-
Name
Nitroethylene
- EINECS
- CAS No. 3638-64-0
- Article Data49
- CAS DataBase
- Density 1.052 g/cm3
- Solubility
- Melting Point -55.5°C
- Formula C2H3NO2
- Boiling Point 98.5 °C at 760 mmHg
- Molecular Weight 73.0513
- Flash Point 23.2 °C
- Transport Information
- Appearance
- Safety
- Risk Codes
-
Molecular Structure
- Hazard Symbols
- Synonyms Ethylene,nitro- (6CI,7CI,8CI);1-Nitroethene;1-Nitroethylene;Nitroethene;
- PSA 45.82000
- LogP 0.92980
Synthetic route

| Conditions | Yield |
|---|---|
| With phthalic anhydride at 150 - 180℃; under 80 Torr; | 89% |
| With phthalic anhydride at 180℃; for 3h; Cooling with acetone-dry ice; | 69% |
| With phthalic anhydride at 150 - 180℃; for 23h; Inert atmosphere; Cooling with ice; | 55% |

| Conditions | Yield |
|---|---|
| at 140 - 180℃; under 80 Torr; Heating; | 80% |
| at 175 - 180℃; under 80 Torr; |

| Conditions | Yield |
|---|---|
| With tert.-butylnitrite; silica gel In acetonitrile at 100℃; under 2250.23 Torr; Time; Temperature; Pressure; Microwave irradiation; | 80% |
| With ferric nitrate for 0.5h; Milling; | 80% |
| With Vilsmeier reagent; potassium nitrate In acetonitrile at 20℃; Reagent/catalyst; Temperature; Sonication; | 75% |
-
-
39221-06-2
1-nitroethanol

-
-
3638-64-0
1-nitroethylene

| Conditions | Yield |
|---|---|
| With phthalic anhydride In tetrahydrofuran at 130 - 180℃; for 3h; | 69% |
| With phthalic anhydride at 140℃; under 80 Torr; |

| Conditions | Yield |
|---|---|
| Leiten ueber mit Bleisalzen aktiviertes Silicagel; | |
| With lead(IV) tetraacetate; silica gel at 250 - 300℃; | |
| With silica gel; lead(II) oxide at 250 - 300℃; | |
| With lead(II) chromate; silica gel at 250 - 300℃; | |
| beim Leiten ueber einen bleisalzhaltigen Silicagel- oder Aluminiumoxydgel Katalysator; |
-
-
60-29-7
diethyl ether

-
-
625-47-8
1-chloro-2-nitroethane

-
-
127-09-3
sodium acetate

-
-
3638-64-0
1-nitroethylene


| Conditions | Yield |
|---|---|
| at 100℃; |

| Conditions | Yield |
|---|---|
| With ethanol at 100℃; |

| Conditions | Yield |
|---|---|
| at 100℃; unter Ausschluss von Alkali; |

| Conditions | Yield |
|---|---|
| With calcium chloride at 250℃; | |
| With calcium carbonate at 300 - 400℃; | |
| With diethyl ether; sodium acetate |

| Conditions | Yield |
|---|---|
| With ethanol at 100℃; |
-
-
421551-02-2
2-nitro-1,4-diphenyl-1,2,3,4-tetrahydro-1,4-epoxido-naphthalene

-
A
-
3638-64-0
1-nitroethylene

-
B
-
5471-63-6
1,3-diphenylisobenzofuran

| Conditions | Yield |
|---|---|
| Erhitzen; |

| Conditions | Yield |
|---|---|
| With phthalic anhydride |

| Conditions | Yield |
|---|---|
| In diphenylether at 250℃; Rate constant; |

| Conditions | Yield |
|---|---|
| at 225℃; Rate constant; activation energy E, logA, ΔS(excit.); other temp.; |

| Conditions | Yield |
|---|---|
| at 225℃; Rate constant; various initial vapour pressures and surface-to-volume ratios; activation energy E, logA, ΔS(excit.); other temp.; |

| Conditions | Yield |
|---|---|
| at 225℃; Rate constant; various initial vapour pressures and surface-to-volume ratios; activation energy E, logA, ΔS(excit.); other temp.; |
-
-
13005-97-5
Trichloressigsaeure-β-nitroaethylester

-
A
-
3638-64-0
1-nitroethylene

-
B
-
76-03-9
trichloroacetic acid

| Conditions | Yield |
|---|---|
| at 225℃; Rate constant; various initial vapour pressures and surface-to-volume ratios; activation energy E, logA, ΔS(excit.); other temp.; |

| Conditions | Yield |
|---|---|
| at 225℃; Rate constant; activation energy E, logA, ΔS(excit.); other temp.; |

-
-
3638-64-0
1-nitroethylene

| Conditions | Yield |
|---|---|
| With glass beads at 350℃; | |
| With aluminum(III) sulfate at 240℃; |

| Conditions | Yield |
|---|---|
| bei der Destillation; |

| Conditions | Yield |
|---|---|
| at 25℃; |

| Conditions | Yield |
|---|---|
| at 275 - 450℃; |


| Conditions | Yield |
|---|---|
| at 275 - 450℃; |


| Conditions | Yield |
|---|---|
| at 250 - 350℃; |


| Conditions | Yield |
|---|---|
| at 250 - 350℃; |


| Conditions | Yield |
|---|---|
| at 250 - 350℃; |

-
-
3638-64-0
1-nitroethylene

-
-
7500-79-0
N-methyl-diphenyl-nitrone

-
-
126399-44-8
2-Methyl-4-nitro-3,3-diphenyl-isoxazolidine

| Conditions | Yield |
|---|---|
| In benzene at 20℃; for 240h; | 100% |
-
-
3638-64-0
1-nitroethylene

-
-
4504-13-6
N-diphenylmethylene-N-pheylnitrone

-
-
126399-36-8
4-nitro-2,3,3-triphenylisoxazolidine

| Conditions | Yield |
|---|---|
| In benzene for 1h; Ambient temperature; | 100% |
| at 20℃; regioselective reaction; |
-
-
3638-64-0
1-nitroethylene

-
-
542-92-7
cyclopenta-1,3-diene

-
-
874-44-2
5-endo-nitrobicyclo<2.2.1>hept-2-ene

| Conditions | Yield |
|---|---|
| In diethyl ether at -15℃; | 99.6% |
| With diethyl ether at 105 - 110℃; | |
| With benzene | |
| With diethyl ether |
-
-
3638-64-0
1-nitroethylene

-
-
16649-50-6
N-tert-Butylhydroxylamine

| Conditions | Yield |
|---|---|
| In dichloromethane for 2h; Ambient temperature; | 99% |
-
-
3638-64-0
1-nitroethylene

-
-
99307-35-4
N-demethyl-N-formylthebaibe

-
-
101077-43-4
4,5α-epoxy-17-formyl-3,6-dimethoxy-7α-nitro-6α,14α-ethenoisomorphinan

| Conditions | Yield |
|---|---|
| In benzene for 19h; Heating; | 99% |

| Conditions | Yield |
|---|---|
| With diisopropylamine; lithium diisopropyl amide In tetrahydrofuran; hexane -78 deg C, 1 h; -78 deg C to room temp., 35 min; | 99% |

| Conditions | Yield |
|---|---|
| Stage #1: methanol; 4-bromo-2-methoxy-5-methylphenol With [bis(acetoxy)iodo]benzene at 0℃; Inert atmosphere; Stage #2: 1-nitroethylene In toluene at 20℃; Diels-Alder reaction; Inert atmosphere; regioselective reaction; | 99% |

-
-
5519-42-6
3,4,5-Trimethylpyrazole

-
-
3638-64-0
1-nitroethylene

-
-
83600-05-9
3,4,5-Trimethyl-1-(2-nitro-ethyl)-1H-pyrazole

| Conditions | Yield |
|---|---|
| In benzene for 12h; Ambient temperature; | 98% |

| Conditions | Yield |
|---|---|
| With indium tribromide In dichloromethane; benzene at 0 - 20℃; Inert atmosphere; | 98% |
-
-
3638-64-0
1-nitroethylene

-
-
137334-09-9, 55623-56-8
tert-butyl 2-oxocyclohexanecarboxylate

-
-
1262669-60-2
(S)-tert-butyl 1-(2-nitroethyl)-2-oxocyclohexanecarboxylate

| Conditions | Yield |
|---|---|
| With C34H22N2Ni2O4 In ethyl acetate; toluene at 40℃; for 18h; optical yield given as %ee; enantioselective reaction; | 98% |
-
-
3638-64-0
1-nitroethylene

| Conditions | Yield |
|---|---|
| Stage #1: C18H15NO With (R)-3,3'-bis(2,4,6-triisopropylphenyl)binol phosphoric acid; magnesium sulfate In dichloromethane at 0℃; for 0.0833333h; Schlenk technique; Inert atmosphere; Stage #2: 1-nitroethylene In dichloromethane; toluene at 0℃; for 60h; Inert atmosphere; Schlenk technique; enantioselective reaction; | 98% |
-
-
3638-64-0
1-nitroethylene

| Conditions | Yield |
|---|---|
| Stage #1: C21H15NO With (R)-3,3'-bis(2,4,6-triisopropylphenyl)binol phosphoric acid; magnesium sulfate In dichloromethane; toluene at 0℃; for 0.0833333h; Schlenk technique; Inert atmosphere; Stage #2: 1-nitroethylene In dichloromethane; toluene at 0℃; for 60h; Schlenk technique; Inert atmosphere; enantioselective reaction; | 98% |
-
-
3638-64-0
1-nitroethylene

-
-
91233-19-1
1-(adamantane-1-carbonyloxy)pyridine-2(1H)-thione

-
-
104543-11-5
2-(1-adamantyl)-1-nitro-1-(pyridine-thiyl)ethane

| Conditions | Yield |
|---|---|
| With camphor-10-sulfonic acid In dichloromethane; toluene at -20 - -10℃; for 0.5h; Irradiation; | 97% |
-
-
3638-64-0
1-nitroethylene

-
-
371-40-4
4-fluoroaniline

-
-
83600-17-3
(4-Fluoro-phenyl)-(2-nitro-ethyl)-amine

| Conditions | Yield |
|---|---|
| In benzene for 12h; Ambient temperature; | 97% |

| Conditions | Yield |
|---|---|
| In benzene | 97% |

| Conditions | Yield |
|---|---|
| In benzene for 12h; Ambient temperature; | 96% |
-
-
67-51-6
3,5-dimethyl-1H-pyrazole

-
-
3638-64-0
1-nitroethylene

-
-
83600-04-8
3,5-Dimethyl-1-(2-nitro-ethyl)-1H-pyrazole

| Conditions | Yield |
|---|---|
| In benzene for 12h; Ambient temperature; | 96% |
-
-
3638-64-0
1-nitroethylene

-
-
18869-43-7
DL-leucine methyl ester

-
-
76919-69-2
N-(2-Nitroethyl)-DL-leucine methyl ester

| Conditions | Yield |
|---|---|
| In benzene for 12h; Ambient temperature; | 96% |
-
-
3638-64-0
1-nitroethylene

-
-
6322-53-8, 5845-53-4, 7517-19-3
leucine methyl ester hydrochloride

-
-
76919-69-2
N-(2-Nitroethyl)-DL-leucine methyl ester

| Conditions | Yield |
|---|---|
| With triethylamine In diethyl ether; benzene at 10℃; for 96h; | 96% |

| Conditions | Yield |
|---|---|
| trifluorormethanesulfonic acid at 0 - 5℃; for 0.25h; | 96% |

| Conditions | Yield |
|---|---|
| 3-nitrobenzoic acid In toluene at 20℃; for 24h; Michael reaction; | 96% |

| Conditions | Yield |
|---|---|
| In benzene for 12h; Ambient temperature; | 95% |
-
-
3638-64-0
1-nitroethylene

| Conditions | Yield |
|---|---|
| With sodium ethanolate In ethanol at 20℃; for 14h; multiple Michael reaction; | 95% |

| Conditions | Yield |
|---|---|
| Stage #1: 1-nitroethylene; propionaldehyde; (2S)-2-{diphenyl[(trimethylsilyl)oxy]methyl}pyrrolidine; 3-nitrobenzoic acid In toluene at 3℃; Michael addition; Cooling with ice; Stage #2: With sodium tetrahydroborate In methanol; toluene at 0℃; Stage #3: With ammonium chloride In methanol; toluene at 0℃; | 95% |
-
-
3638-64-0
1-nitroethylene

-
-
84109-76-2
tert-butyl 2-oxocyclopentanecarboxylate

-
-
1232542-30-1
(S)-tert-butyl 1-(2-nitroethyl)-2-oxocyclopentanecarboxylate

| Conditions | Yield |
|---|---|
| With C34H22N2Ni2O4 In ethyl acetate; toluene at 40℃; for 5h; optical yield given as %ee; enantioselective reaction; | 95% |
-
-
3638-64-0
1-nitroethylene

-
-
1610698-76-4
tert-butyl 3-(1-methyl-1H-indol-3-yl)-2-oxoindoline-1-carboxylate

| Conditions | Yield |
|---|---|
| With 1,1,1,3',3',3'-hexafluoro-propanol; [Ni(IAP1)2] In o-xylene at -20℃; for 25h; Reagent/catalyst; Concentration; Time; Temperature; Inert atmosphere; enantioselective reaction; | 95% |
-
-
3638-64-0
1-nitroethylene

-
-
1610698-77-5
C22H21BrN2O3

| Conditions | Yield |
|---|---|
| With 1,1,1,3',3',3'-hexafluoro-propanol; [Ni(IAP1)2] In o-xylene at -20℃; for 20h; Inert atmosphere; enantioselective reaction; | 95% |
Nitroethylene Specification
The CAS register number of Nitroethylene is 3638-64-0. It also can be called as Ethene, nitro- and the IUPAC name about this chemical is 1-nitroethene. The molecular formula about this chemical is C2H3NO2.
Physical properties about Nitroethylene are: (1)ACD/LogP: 0.01; (2)ACD/LogD (pH 5.5): 0.01; (3)ACD/LogD (pH 7.4): 0.01 ; (4)#H bond acceptors: 3; (5)#Freely Rotating Bonds: 1; (6)Polar Surface Area: 45.82Å2; (7)Index of Refraction: 1.406; (8)Molar Refractivity: 17.06 cm3; (9)Molar Volume: 69.4 cm3; (10)Polarizability: 6.76x10-24cm3; (11)Surface Tension: 27.7 dyne/cm; (12)Enthalpy of Vaporization: 32.39 kJ/mol; (13)Boiling Point: 98.5 °C at 760 mmHg; (14)Vapour Pressure: 45.8 mmHg at 25°C.
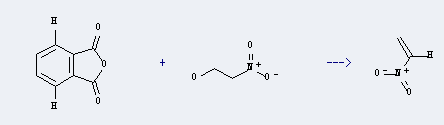
Preparation: this chemical can be prepared by phthalic acid anhydride and 2-nitro-ethanol. The reaction temperature is 140 - 180 ℃. The reaction pressure is 80.
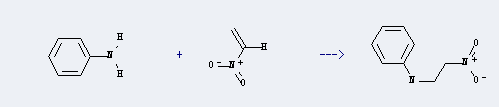
Uses of Nitroethylene: it can be used to produce N-(2-nitro-ethyl)-aniline with aniline at Ambient temperature. This reaction will need solvent benzene with reaction time of 12 hours. The yield is about 90%.
You can still convert the following datas into molecular structure:
(1)SMILES: C=C[N+](=O)[O-]
(2)InChI: InChI=1/C2H3NO2/c1-2-3(4)5/h2H,1H2
(3)InChIKey: RPMXALUWKZHYOV-UHFFFAOYAU
(4)Std. InChI: InChI=1S/C2H3NO2/c1-2-3(4)5/h2H,1H2
(5)Std. InChIKey: RPMXALUWKZHYOV-UHFFFAOYSA-N
The toxicity data is as follows:
| Organism | Test Type | Route | Reported Dose (Normalized Dose) | Effect | Source |
|---|---|---|---|---|---|
| mouse | LDLo | oral | 300mg/kg (300mg/kg) | PERIPHERAL NERVE AND SENSATION: FLACCID PARALYSIS WITHOUT ANESTHESIA (USUALLY NEUROMUSCULAR BLOCKAGE) BEHAVIORAL: TREMOR BEHAVIORAL: CONVULSIONS OR EFFECT ON SEIZURE THRESHOLD | National Technical Information Service. Vol. OTS0555773, |
Related Products
- Nitroethylene
- Nitroethylene polymer
- 3638-73-1
- 36389-07-8
- 3639-21-2
- 36393-20-1
- 36393-27-8
- 36393-42-7
- 36394-75-9
- 36395-07-0
- 363-95-1
- 36397-51-0
Hot Products
About|Contact|Cas|Product Name|Molecular|Country|Encyclopedia
Message|New Cas|MSDS|Service|Advertisement|CAS DataBase|Article Data|Manufacturers | Chemical Catalog
©2008 LookChem.com,License: ICP
NO.:Zhejiang16009103
complaints:service@lookchem.com Desktop View