mercury acetate Specification
1.Reactivity Profile
Mercuric acetate (1600-27-7) is incompatible with acetylene, ammonia, chlorine dioxide, azides, calcium (amalgam formation), sodium carbide, lithium, rubidium, and copper .
Related Products
- Mercury
- Mercury (II) fluoroacetate
- Mercury acetate
- MERCURY ACETYLIDE (DOT)
- Mercury alloy, base, Hg 60-100,Zn 0-40 (9CI)
- Mercury azide
- Mercury chloride
- Mercury chloride
- Mercury cyanide hydroxide
- Mercury cyanide oxide
- 1600-29-9
- 160033-52-3
- 160037-58-1
- 16004-15-2
- 1600-44-8
- 160047-24-5
- 160047-56-3
- 1600-49-3
- 16006-62-5
- 160081-62-9
Hot Products
About|Contact|Cas|Product Name|Molecular|Country|Encyclopedia
Message|New Cas|MSDS|Service|Advertisement|CAS DataBase|Article Data|Manufacturers | Chemical Catalog
©2008 LookChem.com,License: ICP
NO.:Zhejiang16009103
complaints:service@lookchem.com Desktop View