-
Name
VITAMIN A
- EINECS 200-683-7
- CAS No. 68-26-8
- Article Data126
- CAS DataBase
- Density 0.954 g/cm3
- Solubility Practically insoluble inwaterorglycerol; soluble in absolute alcohol,methanol,℃hloroform, ether, fats and oils.
- Melting Point 63.5 ºC
- Formula C20H30O
- Boiling Point 421.2 ºC at 760 mmHg
- Molecular Weight 286.458
- Flash Point 147.3 ºC
- Transport Information UN 1208 3/PG 2
- Appearance yellow to orange crystalline solid
- Safety 36/37-61-62
- Risk Codes R22-38-67-65-62-51/53-48/20-11
-
Molecular Structure
-
Hazard Symbols
 Xn,
Xn, N,
N, F
F
- Synonyms Retinol,all-trans- (8CI);2,4,6,8-Nonatetraen-1-ol, 3,7-dimethyl-9-(2,6,6-trimethyl-1-cyclohexen-1-yl)-,(all-E)-;A-Mulsal;A-Vi-Pel;Acon;Agiolan;Alcovit A;Alphalin;Anatola;Anti-Infective vitamin;Aoral;Apostavit;Aquasol A Parenteral;Avibon;Avitol;Axerophthol;Biosterol;Disatabs Tabs;Dofsol;Epiteliol;Lard Factor;NSC 122759;Oleovitamin a;Ophthalamin;PlivitA;Retinol 50C;Tegosphere VitA;Thalasphere;Veroftal;Vi-Dom-A;Vitamin Aalcohol, all-trans-;Vitamin A1 alcohol;Vitamin A1, all-trans-;Vitpex;all-trans-Retinol;all-trans-Vitamin A;all-trans-Vitamin A alcohol;trans-VitaminA alcohol;
- PSA 20.23000
- LogP 5.51030
Synthetic route
-
-
557-75-5
ethenol

-
-
39668-33-2
(+/-)-7-hydroxy-3.7-dimethyl-1-(2.2.6-trimethyl-cyclohexen-(6)-yl)-nonatetraene-(1.3.5.8)

-
-
68-26-8
RETINOL

| Conditions | Yield |
|---|---|
| With hydrogenchloride In toluene at 50℃; for 1h; Temperature; | 95% |
-
-
50465-60-6
9-(2,6,6-Trimethyl-cyclohex-1-enyl)-5-phenyl-sulphonyl-3,7-dimethyl-1-acetoxy-nona-2,6,8-triene

-
-
68-26-8
RETINOL

| Conditions | Yield |
|---|---|
| With sodium ethanolate In ethanol Heating; | 94% |
-
-
3917-41-7
(2E,4E)-3-methyl-5-(2,6,6-trimethyl-1-cyclohexen-1-yl)-2,4-pentadienal

-
-
53170-97-1
(E)-4-chloro-3-methyl-2-buten-1-ol

-
-
68-26-8
RETINOL

| Conditions | Yield |
|---|---|
| Stage #1: (E)-4-chloro-3-methyl-2-buten-1-ol With triphenylphosphine In methanol at 45℃; for 1h; Stage #2: (2E,4E)-3-methyl-5-(2,6,6-trimethyl-1-cyclohexen-1-yl)-2,4-pentadienal With sodium hydroxide In methanol; water at 45℃; for 1h; Reagent/catalyst; | 90% |
-
-
138922-13-1
tert-Butyl-[(2E,4E,6E,8E)-3,7-dimethyl-9-(2,6,6-trimethyl-cyclohex-1-enyl)-nona-2,4,6,8-tetraenyloxy]-diphenyl-silane

-
-
68-26-8
RETINOL

| Conditions | Yield |
|---|---|
| With tetrabutyl ammonium fluoride In tetrahydrofuran | 84% |

| Conditions | Yield |
|---|---|
| With methyllithium In tetrahydrofuran at -15℃; for 0.75h; Inert atmosphere; | 83% |
| With water | |
| With methyllithium In diethyl ether at -15℃; for 2h; | 600 mg |
-
-
120040-83-7
(E,E)-(5-hydroxy-3-methylpenta-1,3-dienyl)boronic acid

-
-
138846-06-7
2-[(1’E,3’E)-3’-methyl-4’-iodobuta-1’,3’-dien-1’yl]-1,3,3-trimethylcyclohex-1-ene

-
-
68-26-8
RETINOL

| Conditions | Yield |
|---|---|
| With tetrakis(triphenylphosphine) palladium(0); TlOH In tetrahydrofuran for 0.5h; Ambient temperature; | 83% |
-
-
1430344-83-4
(2E,4E)-5-(benzyldimethylsilyl)-3-methylpenta-2,4-dien-1-ol

-
-
138846-06-7
2-[(1’E,3’E)-3’-methyl-4’-iodobuta-1’,3’-dien-1’yl]-1,3,3-trimethylcyclohex-1-ene

-
-
68-26-8
RETINOL

| Conditions | Yield |
|---|---|
| Stage #1: (2E,4E)-5-(benzyldimethylsilyl)-3-methylpenta-2,4-dien-1-ol With tetrabutyl ammonium fluoride In tetrahydrofuran at 0℃; for 0.5h; Hiyama Coupling; Inert atmosphere; Stage #2: 2-[(1’E,3’E)-3’-methyl-4’-iodobuta-1’,3’-dien-1’yl]-1,3,3-trimethylcyclohex-1-ene With tris(dibenzylideneacetone)dipalladium(0) chloroform complex In tetrahydrofuran at 0 - 25℃; for 3.5h; Hiyama Coupling; Inert atmosphere; | 82% |
| Stage #1: (2E,4E)-5-(benzyldimethylsilyl)-3-methylpenta-2,4-dien-1-ol With tetrabutyl ammonium fluoride In tetrahydrofuran at 20℃; for 0.5h; Hiyama Coupling; Inert atmosphere; Darkness; Stage #2: 2-[(1’E,3’E)-3’-methyl-4’-iodobuta-1’,3’-dien-1’yl]-1,3,3-trimethylcyclohex-1-ene With tris(dibenzylideneacetone)dipalladium(0) chloroform complex In tetrahydrofuran for 1h; Hiyama Coupling; Inert atmosphere; Darkness; | 77% |
-
-
118353-70-1
tBDMS-retinol

-
-
68-26-8
RETINOL

| Conditions | Yield |
|---|---|
| With tetrabutyl ammonium fluoride | 80% |
| With tetrabutyl ammonium fluoride In tetrahydrofuran for 2h; Ambient temperature; | 80% |
-
-
127-47-9
Retinol acetate

-
-
57-10-3
1-hexadecylcarboxylic acid

-
A
-
79-81-2
retinyl palmitate

-
B
-
68-26-8
RETINOL

| Conditions | Yield |
|---|---|
| With Novozyme 435 (from Candida antarctica immobilized on acrylic resin); Amberlyst A-21 In toluene at 20℃; for 15h; Product distribution / selectivity; Enzymatic reaction; | A 78% B n/a |

-
-
103905-07-3
1-acetoxy-3,7-dimethyl-8-(tetrahydropyran-2-yl)oxy-9-phenylsulfonyl-9-(2,6,6-trimethyl-1-cyclohexen-1-yl)-2(E),6(E)-nonadiene

-
A
-
2052-63-3
2-cis-Vitamin-A


-
C
-
22737-96-8
11-cis-retinol

-
D
-
68-26-8
RETINOL

| Conditions | Yield |
|---|---|
| With potassium methanolate In cyclohexane at 38℃; for 2h; | A n/a B n/a C n/a D 77% |
| With potassium methanolate In cyclohexane at 38℃; for 2h; Title compound not separated from byproducts; |


| Conditions | Yield |
|---|---|
| With diisobutylaluminium hydride | 76% |
-
-
138842-95-2
trans-tetrahydropyran-2-yl retinyl ether

-
-
68-26-8
RETINOL

| Conditions | Yield |
|---|---|
| With methanol; chloro-trimethyl-silane; water In tetrahydrofuran for 0.0833333h; | 74% |
| With chloro-trimethyl-silane; water In methanol for 0.166667h; Inert atmosphere; | 74% |
| With chloro-trimethyl-silane; water In methanol for 0.166667h; | 74% |

| Conditions | Yield |
|---|---|
| With Novozyme 435 (from Candida antarctica immobilized on acrylic resin); Amberlyst A-21 In toluene at 20℃; for 21h; Product distribution / selectivity; Enzymatic reaction; Sonication; | A 71% B n/a |


| Conditions | Yield |
|---|---|
| With Novozyme 435 (from Candida antarctica immobilized on acrylic resin); Amberlyst A-21 In toluene at 20℃; for 2 - 50h; Product distribution / selectivity; Enzymatic reaction; | A 71% B n/a |
| With Novozyme 435 (from Candida antarctica immobilized on acrylic resin) In toluene at 20 - 50℃; for 1 - 2h; Product distribution / selectivity; Enzymatic reaction; | |
| With Lipozyme TI IM; Amberlyst A-21 In toluene at 20℃; for 45h; Product distribution / selectivity; Enzymatic reaction; |

-
-
112-80-1
cis-Octadecenoic acid

-
-
127-47-9
Retinol acetate

-
A
-
631-88-9
retinyl stearate

-
B
-
68-26-8
RETINOL

| Conditions | Yield |
|---|---|
| With Novozyme 435 (from Candida antarctica immobilized on acrylic resin); Amberlyst A-21 In toluene at 20℃; for 15h; Product distribution / selectivity; Enzymatic reaction; | A 69% B n/a |

-
-
50-00-0
formaldehyd

-
-
75-24-1
trimethylaluminum

-
-
111917-89-6
1-((E,E,E)-3-methyl-1,3,5-octatrien-7-ynyl)-2,6,6-trimethyl-1-cyclohexene

-
-
68-26-8
RETINOL

| Conditions | Yield |
|---|---|
| Multistep reaction.; | 67% |

-
-
138846-06-7
2-[(1’E,3’E)-3’-methyl-4’-iodobuta-1’,3’-dien-1’yl]-1,3,3-trimethylcyclohex-1-ene

-
-
128426-10-8
(2E,4E)-3-methyl-5-(tri-n-butylstannyl)penta-2,4-dien-1-ol

-
-
68-26-8
RETINOL

| Conditions | Yield |
|---|---|
| Stage #1: 2-[(1’E,3’E)-3’-methyl-4’-iodobuta-1’,3’-dien-1’yl]-1,3,3-trimethylcyclohex-1-ene With 1-methyl-pyrrolidin-2-one; tris-(dibenzylideneacetone)dipalladium(0); triphenyl-arsane at 25℃; for 0.166667h; Stille Cross-Coupling (Migita-Kosugi-Stille Coupling); Inert atmosphere; Stage #2: (2E,4E)-5-(tri-n-butylstannyl)-3-methylpenta-2,4-dien-1-ol at 25℃; Stille Cross-Coupling (Migita-Kosugi-Stille Coupling); Inert atmosphere; | 60% |

| Conditions | Yield |
|---|---|
| With diisobutylaluminium hydride | |
| With triethylaluminum | |
| With sodium tetrahydroborate In ethanol Ambient temperature; 2-3 h; |

| Conditions | Yield |
|---|---|
| With lithium aluminium tetrahydride; diethyl ether |

-
-
68-26-8
RETINOL
-
-
3230-76-0, 34255-07-7, 60102-36-5, 62653-03-6
(+/-)-3,7-dimethyl-9-(2,6,6-trimethyl-cyclohex-1-enyl)-nona-2c,4c,7ξ-triene-1,6-diol

-
-
98-88-4
benzoyl chloride

-
-
68-26-8
RETINOL

| Conditions | Yield |
|---|---|
| With benzene weiteres Edukt: N.N-Dimethyl-anilin; ueber mehrere Stufen; | |
| With benzene weiteres Edukt: Chinolin; ueber mehrere Stufen; | |
| With benzene weiteres Edukt: Pyridin; ueber mehrere Stufen; |
-
-
3230-76-0, 34255-07-7, 60102-36-5, 62653-03-6
(+/-)-3,7-dimethyl-9-(2,6,6-trimethyl-cyclohex-1-enyl)-nona-2c,4c,7ξ-triene-1,6-diol

-
-
75-36-5
acetyl chloride

-
-
68-26-8
RETINOL

| Conditions | Yield |
|---|---|
| With benzene weiteres Edukt: Chinolin; ueber mehrere Stufen; | |
| With benzene weiteres Edukt: Pyridin; ueber mehrere Stufen; | |
| With benzene weiteres Edukt: N.N-Dimethyl-anilin; ueber mehrere Stufen; |
-
-
3230-76-0, 34255-07-7, 60102-36-5, 62653-03-6
(+/-)-3,7-dimethyl-9-(2,6,6-trimethyl-cyclohex-1-enyl)-nona-2c,4c,7ξ-triene-1,6-diol

-
-
141-75-3
butyryl chloride

-
-
68-26-8
RETINOL

| Conditions | Yield |
|---|---|
| With benzene weiteres Edukt: Pyridin; ueber mehrere Stufen; | |
| With benzene weiteres Edukt: N.N-Dimethyl-anilin; ueber mehrere Stufen; | |
| With benzene weiteres Edukt: Chinolin; ueber mehrere Stufen; |
-
-
3230-76-0, 34255-07-7, 60102-36-5, 62653-03-6
(+/-)-3,7-dimethyl-9-(2,6,6-trimethyl-cyclohex-1-enyl)-nona-2c,4c,7ξ-triene-1,6-diol

-
-
112-67-4
n-hexadecanoyl chloride

-
-
68-26-8
RETINOL

| Conditions | Yield |
|---|---|
| With benzene weiteres Edukt: N.N-Dimethyl-anilin; ueber mehrere Stufen; | |
| With benzene weiteres Edukt: Chinolin; ueber mehrere Stufen; | |
| With benzene weiteres Edukt: Pyridin; ueber mehrere Stufen; |
-
-
83802-77-1
retinoyl fluoride

-
-
68-26-8
RETINOL

| Conditions | Yield |
|---|---|
| With lithium aluminium tetrahydride Yield given; |
-
-
103905-07-3, 105615-50-7
Acetic acid (2Z,6E)-9-benzenesulfonyl-3,7-dimethyl-8-(tetrahydro-pyran-2-yloxy)-9-(2,6,6-trimethyl-cyclohex-1-enyl)-nona-2,6-dienyl ester

-
A
-
2052-63-3
2-cis-Vitamin-A

-
B
-
17706-49-9
11,13-Di-cis-vitamin A

-
C
-
29444-25-5
(9Z,13Z)-retinol

-
D
-
68-26-8
RETINOL

| Conditions | Yield |
|---|---|
| With potassium methanolate In cyclohexane at 38℃; for 2h; Yield given. Title compound not separated from byproducts; | |
| With potassium methanolate In cyclohexane at 38℃; for 2h; Title compound not separated from byproducts; |

-
-
103905-07-3
1-acetoxy-3,7-dimethyl-8-(tetrahydropyran-2-yl)oxy-9-phenylsulfonyl-9-(2,6,6-trimethyl-1-cyclohexen-1-yl)-2(E),6(E)-nonadiene


-
B
-
22737-96-8
11-cis-retinol

-
C
-
68-26-8
RETINOL

| Conditions | Yield |
|---|---|
| With potassium methanolate In cyclohexane at 38℃; for 2h; |


| Conditions | Yield |
|---|---|
| In hexane at 35℃; for 1h; Solvent; Temperature; | 99.5% |
| In hexane at 35℃; for 1h; | 99.5% |
| With triethylamine In hexane at 25℃; for 24h; Inert atmosphere; Darkness; | 76% |

| Conditions | Yield |
|---|---|
| In toluene at 35℃; for 1h; | 99% |
| With pyridine In 1,2-dichloro-ethane |

| Conditions | Yield |
|---|---|
| With aluminum oxide In Petroleum ether at 40℃; for 2h; | 98.73% |

| Conditions | Yield |
|---|---|
| With toluene-4-sulfonic acid In cyclohexane at 70℃; for 2h; | 98% |

| Conditions | Yield |
|---|---|
| With aluminum oxide In Petroleum ether at 40℃; for 2h; Solvent; | 97.62% |

| Conditions | Yield |
|---|---|
| With aluminum oxide In Petroleum ether at 40℃; for 2h; | 97.48% |

| Conditions | Yield |
|---|---|
| With aluminum oxide In Petroleum ether at 40℃; for 2h; | 97.09% |

| Conditions | Yield |
|---|---|
| With aluminum oxide In Petroleum ether at 40℃; for 2h; | 97.01% |

| Conditions | Yield |
|---|---|
| Novozym 435 In toluene at 20℃; for 26h; Inert atmosphere; | 97% |

| Conditions | Yield |
|---|---|
| With aluminum oxide In Petroleum ether at 40℃; for 2h; | 96.85% |

| Conditions | Yield |
|---|---|
| With aluminum oxide In Petroleum ether at 40℃; for 2h; | 96.73% |

| Conditions | Yield |
|---|---|
| at 60℃; under 0.750075 Torr; for 3h; Temperature; Large scale; | 94% |

| Conditions | Yield |
|---|---|
| With triethylamine In dichloromethane | 92% |

| Conditions | Yield |
|---|---|
| With tris(triphenylphosphine)ruthenium(II) chloride; oxygen; bis-(3-methyl-1-imidazolyl)ethylene tetrafluoroborate In hexane at 50℃; under 760.051 Torr; for 3h; Reagent/catalyst; Darkness; | 90.2% |
| Multi-step reaction with 2 steps 1: MnO2 / CH2Cl2 / 2 h / Ambient temperature 2: 80 percent / AgO, NaCN / methanol / 18 h / Ambient temperature View Scheme | |
| Multi-step reaction with 2 steps 1: 90 percent / MnO2 / CH2Cl2 / Ambient temperature 2: AgO / CNNa / methanol View Scheme |

| Conditions | Yield |
|---|---|
| With manganese(IV) oxide In dichloromethane Ambient temperature; | 90% |
| With manganese(IV) oxide; sodium carbonate In dichloromethane for 4h; Inert atmosphere; | 90% |
| With manganese(IV) oxide | 90% |
-
-
4233-33-4
4-Phenyl-1,2,4-triazolidine-3,5-dione

-
-
68-26-8
RETINOL

-
-
135154-45-9
5-Hydroxymethyl-6-methyl-8-[(1E,3E)-2-methyl-4-(2,6,6-trimethyl-cyclohex-1-enyl)-buta-1,3-dienyl]-2-phenyl-5,8-dihydro-[1,2,4]triazolo[1,2-a]pyridazine-1,3-dione

| Conditions | Yield |
|---|---|
| In dichloromethane at -78℃; | 90% |
-
-
68-26-8
RETINOL

-
-
132788-52-4
4-<2-(6,7-dimethoxy-4-methyl-3-oxo-3,4-dihydroquinoxalyl)ethyl>-1,2,4-triazoline-3,5-dione

-
-
135154-50-6
2-[2-(6,7-Dimethoxy-4-methyl-3-oxo-3,4-dihydro-quinoxalin-2-yl)-ethyl]-5-hydroxymethyl-6-methyl-8-[(1E,3E)-2-methyl-4-(2,6,6-trimethyl-cyclohex-1-enyl)-buta-1,3-dienyl]-5,8-dihydro-[1,2,4]triazolo[1,2-a]pyridazine-1,3-dione

| Conditions | Yield |
|---|---|
| In dichloromethane at -78℃; | 90% |
| In dichloromethane at 0℃; for 1h; |

| Conditions | Yield |
|---|---|
| With manganese(IV) oxide In dichloromethane for 12h; Inert atmosphere; Fluorescence light; | A 8% B 90% |

| Conditions | Yield |
|---|---|
| With dicyclohexyl-carbodiimide In Petroleum ether at 50℃; | 89% |
| With dicyclohexyl-carbodiimide In Petroleum ether at 50℃; | 89% |
| With dicyclohexyl-carbodiimide In Petroleum ether at 50℃; | 89% |

| Conditions | Yield |
|---|---|
| With dmap; triethylamine In dichloromethane for 12h; Ambient temperature; | 88% |
| With dmap In dichloromethane at 20℃; | 85% |
| With dmap; triethylamine In dichloromethane at 20℃; for 12h; | 3.4 g |
| With 1-pentyl-3-methylimidazolium nitrate at 50℃; for 3h; Darkness; Inert atmosphere; Green chemistry; |
-
-
68-26-8
RETINOL

-
-
630-19-3
pivalaldehyde

-
A
-
75-84-3
2,2-dimethyl-propanol-1

-
B
-
116-31-4
all-trans-Retinal

| Conditions | Yield |
|---|---|
| With aluminum isopropoxide In water | A n/a B 87% |
| With aluminum isopropoxide In water |

-
-
5497-67-6
2,2-dimethyl-4-pentenal

-
-
68-26-8
RETINOL

-
A
-
3420-42-6
2,2-dimethylpent-4-en-1-ol

-
B
-
116-31-4
all-trans-Retinal

| Conditions | Yield |
|---|---|
| With aluminum isopropoxide In water | A n/a B 87% |


| Conditions | Yield |
|---|---|
| In methanol at 20℃; for 1h; | 86% |
Retinol Chemical Properties
The Molecular Structure of Vitamin A (CAS NO.68-26-8):
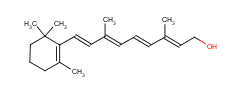
Empirical Formula: C20H30O
Molecular Weight: 286.4516
IUPAC Name: (2E,4E,6E,8E)-3,7-Dimethyl-9-(2,6,6-trimethylcyclohexen-1-yl)nona-2,4,6,8-tetraen-1-ol
Appearance: Yellow-Orange Powder
Product Categories: Intermediates & Fine Chemicals;Pharmaceuticals;Retinoids;Intracellular receptor
Nominal Mass: 286
Average Mass: 286.4516
Monoisotopic Mass: 286.229666
Index of Refraction: 1.549
Molar Refractivity: 95.49 cm3
Molar Volume: 300 cm3
Surface Tension: 36.6 dyne/cm
Density: 0.954 g/cm3
Flash Point: 147.3 °C
Enthalpy of Vaporization: 77.99 kJ/mol
Boiling Point: 421.2 °C at 760 mmHg
Vapour Pressure: 7.35E-09 mmHg at 25°C
Stability: Stable, but light and air sensitive. Incompatible with strong acids, strong oxidizing agents
InChI: InChI=1/C20H30O/c1-16(8-6-9-17(2)13-15-21)11-12-19-18(3)10-7-14-20(19,4)5/h6,8-9,11-13,21H,7,10,14-15H2,1-5H3/b9-6+,12-11+,16-8+,17-13+
Smiles: C=1(C(CCCC1C)(C)C)\C=C\C(=C\C=C\C(=C\CO)C)C
Melting Point: 63.5 °C
log P (octanol-water): 5.68
Water Solubility: 0.671 mg/L at 25 °C
Henry's Law Constant: 1.16E-04 atm-m3/mole at 25 °C
Atmospheric OH Rate Constant: 4.00E-10 cm3/molecule-sec at 25 °C
Retinol History
Vitamin A (CAS NO.68-26-8) was first synthesized in 1947 by two Dutch chemists, David Adriaan van Dorp and Jozef Ferdinand Arens. The discovery of vitamin A may have stemmed from research dating back to 1906, indicating that factors other than carbohydrates, proteins, and fats were necessary to keep cattle healthy.By 1917 one of these substances was independently discovered by Elmer McCollum at the University of Wisconsin-Madison, and Lafayette Mendel and Thomas Burr Osborne at Yale University. Since "water-soluble factor B" ( Vitamin B ) had recently been discovered, the researchers chose the name "fat-soluble factor A" (vitamin A).
Retinol Uses
Vitamin A (CAS NO.68-26-8) is important in vision and bone growth,and appears to function in maintaining normal skin health.It is used in medicine and feed industry.
Retinol Toxicity Data With Reference
| Organism | Test Type | Route | Reported Dose (Normalized Dose) | Effect | Source |
|---|---|---|---|---|---|
| child | TDLo | oral | 912ku/kg/2Y-I (912000iu/kg) | SENSE ORGANS AND SPECIAL SENSES: OTHER: EYE LIVER: OTHER CHANGES SKIN AND APPENDAGES (SKIN): "DERMATITIS, OTHER: AFTER SYSTEMIC EXPOSURE" | New England Journal of Medicine. Vol. 294, Pg. 805, 1976. |
| child | TDLo | oral | 5475ku/kg/1Y- (5475000iu/kg) | LUNGS, THORAX, OR RESPIRATION: PLEURAL EFFUSION GASTROINTESTINAL: OTHER CHANGES MUSCULOSKELETAL: OTHER CHANGES | Clinical Pediatrics Vol. 21, Pg. 435, 1982. |
| infant | LDLo | oral | 442ku/kg/11D- (442000iu/kg) | BEHAVIORAL: ANOREXIA (HUMAN BEHAVIORAL: CHANGES IN MOTOR ACTIVITY (SPECIFIC ASSAY) KIDNEY, URETER, AND BLADDER: "CHANGES IN TUBULES (INCLUDING ACUTE RENAL FAILURE, ACUTE TUBULAR NECROSIS)" | Archives of Pathology and Laboratory Medicine. Vol. 108, Pg. 838, 1984. |
| man | TDLo | oral | 1217ku/kg/1Y- (1217000iu/kg) | LIVER: "HEPATITIS, FIBROUS (CIRRHOSIS, POST-NECROTIC SCARRING)" BLOOD: HEMORRHAGE BLOOD: CHANGES IN SPLEEN | Liver Annual. Vol. 12, Pg. 381, 1992. |
| mouse | LD50 | intraperitoneal | 1510mg/kg (1510mg/kg) | Journal of the American Academy of Dermatology. Vol. 6, Pg. 652, 1982. | |
| mouse | LD50 | oral | 1510mg/kg (1510mg/kg) | "The Retinoids, Vol.2," Sporn, M.B., et al., eds., New York, Academic Press, Inc., 1984Vol. 2, Pg. 287, 1984. | |
| rat | LD50 | oral | 2gm/kg (2000mg/kg) | Acta Dermato-Venereologica, Supplementum. Vol. 74, Pg. 29, 1975. | |
| women | TDLo | oral | 91000iu/kg/26 (91000iu/kg) | BEHAVIORAL: ANOREXIA (HUMAN LIVER: "JAUNDICE, OTHER OR UNCLASSIFIED" SKIN AND APPENDAGES (SKIN): "DERMATITIS, OTHER: AFTER SYSTEMIC EXPOSURE" | American Journal of Medicine. Vol. 97, Pg. 523, 1994. |
Retinol Consensus Reports
Reported in EPA TSCA Inventory. EPA Genetic Toxicology Program.
Retinol Safety Profile
Moderately toxic by ingestion. Human teratogenic effects by ingestion: developmental abnormalities of the craniofacial area and urogenital system. An experimental teratogen. Experimental reproductive effects. Human mutation data reported. When heated to decomposition it emits acrid smoke and irritating fumes.
Hazard Codes:  Xn
Xn N
N F
F
Risk Statements: 22-38-67-65-62-51/53-48/20-11
R22: Harmful if swallowed
R38: Irritating to skin
R67: Vapours may cause drowsiness and dizziness
R65: Harmful: may cause lung damage if swallowed
R62: Risk of impaired fertility
R51/53: Toxic to aquatic organisms, may cause long-term adverse effects in the aquatic environment
R48/20: Harmful: danger of serious damage to health by prolonged exposure through inhalation
R11: Highly flammable
Safety Statements: 36/37-61-62
S36/37: Wear suitable protective clothing and gloves
S61: Avoid release to the environment. Refer to special instructions / safety data sheets
S62: If swallowed, do not induce vomitting; seek medical advice immediately and show this container or label
RIDADR: UN 1208 3/PG 2
WGK Germany: 3
RTECS: VH6750000
F: 8-10-16-23
F 8: Photosensitive
F 10: Keep under argon
F 16: Decomposes easily
F 23: Sensitive to air
Retinol Specification
Vitamin A (CAS NO.68-26-8) is also called as Retinol [INN:BAN] ; (all-E)-3,7-Dimethyl-9-(2,6,6-trimethyl-1-cyclohexen-1-yl)-2,4,6,8-nonatetraen-1-ol ; 2,4,6,8-Nonatetraen-1-ol, 3,7-dimethyl-9-(2,6,6-trimethyl-1-cyclohexen-1-yl)-, (all-E)- ; 3,7-Dimethyl-9-(2,6,6-trimethyl-1-cyclchexen-1-yl)-2,4,6,8-nonatetraen-1-ol ; 3,7-Dimethyl-9-(2,6,6-trimethyl-1-cyclohexen-1-yl)-2,4,6,8-nonatetraen-1-ol, (all-E)- ; A-Mulsal ; A-Sol ; A-Vi-Pel ; A-Vitan ; ACON ; ATAV ; Afaxin ; Agiolan ; Agoncal ; Alcovit A ; All-trans retinol ; Alphalin ; Alphasterol ; Anatola ; Anatola A ; Anti-infective vitamin ; Antixerophthalmic vitamin ; Antixerophthalmisches Vitamin ; Aoral ; Apexol ; Apostavit ; Aquasol A ; Aquasynth ; Atars ; Avibon ; Avita ; Avitol ; Axerol ; Axerophthol ; Axerophtholum ; BRN 0403040 ; Bentavit A ; Biosterol ; Chocola A ; Del-VI-A ; Disatabs Tabs ; Dofsol ; Dohyfral A ; EINECS 200-683-7 ; Epiteliol ; HI-A-Vita ; HSDB 815 ; Homagenets Aoral ; Lard factor ; Myvpack ; NSC 122759 ; Nio-A-let ; Oleovitamin A ; Ophthalamin ; Plivit A ; Prepalin ; Retinol ; Retinol, all trans- ; Retinolo ; Retinolo [DCIT] ; Retinolum ; Retinolum [INN-Latin] ; Retrovitamin A ; Sehkraft A ; Solu-A ; Super A ; Testavol ; Testavol S ; UNII-81G40H8B0T ; UNII-G2SH0XKK91 ; VI-alpha ; Vaflol ; Vafol ; Veroftal ; Vi-Dom-A ; Vi-alpha ; Vi-alpha; Vi-alpha ; Vio-A ; Vitamin A alcohol (VAN) ; Vitamin A alcohol, all-trans- ; Vitamin A1 ; Vitamin A1 alcohol ; Vitamin A1 alcohol, all trans ; Vitamin A1, all-trans- ; Wachstumsvitamin ; all-trans-Retinol ; all-trans-Retinyl alcohol ; all-trans-Vitamin A ; all-trans-Vitamin A alcohol ; 3,7-Dimethyl-9-(2,6,6-trimethyl-1-cyclohexen-1-yl)-2,4,6,8-nonate-traen-1-ol ; Alcohol 9,13-dimethyl-7-(1,1,5-trimethyl-6-cyclohexen-5-yl)-7,9,11,13-nonatetraen-15-ol .
Related Products
- Retinol
- Retinol palmitate
- Retinol, 13-cis-
- 68269-83-0
- 682732-81-6
- 682748-53-4
- 68274-97-5
- 68275-05-8
- 682757-49-9
- 68277-01-0
- 68278-23-9
- 682802-92-2
- 682803-80-1
Hot Products
About|Contact|Cas|Product Name|Molecular|Country|Encyclopedia
Message|New Cas|MSDS|Service|Advertisement|CAS DataBase|Article Data|Manufacturers | Chemical Catalog
©2008 LookChem.com,License: ICP
NO.:Zhejiang16009103
complaints:service@lookchem.com Desktop View