-
Name
CHOLIC ACID METHYL ESTER
- EINECS 215-903-7
- CAS No. 1448-36-8
- Article Data132
- CAS DataBase
- Density 1.141g/cm3
- Solubility Insoluble in water. Soluble in medium polarity organic solvents.
- Melting Point 155-156°C
- Formula C25H42 O5
- Boiling Point 541.6°Cat760mmHg
- Molecular Weight 422.605
- Flash Point 174.9°C
- Transport Information
- Appearance Off-White Solid
-
Safety
Hazard Codes Xi Safety Statements 22-24/25 - Risk Codes
-
Molecular Structure
-
Hazard Symbols

- Synonyms Cholicacid, methyl ester (6CI,7CI,8CI); Methyl 3a,7a,12a-trihydroxy-5b-cholan-24-oate; Methyl 3a,7a,12a-trihydroxy-5b-cholanoate; Methyl cholate; NSC 126794
- PSA 86.99000
- LogP 3.53710
Synthetic route

| Conditions | Yield |
|---|---|
| With diazomethyl-trimethyl-silane In methanol; toluene at 20℃; for 2h; | 100% |
| With acetyl chloride at 20℃; for 72h; | 100% |
| With acetyl chloride at 20℃; for 0.75h; | 100% |

| Conditions | Yield |
|---|---|
| In tetrahydrofuran | A 100% B n/a |


| Conditions | Yield |
|---|---|
| In methanol; hexane; toluene at 20℃; | 100% |

| Conditions | Yield |
|---|---|
| In methanol for 12.5h; Cooling with ice; | 99% |
| In methanol |

| Conditions | Yield |
|---|---|
| With potassium carbonate In N,N-dimethyl-formamide at 20℃; | 97% |
| With cesium fluoride In N,N-dimethyl-formamide |
-
-
55319-79-4
7-α,12-α-dihydroxycholin-4-ene-3-one carboxylic acid methyl ester

-
A
-
14772-93-1
methyl 3β,7α,12α-trihydroxy-5α-cholan-24-oate

-
B
-
1448-36-8
methyl cholate

| Conditions | Yield |
|---|---|
| With sodium tetrahydroborate In pyridine for 20h; Ambient temperature; | A 77% B 23% |
| With sodium tetrahydroborate In pyridine for 20h; Ambient temperature; | A 77% B 23% |
-
-
3749-87-9
methyl 3α,7α-diacetoxy-12α-hydroxy cholanate


-
B
-
1249-70-3
Methyl 12α-hydroxy-5β-cholanate

-
C
-
3701-54-0
methyl 7α,12a-dihydroxy-5β-cholan-24-oate

-
D
-
1448-36-8
methyl cholate

| Conditions | Yield |
|---|---|
| With potassium Sodium; iso-butanol; Tris(3,6-dioxaheptyl)amine In tetrahydrofuran Ambient temperature; var. cat.: 18-crown-6; Yield given. Yields of byproduct given; | A n/a B 58% C n/a D n/a |

-
-
3749-87-9
methyl 3α,7α-diacetoxy-12α-hydroxy cholanate

-
A
-
1249-70-3
Methyl 12α-hydroxy-5β-cholanate

-
B
-
3245-38-3
methyl deoxycholate

-
C
-
3701-54-0
methyl 7α,12a-dihydroxy-5β-cholan-24-oate

-
D
-
1448-36-8
methyl cholate

| Conditions | Yield |
|---|---|
| With potassium Sodium; iso-butanol; Tris(3,6-dioxaheptyl)amine In tetrahydrofuran Ambient temperature; var. cat.: 18-crown-6; Yields of byproduct given; | A 58% B n/a C n/a D n/a |


| Conditions | Yield |
|---|---|
| With methanol; sodium tetrahydroborate |
-
-
10538-64-4
methyl 3α,7α-dihydroxy-12-oxo-5β-cholan-24-oate

-
A
-
1448-36-8
methyl cholate

| Conditions | Yield |
|---|---|
| With borane tert-butylamine In methanol for 3h; Ambient temperature; Yield given. Yields of byproduct given; |
-
-
21059-36-9
3α-ethoxycarbonyloxy-7α,12α-dihydroxy-5β-cholan-24-oic acid methyl ester

-
A
-
1448-36-8
methyl cholate

-
B
-
10538-65-5, 15073-97-9, 15180-34-4, 81644-39-5, 81644-40-8
methyl 3α,12α-dihydroxy-7-oxo-5β-cholanate

| Conditions | Yield |
|---|---|
| With N-Bromosuccinimide Multistep reaction; |

| Conditions | Yield |
|---|---|
| at 85℃; Rate constant; Kinetics; Thermodynamic data; Heating; Ea; var. temp.; |

-
-
1448-36-8
methyl cholate

| Conditions | Yield |
|---|---|
| With methanol; sodium tetrahydroborate |

-
A
-
1448-36-8
methyl cholate

-
B
-
28050-54-6
3β,7α,12α-trihydroxy-5β-cholan-24-oic acid methyl ester

| Conditions | Yield |
|---|---|
| With methanol; sodium tetrahydroborate |
-
-
14772-99-7
3-oxo cholic acid methyl ester

-
-
1448-36-8
methyl cholate

| Conditions | Yield |
|---|---|
| Multi-step reaction with 3 steps 1: 25 percent / DDQ / dioxane / 10 h / Heating 2: H2 / tris(triphenylphosphine)-rhodium chloride / benzene 3: 23 percent / NaBH4 / pyridine / 20 h / Ambient temperature View Scheme | |
| Multi-step reaction with 4 steps 1: 25 percent / DDQ / dioxane / 10 h / Heating 2: NaBH4 / methanol / 2 h / Ambient temperature 3: MnO2 / CHCl3 / 60 h 4: 23 percent / NaBH4 / pyridine / 20 h / Ambient temperature View Scheme |
-
-
111102-58-0
methyl 3-oxo-7α,12α-dihydroxy-1,4-choladienate

-
-
1448-36-8
methyl cholate

| Conditions | Yield |
|---|---|
| Multi-step reaction with 2 steps 1: H2 / tris(triphenylphosphine)-rhodium chloride / benzene 2: 23 percent / NaBH4 / pyridine / 20 h / Ambient temperature View Scheme | |
| Multi-step reaction with 3 steps 1: NaBH4 / methanol / 2 h / Ambient temperature 2: MnO2 / CHCl3 / 60 h 3: 23 percent / NaBH4 / pyridine / 20 h / Ambient temperature View Scheme |
-
-
125626-89-3
(R)-4-((7R,8R,9S,10R,12S,13R,14S,17R)-3,7,12-Trihydroxy-10,13-dimethyl-2,3,6,7,8,9,10,11,12,13,14,15,16,17-tetradecahydro-1H-cyclopenta[a]phenanthren-17-yl)-pentanoic acid methyl ester

-
-
1448-36-8
methyl cholate

| Conditions | Yield |
|---|---|
| Multi-step reaction with 2 steps 1: MnO2 / CHCl3 / 60 h 2: 23 percent / NaBH4 / pyridine / 20 h / Ambient temperature View Scheme |

| Conditions | Yield |
|---|---|
| In methanol |

| Conditions | Yield |
|---|---|
| With hydrogenchloride In methanol at 20℃; for 12h; |

| Conditions | Yield |
|---|---|
| With pyridinium dichromate | 100% |
| With pyridinium dichromate | 100% |
| With pyridinium dichromate | 100% |

| Conditions | Yield |
|---|---|
| With pyridine; dmap at 20℃; for 1h; | 100% |
| With pyridine; dmap at 20℃; for 1h; | 100% |
| With pyridine; dmap at 20℃; for 3h; | 98% |
-
-
111-82-0
methyl n-dodecanoate

-
-
1448-36-8
methyl cholate

-
-
151112-03-7
Dodecanoic acid (3R,5R,7R,8R,9S,10S,12S,13R,14S,17R)-7,12-dihydroxy-17-((R)-3-methoxycarbonyl-1-methyl-propyl)-10,13-dimethyl-hexadecahydro-cyclopenta[a]phenanthren-3-yl ester

| Conditions | Yield |
|---|---|
| at 60℃; under 8 Torr; for 20h; Candida antarctica lipase immobilized on Florisil with Triton X-100; | 100% |

| Conditions | Yield |
|---|---|
| at 60℃; under 8 Torr; for 20h; Candida antarctica lipase immobilized on Florisil with Triton X-100; | 100% |

| Conditions | Yield |
|---|---|
| at 60℃; under 8 Torr; for 20h; Candida antarctica lipase immobilized on Florisil with Triton X-100; | 99% |

| Conditions | Yield |
|---|---|
| With potassium hydroxide In methanol; water for 0.0666667h; microwave irradiation; | 99% |
-
-
1448-36-8
methyl cholate

-
-
18162-48-6
tert-butyldimethylsilyl chloride

-
-
73677-12-0
methyl 3α-O-(tert-butyl-dimethylsilanyloxy)-7α,12α-dihydroxy-5β-cholan-24-oate

| Conditions | Yield |
|---|---|
| With 1H-imidazole In N,N-dimethyl-formamide at 20℃; for 2h; | 99% |
| With pyridine; 1H-imidazole In dichloromethane at 50℃; for 18h; | 99% |
| With 1H-imidazole In N,N-dimethyl-formamide at 20℃; for 3h; | 92% |
-
-
1448-36-8
methyl cholate

-
-
14772-99-7
3-oxo cholic acid methyl ester

| Conditions | Yield |
|---|---|
| With Ag2CO3 on Celite In toluene Reflux; Inert atmosphere; | 98% |
| With silver carbonate In toluene for 12h; Reflux; Dean-Stark; | 94% |
| With 4-methyl-morpholine; 6C6H15P*4CF3O3S(1-)*2Ru(2+); acetone at 35℃; for 4h; Inert atmosphere; Sealed tube; | 94% |

| Conditions | Yield |
|---|---|
| With lithium aluminium tetrahydride In tetrahydrofuran | 98% |
| With lithium aluminium tetrahydride In tetrahydrofuran for 48h; Heating / reflux; | 98% |
| With lithium aluminium tetrahydride In tetrahydrofuran for 48h; Heating / reflux; | 98% |
-
-
1448-36-8
methyl cholate

-
-
107-15-3
ethylenediamine

-
-
78793-09-6
N-(2-aminoethyl)(3α,5β,7α,12α)-3,7,12-trihydroxycholan-24-amide

| Conditions | Yield |
|---|---|
| In methanol at 20℃; for 48h; | 98% |
| In methanol at 69 - 71℃; | 93% |
| at 73℃; for 16h; | 93% |
-
-
1448-36-8
methyl cholate

-
-
107-30-2
chloromethyl methyl ether

-
-
637350-96-0
methyl 3α,7α,12α-tri(methoxymethoxy)-5β-cholan-24-oate

| Conditions | Yield |
|---|---|
| With N-ethyl-N,N-diisopropylamine In benzene at 20℃; for 240h; | 98% |

| Conditions | Yield |
|---|---|
| at 20℃; for 72h; | 96% |
| With perchloric acid at 50℃; for 1.5h; | 86% |

| Conditions | Yield |
|---|---|
| With pyridine at -10 - -6℃; for 15h; | 95.3% |
-
-
1448-36-8
methyl cholate

-
-
67-68-5
dimethyl sulfoxide

-
-
168131-38-2
3α,7α,12α-trihydroxy-24-methylsulfinylmethyl-5β-cholan-24-one

| Conditions | Yield |
|---|---|
| With sodium hydride In tetrahydrofuran at 0℃; for 0.666667h; | 95% |
-
-
1448-36-8
methyl cholate

-
-
124-63-0
methanesulfonyl chloride

-
-
160836-52-2
Methyl 3α-methanesulphonyloxy-7α,12α-dihydroxy-5β-cholan-24-oate

| Conditions | Yield |
|---|---|
| With pyridine; dmap at 20℃; Cooling with ice; | 95% |
| With triethylamine In dichloromethane at 0℃; for 0.166667h; | 87% |
| With triethylamine In dichloromethane at 0 - 20℃; for 2.5h; Inert atmosphere; | 84% |
-
-
124-09-4
1,6-Hexanediamine

-
-
1448-36-8
methyl cholate

-
-
142970-26-1
N-hexylamino-3α,7α,12α-trihydroxy-5β-cholan-24-cholanoamide

| Conditions | Yield |
|---|---|
| In methanol at 69 - 71℃; | 95% |
| at 73℃; for 16h; | 95% |
-
-
32315-10-9
bis(trichloromethyl) carbonate

-
-
1448-36-8
methyl cholate

| Conditions | Yield |
|---|---|
| With pyridine In benzene at 25℃; for 24h; | 95% |
-
-
1448-36-8
methyl cholate

-
-
74-88-4
methyl iodide

-
-
1351984-85-4
methyl 3α,7α-dimethoxy-12α-hydroxy-5β-cholan-24-oate

| Conditions | Yield |
|---|---|
| With sodium hydride In tetrahydrofuran at 20℃; | 94% |
| With sodium hydride In tetrahydrofuran at 20℃; for 12h; | 94% |
| Stage #1: methyl cholate With sodium hydride In tetrahydrofuran at 0℃; for 0.25h; Inert atmosphere; Stage #2: methyl iodide In tetrahydrofuran at 20℃; for 24h; Inert atmosphere; | 94% |
-
-
1448-36-8
methyl cholate

-
-
108-24-7
acetic anhydride

-
-
3749-87-9
methyl 3α,7α-diacetoxy-12α-hydroxy cholanate

| Conditions | Yield |
|---|---|
| With dmap; triethylamine In dichloromethane at 25℃; for 5h; | 92% |
| With dmap; triethylamine In dichloromethane at 28℃; | 92% |
| With dmap; triethylamine In dichloromethane at 0 - 28℃; | 92% |
-
-
1448-36-8
methyl cholate

-
-
141-43-5
ethanolamine

-
-
63266-83-1
N-(2-hydroxyethyl)-3α,7α,12α-trihydroxy-5β-cholan-24-amide

| Conditions | Yield |
|---|---|
| In methanol Heating; | 92% |
-
-
1448-36-8
methyl cholate

-
-
124-63-0
methanesulfonyl chloride

-
-
1204197-58-9
methyl 3α-methanesulfonyl-7α,12α-dihydroxy-5β-cholan-24-oate

| Conditions | Yield |
|---|---|
| With pyridine at 20℃; Inert atmosphere; | 92% |
-
-
1448-36-8
methyl cholate

-
-
98-59-9
p-toluenesulfonyl chloride

-
-
28192-77-0
7α,12α-dihydroxy-3α-(toluene-sulfonyl-(4)-oxy)-5β-cholanoic acid-(24)-methyl ester

| Conditions | Yield |
|---|---|
| In pyridine; benzene Ambient temperature; | 91% |
| In pyridine at 10℃; for 16h; | 90% |
| With pyridine | 88% |

| Conditions | Yield |
|---|---|
| In chloroform for 16h; | 91% |

| Conditions | Yield |
|---|---|
| With triphenylphosphine; diethylazodicarboxylate In tetrahydrofuran at 40℃; for 24h; | 90% |
| With di-isopropyl azodicarboxylate; triphenylphosphine In tetrahydrofuran at 40 - 50℃; for 24h; | |
| With dmap; triphenylphosphine; diethylazodicarboxylate In tetrahydrofuran at 20℃; for 48h; Mitsunobu reaction; | |
| With dmap; di-isopropyl azodicarboxylate; triphenylphosphine In tetrahydrofuran at 40℃; for 8h; Mitsunobu Displacement; | |
| With di-isopropyl azodicarboxylate; triphenylphosphine |

| Conditions | Yield |
|---|---|
| In methanol at 65℃; for 72h; | 89% |
| at 20℃; for 168h; |
Methyl cholate Chemical Properties
Molecular Structure of Methyl cholate (CAS NO.1448-36-8):
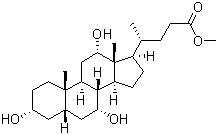
IUPAC Name: Methyl(4R)-4-[(3R,5S,8R,9S,10S,12S,13R,14S,17R)-3,7,12-trihydroxy-10,13-dimethyl-2,3,4,5,6,7,8,9,11,12,14,15,16,17-tetradecahydro-1H-cyclopenta[a]phenanthren-17-yl]pentanoate
Molecular Weight: 422.59798 [g/mol]
Molecular Formula: C25H42O5
XLogP3-AA: 3.9
H-Bond Donor: 3
H-Bond Acceptor: 5
EINECS: 215-903-7
Appearance: Off-White Solid
Melting Point: 155-156 °C
Index of Refraction: 1.539
Molar Refractivity: 116.08 cm3
Molar Volume: 370.2 cm3
Surface Tension: 46 dyne/cm
Density: 1.141 g/cm3
Flash Point: 174.9 °C
Enthalpy of Vaporization: 94.23 kJ/mol
Boiling Point: 541.6 °C at 760 mmHg
Vapour Pressure: 5.49E-14 mmHg at 25 °C
Product Categories: Steroids; Steroids & Hormones - 13C & 2H
Methyl cholate Safety Profile
Safety Information of Methyl cholate (CAS NO.1448-36-8):
Hazard Codes:  Xi
Xi
Safety Statements: 22-24/25
S22:Do not breathe dust.
S24/25:Avoid contact with skin and eyes.
Methyl cholate Specification
Methyl cholate (CAS NO.1448-36-8), its Synonyms are Methyl 4-(3,7,12-trihydroxy-10,13-dimethylperhydrocyclopenta[a]phenanthren-17-yl)pen ; 5(beta)-Cholmic acid-3alpha,7alpha,12alpha-triol methyl ester ; 5-beta-Cholanic acid-3-alpha, 7-alpha, 12-alpha-triol methyl ester ; Methyl 4-(3,7,12-trihydroxy-10,13-dimethylperhydrocyclopenta[a]phenanthren-17-yl)pentanoate ; Cholic acid methyl ester .
Related Products
- Methyl 1-Benzyl-5-oxopyrrolidine-3-carboxylate
- Methyl (((methoxymethylphosphinothioyl)thio)acetyl)methylcarbamate
- Methyl (+)-(3R)-7-[4-(4-fluorophenyl)-6-isopropyl-2-(N-methyl-N-methanesulfonylamino)pyrimidin-5-yl]-3-hydroxy-5-oxo-(6E)-heptenoate
- Methyl (2-amino-5-methyl-1,3-thiazol-4-yl)acetate
- Methyl (2-chloromethyl)oxazole-4-carboxylate
- Methyl (2E)-3-(4-methylphenyl)propenoate
- Methyl (2E)-3-cyclohexylprop-2-enoate
- Methyl (2R)-2-[(tert-butoxycarbonyl)amino]-3-iodopropanoate
- Methyl (2R)-2-[4-(2,4-dichlorophenoxy)phenoxy]propanoate
- Methyl (2R)-2-amino-2-cyclohexylethanoate hydrochloride
- 14484-47-0
- 14484-64-1
- 14484-69-6
- 144851-59-2
- 144851-61-6
- 144851-82-1
- 14485-80-4
- 144860-30-0
- 14486-03-4
- 14486-08-9
Hot Products
About|Contact|Cas|Product Name|Molecular|Country|Encyclopedia
Message|New Cas|MSDS|Service|Advertisement|CAS DataBase|Article Data|Manufacturers | Chemical Catalog
©2008 LookChem.com,License: ICP
NO.:Zhejiang16009103
complaints:service@lookchem.com Desktop View