-
Name
phenylacetyl chloride
- EINECS 203-146-5
- CAS No. 103-80-0
- Article Data166
- CAS DataBase
- Density 1.16 g/cm3
- Solubility
- Melting Point 91oC
- Formula C8H7ClO
- Boiling Point 218.7 °C at 760 mmHg
- Molecular Weight 154.596
- Flash Point 90 °C
- Transport Information UN 2577 8/PG 2
- Appearance Colourless liquid
- Safety 26-36/37/39-45-25-27
- Risk Codes 34-37-14
-
Molecular Structure
-
Hazard Symbols
 C
C
- Synonyms Benzeneacetyl chloride;Acetylchloride, phenyl- (6CI,7CI,8CI);2-Phenylacetyl chloride;2-Phenylethanoylchloride;Phenacetyl chloride;Phenylacetic acid chloride;Phenylaceticchloride;a-Phenylacetyl chloride;
- PSA 17.07000
- LogP 1.99450
Synthetic route

| Conditions | Yield |
|---|---|
| With thionyl chloride In dichloromethane | 100% |
| With thionyl chloride; N,N-dimethyl-formamide In dichloromethane at 23℃; | 100% |
| With thionyl chloride In dichloromethane; N,N-dimethyl-formamide at 20℃; for 1h; Inert atmosphere; | 100% |
-
-
383865-57-4
4-methoxy-7-morpholin-4-yl-benzothiazol-2-yl-amine

-
B
-
103-80-0
phenylacetyl chloride

| Conditions | Yield |
|---|---|
| A 37% B n/a |

| Conditions | Yield |
|---|---|
| With benzene |
-
-
937611-56-8
4-chloro-1,3,4-triphenyl-butan-2-one

-
A
-
588-59-0
stilbene

-
B
-
103-80-0
phenylacetyl chloride

| Conditions | Yield |
|---|---|
| With Nitroethane; chlorine |

| Conditions | Yield |
|---|---|
| With sulfuryl dichloride |
-
-
79-37-8
oxalyl dichloride

-
-
108-88-3
toluene

-
-
94-36-0
dibenzoyl peroxide

-
-
103-80-0
phenylacetyl chloride

-
-
103-82-2
phenylacetic acid

-
-
79-37-8
oxalyl dichloride

-
-
71-43-2
benzene

-
A
-
1555-80-2
phenylacetic anhydride

-
B
-
103-80-0
phenylacetyl chloride


| Conditions | Yield |
|---|---|
| With thionyl chloride; sulfuric acid Multistep reaction; |

| Conditions | Yield |
|---|---|
| With tetrachloromethane; cross-linked polymer (containing 2.50 mmol of phosphine/g) for 4h; Heating; Yield given; |
-
-
88211-07-8
Benzylchlorocarbene

-
-
103-80-0
phenylacetyl chloride

| Conditions | Yield |
|---|---|
| With methyloxirane In cyclohexane at 22℃; Rate constant; other oxygen donors; also in acetonitrile; |

| Conditions | Yield |
|---|---|
| at 60℃; |



-
-
103-80-0
phenylacetyl chloride

| Conditions | Yield |
|---|---|
| Multi-step reaction with 2 steps 1: aq. barium hydroxide solution 2: PCl5 View Scheme |

| Conditions | Yield |
|---|---|
| In toluene |


-
-
103-80-0
phenylacetyl chloride

| Conditions | Yield |
|---|---|
| With chlorine In dichloromethane byproducts: (P(C6H5)3)2PdCl2; addition of Cl2 to Pd(P(C6H5)3)2(C6H5CH2CO)Cl in CH2Cl2; determination : IR; |

-
-
103-80-0
phenylacetyl chloride

| Conditions | Yield |
|---|---|
| With Iodine monochloride In dichloromethane byproducts: (PPh)2PtClI; addition of ICl to trans-(Pt(PPh3)2(PhCH2CO)Cl) in CH2Cl2; determination:IR; | |
| With chlorine In dichloromethane byproducts: (PPh)2PtCl2; addition of Cl2 to trans-(Pt(PPh3)2(PhCH2CO)Cl) in CH2Cl2; determination:IR; |

| Conditions | Yield |
|---|---|
| Multi-step reaction with 2 steps 1: methanol; sodium hydroxide / water / 3 h / 70 °C 2: thionyl chloride / 1 h / 20 °C View Scheme | |
| Multi-step reaction with 2 steps 1: sodium hydroxide; water / 70 - 85 °C 2: thionyl chloride; N,N-dimethyl-formamide / dichloromethane / 40 °C View Scheme | |
| Multi-step reaction with 2 steps 1: sodium hydroxide / water / 3 h / 70 °C 2: thionyl chloride / 1 h / 20 °C View Scheme | |
| Multi-step reaction with 2 steps 1: sodium hydroxide / methanol; water / 3 h / Reflux 2: chlorinating agent / dichloromethane / 0 °C View Scheme |

| Conditions | Yield |
|---|---|
| With trichloroisocyanuric acid In dichloromethane at 20℃; for 120h; Inert atmosphere; | |
| With trichloroisocyanuric acid In dichloromethane at 20℃; for 4h; Inert atmosphere; Irradiation; |

| Conditions | Yield |
|---|---|
| With triethylamine In dichloromethane at 0 - 20℃; | 100% |
| With triethylamine In dichloromethane at 20℃; for 14h; Inert atmosphere; | 100% |
| With triethylamine In dichloromethane at 20℃; | 92% |

| Conditions | Yield |
|---|---|
| In dichloromethane | 100% |
| at 20℃; for 1h; | 91% |
| In chloroform Cooling with ice; | 90% |
-
-
67-51-6
3,5-dimethyl-1H-pyrazole

-
-
103-80-0
phenylacetyl chloride

-
-
36140-84-8
1-(3,5-dimethyl-1H-pyrazol-1-yl)-2-phenylethan-1-one

| Conditions | Yield |
|---|---|
| In dichloromethane at 0 - 20℃; for 3h; Inert atmosphere; | 100% |
| With triethylamine In benzene for 5h; Heating; | 65% |
| With pyridine; benzene | |
| With triethylamine | |
| With triethylamine |

| Conditions | Yield |
|---|---|
| In N,N-dimethyl acetamide at 0℃; for 4h; | 100% |
| In N,N-dimethyl acetamide for 0.333333h; Cooling with ice; | 92% |
| With pyridine for 2h; Cooling with ice; | 91.43% |

| Conditions | Yield |
|---|---|
| With triethylamine In dichloromethane at 0 - 20℃; Inert atmosphere; | 100% |
| With samarium In acetonitrile at 70℃; for 0.0333333h; | 92% |
| With triethylamine In dichloromethane at 20℃; for 12h; | 70.1% |
-
-
103-67-3
benzyl-methyl-amine

-
-
103-80-0
phenylacetyl chloride

-
-
34317-22-1
N-benzyl-N-methyl-2-phenylacetamide

| Conditions | Yield |
|---|---|
| With triethylamine In dichloromethane at 0 - 20℃; for 14h; Inert atmosphere; | 100% |
| In diethyl ether | |
| With sodium hydroxide In dichloromethane for 1h; Yield given; | |
| With TEA; N-methylglycine potassium salt 1.) DMF, 4 h; 2.) DMF, 0.5 h; Yield given. Multistep reaction; |
-
-
1071-46-1
hydrogen ethyl malonate

-
-
103-80-0
phenylacetyl chloride

-
-
718-08-1
ethyl 3-oxo-4-phenylbutyrate

| Conditions | Yield |
|---|---|
| Stage #1: hydrogen ethyl malonate With n-butyllithium In tetrahydrofuran; hexane at -78 - -10℃; Stage #2: phenylacetyl chloride In tetrahydrofuran; hexane at -78℃; for 0.166667h; Further stages.; | 100% |
| With [2,2]bipyridinyl; n-butyllithium In tetrahydrofuran; hexane at -65℃; for 0.0833333h; | 85% |
| 75% |

| Conditions | Yield |
|---|---|
| With triethylamine In dichloromethane at 20℃; for 14h; Inert atmosphere; | 100% |
| With triethylamine In dichloromethane at 20℃; | 60% |
-
-
2033-24-1
cycl-isopropylidene malonate

-
-
103-80-0
phenylacetyl chloride

-
-
66696-84-2
5-(1-hydroxy-2-phenylethylidene)-2,2-dimethyl-1,3-dioxane-4,6-dione

| Conditions | Yield |
|---|---|
| With pyridine In dichloromethane at 0 - 20℃; Acylation; | 100% |
| With dmap Inert atmosphere; | 75% |
| Stage #1: cycl-isopropylidene malonate With pyridine In dichloromethane at 0℃; Stage #2: phenylacetyl chloride In dichloromethane at 0 - 20℃; Stage #3: With hydrogenchloride In dichloromethane; water Cooling with ice; | 71% |
-
-
100-01-6
4-nitro-aniline

-
-
103-80-0
phenylacetyl chloride

-
-
13140-77-7
N-(4-nitrophenyl)-2-phenylacetamide

| Conditions | Yield |
|---|---|
| With pyridine In tetrahydrofuran at 0 - 20℃; for 2h; | 100% |
| With potassium phosphate In tetrahydrofuran at 20℃; for 1h; Inert atmosphere; | 95% |
| In dichloromethane at 20℃; for 1h; | 70% |
-
-
99-09-2
3-nitro-aniline

-
-
103-80-0
phenylacetyl chloride

-
-
13140-76-6
N-(3-nitrophenyl)-2-phenylacetamide

| Conditions | Yield |
|---|---|
| With pyridine In tetrahydrofuran at 0 - 20℃; for 2h; | 100% |
-
-
17639-64-4
N-p-toluenesulfonylpyrrole

-
-
103-80-0
phenylacetyl chloride

-
-
152171-07-8
2-phenyl-1-[1-(toluene-4-sulfonyl)-1H-pyrrol-3-yl]ethanone

| Conditions | Yield |
|---|---|
| With aluminum (III) chloride In 1,2-dichloro-ethane at 0 - 20℃; for 1h; Friedel Crafts acylation; | 100% |
| With aluminium trichloride 1.) CH2Cl2, 15 min, 2.) CH2Cl2, 25 deg C, 2 h; Yield given. Multistep reaction; |
-
-
86945-25-7
4-(2-aminoethyl)-1-(phenylmethyl)piperidine

-
-
103-80-0
phenylacetyl chloride

| Conditions | Yield |
|---|---|
| With triethylamine In tetrahydrofuran at 0℃; for 1h; | 100% |
-
-
6638-79-5
N,O-dimethylhydroxylamine*hydrochloride

-
-
103-80-0
phenylacetyl chloride

-
-
95092-10-7
N-methoxy-N-methyl-2-phenylacetamide

| Conditions | Yield |
|---|---|
| With pyridine In dichloromethane at 0 - 20℃; for 4h; | 100% |
| With pyridine In dichloromethane at 0 - 20℃; for 1.25h; Inert atmosphere; | 99% |
| With hydrogenchloride; potassium carbonate In tert-butyl methyl ether; water; toluene | 95% |
-
-
15690-38-7
(6R,7R)-7-amino-3-hydroxymethyl-8-oxo-5-thia-1-aza-bicyclo[4.2.0]oct-2-ene-2-carboxylic acid

-
-
103-80-0
phenylacetyl chloride

-
-
28240-15-5
3-hydroxymethyl-8-oxo-7-phenylacetylamino-5-thia-1-aza-bicyclo[4.2.0]oct-2-ene-2-carboxylic acid

| Conditions | Yield |
|---|---|
| Stage #1: (6R,7R)-7-amino-3-hydroxymethyl-8-oxo-5-thia-1-aza-bicyclo[4.2.0]oct-2-ene-2-carboxylic acid With benzenesulfonamide In N,N-dimethyl acetamide at 20℃; for 0.5h; Stage #2: phenylacetyl chloride In N,N-dimethyl acetamide at -20 - -10℃; for 1.5h; | 100% |
| Stage #1: (6R,7R)-7-amino-3-hydroxymethyl-8-oxo-5-thia-1-aza-bicyclo[4.2.0]oct-2-ene-2-carboxylic acid With N,O-bis-(trimethylsilyl)-acetamide In N,N-dimethyl acetamide at 20℃; for 0.5h; Stage #2: phenylacetyl chloride In N,N-dimethyl acetamide at -20 - -10℃; for 1.5h; | 51.9% |
| In acetone at 0℃; for 1h; | |
| With triethylamine Acylation; | |
| In water; acetone pH=7.5 - 8.5; |

| Conditions | Yield |
|---|---|
| With hydroxylamine hydrochloride; potassium hydroxide In methanol at 0 - 20℃; Inert atmosphere; | 100% |
| With hydroxylamine hydrochloride; potassium carbonate In diethyl ether; water 1.) 0 deg.C, 7 h, 2.) room temp.; | 82% |
| With hydroxylamine hydrochloride; potassium carbonate In water; ethyl acetate at 0 - 23℃; for 2h; | 78% |

| Conditions | Yield |
|---|---|
| With pyridine In tetrahydrofuran at 0 - 20℃; for 2h; | 100% |
| With triethylamine at 60℃; for 5h; |
-
-
103-80-0
phenylacetyl chloride

-
-
57260-71-6
1-t-Butoxycarbonylpiperazine

| Conditions | Yield |
|---|---|
| With N-ethyl-N,N-diisopropylamine In dichloromethane; water | 100% |

-
-
103-80-0
phenylacetyl chloride

-
-
910540-10-2
4-(1-phenylacetyl-piperidin-4-yl)-butyric acid methyl ester

| Conditions | Yield |
|---|---|
| With N-ethyl-N,N-diisopropylamine In dichloromethane at 0 - 20℃; for 2h; | 100% |
| With N-ethyl-N,N-diisopropylamine In dichloromethane at 0 - 20℃; for 2h; | 100% |
| With N-ethyl-N,N-diisopropylamine In dichloromethane |
-
-
852691-00-0
4-(1,3-dioxolan-2-yl)-N1-hydroxybenzenecarboximidamide

-
-
103-80-0
phenylacetyl chloride

-
-
1003867-83-1
(4-[1,3]-dioxolan-2-yl)-O-phenylacetylbenzamidoxime

| Conditions | Yield |
|---|---|
| With pyridine In dichloromethane at 20℃; for 16h; | 100% |

-
-
103-80-0
phenylacetyl chloride

| Conditions | Yield |
|---|---|
| With Et3N; Me3N In tetrahydrofuran byproducts: Et3NHCl; dissolving of Os3(CO)9(NH2C9H5N)H in THF, addn. of stoich. amts. of Et3N, PhCH2COCl and 10% excess of trimethylamine; pptn., filtration, rotary evapn. of solvent; elem. anal.; | 100% |
-
-
103-80-0
phenylacetyl chloride

-
-
2835-77-0
(2-aminophenyl)(phenyl)methanone

-
-
342603-80-9
N-(2-benzoylphenyl)-2-phenylacetamide

| Conditions | Yield |
|---|---|
| With pyridine In dichloromethane for 3h; | 100% |

| Conditions | Yield |
|---|---|
| With pyridine In dichloromethane at 20 - 25℃; for 17h; Inert atmosphere; | 100% |
| With Fe/SWCNTs at 20℃; for 0.333333h; | 89% |
| With sodium hydroxide In water for 2h; |
-
-
765-39-9
1H-pyrrol-1-amine

-
-
103-80-0
phenylacetyl chloride

-
-
1357159-02-4
2-phenyl-N-(1H-pyrrol-1-yl)acetamide

| Conditions | Yield |
|---|---|
| With N-ethyl-N,N-diisopropylamine In dichloromethane at 0 - 20℃; for 1h; Inert atmosphere; | 100% |
-
-
5003-71-4
3-bromopropylamine hydrochloride

-
-
103-80-0
phenylacetyl chloride

-
-
910794-36-4
N-(3'-bromopropyl)-2-phenylacetamide

| Conditions | Yield |
|---|---|
| Stage #1: 3-bromopropylamine hydrochloride With sodium carbonate In dichloromethane; water at 0℃; for 0.0833333h; Inert atmosphere; Stage #2: phenylacetyl chloride In dichloromethane; water at 0 - 20℃; for 4h; Inert atmosphere; | 100% |

-
-
103-80-0
phenylacetyl chloride

| Conditions | Yield |
|---|---|
| With pyridine at 20℃; for 2h; | 100% |

-
-
103-80-0
phenylacetyl chloride

| Conditions | Yield |
|---|---|
| With triethylamine In dichloromethane at 20℃; for 1h; | 100% |

| Conditions | Yield |
|---|---|
| With triethylamine In dichloromethane at 20℃; for 3h; Inert atmosphere; | 100% |

| Conditions | Yield |
|---|---|
| With pyridine In dichloromethane at 20 - 25℃; for 4h; Inert atmosphere; | 100% |
| With triethylamine In tetrahydrofuran; dichloromethane at 0 - 25℃; Inert atmosphere; | 98% |
Phenylacetyl chloride Specification
The Phenylacetyl chloride with CAS registry number of 103-80-0 is also known as Phenylacetic acid chloride. The IUPAC name is 2-Phenylacetyl chloride. It belongs to product categories of Pharmaceutical Intermediates; Acid Halides; Carbonyl Compounds; Organic Building Blocks. Its EINECS registry number is 203-146-5. In addition, the formula is C8H7ClO and the molecular weight is 154.60. This chemical is a colourless liquid that soluble in water and ether. It may destroy living tissue on contact and should be sealed in ventilated, cool, dry place away from oxidants, alkali, alcohols and water. What's more, this chemical is used as intermediates for pharmaceuticals, pesticides, spices and organic synthesis.
Physical properties about Phenylacetyl chloride are: (1)ACD/LogP: 1.628; (2)ACD/LogD (pH 5.5): 1.63; (3)ACD/LogD (pH 7.4): 1.63; (4)ACD/BCF (pH 5.5): 10.17; (5)ACD/BCF (pH 7.4): 10.17; (6)ACD/KOC (pH 5.5): 183.07; (7)ACD/KOC (pH 7.4): 183.07; (8)#H bond acceptors: 1; (9)#Freely Rotating Bonds: 2; (10)Index of Refraction: 1.535; (11)Molar Refractivity: 40.681 cm3; (12)Molar Volume: 130.755 cm3; (13)Polarizability: 16.127 10-24cm3; (14)Surface Tension: 39.2970008850098 dyne/cm; (15)Density: 1.182 g/cm3; (16)Flash Point: 89.971 °C; (17)Enthalpy of Vaporization: 45.51 kJ/mol; (18)Boiling Point: 218.704 °C at 760 mmHg; (19)Vapour Pressure: 0.123999997973442 mmHg at 25°C
Preparation of Phenylacetyl chloride: it is prepared by reaction of benzeneacetic acid with thionyl chloride. The reaction releases sulfur dioxide and hydrogen chloride gas at room temperature. When reaction is complete, the reaction mixture is heated to 30-40 °C for 25 hours and benzene is added. After steaming volatile matter, product is obtained by collecting distillate at 55-57 °C (0.133kPa). The yield is about 80-85%.
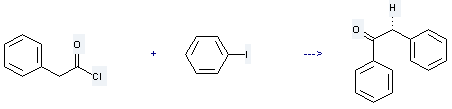
Uses of Phenylacetyl chloride: it is used to produce 1,2-diphenyl-ethanone by reaction with iodobenzene. The reaction occurs with reagent NBu4BF4, catalyst NiBr2bpy and solvent acetonitrile with ambient temperature. The yield is about 35%.
When you are using this chemical, please be cautious about it. As a chemical, it is irritating to respiratory system and reacts violently with water. It can cause burns. During using it, wear suitable protective clothing, gloves and eye/face protection. Avoid contact with eyes. After using it, take off immediately all contaminated clothing. In case of contact with eyes, rinse immediately with plenty of water and seek medical advice. If accident happens or you feel unwell seek medical advice immediately.
You can still convert the following datas into molecular structure:
1. Canonical SMILES: C1=CC=C(C=C1)CC(=O)Cl
2. InChI: InChI=1S/C8H7ClO/c9-8(10)6-7-4-2-1-3-5-7/h1-5H,6H2
3. InChIKey: VMZCDNSFRSVYKQ-UHFFFAOYSA-N
About|Contact|Cas|Product Name|Molecular|Country|Encyclopedia
Message|New Cas|MSDS|Service|Advertisement|CAS DataBase|Article Data|Manufacturers | Chemical Catalog
©2008 LookChem.com,License: ICP
NO.:Zhejiang16009103
complaints:service@lookchem.com Desktop View