-
Name
Tetrabutyl ammonium chloride
- EINECS 214-195-7
- CAS No. 1112-67-0
- Article Data21
- CAS DataBase
- Density 0.98
- Solubility soluble in water
- Melting Point 83-86 °C
- Formula C16H36ClN
- Boiling Point
- Molecular Weight 277.922
- Flash Point >110°C
- Transport Information
- Appearance white crystals, granules or powder
- Safety 26-37/39-36
- Risk Codes 36/37/38
-
Molecular Structure
-
Hazard Symbols
 Xi
Xi
- Synonyms 1-Butanaminium,N,N,N-tributyl-, chloride (9CI);Ammonium, tetrabutyl-, chloride (8CI);Tetrabutylammonium chloride (6CI);N,N,N-Tributyl-1-butanaminium chloride;TBAC 100;Tetra-n-butylammonium chloride;1-Butanaminium, N,N,N-tributyl-, chloride;1-Butanaminium, N,N,N-tributyl-, chloride (1:1);N,N,N-Tributyl-1-butanaminium chloride;N,N,N-Tributylbutan-1-aminium chloride;Tetrabutylammonium Chloride;
- PSA 0.00000
- LogP 2.00760
Synthetic route

| Conditions | Yield |
|---|---|
| In ethanol; dichloromethane soln. of NMe4Cl in EtOH added to a soln. of (n-Bu4N)2(OsO2(CN)4) in CH2Cl2; pptd. Os complex recrystd. from CH3CN; | A 80% B n/a |


| Conditions | Yield |
|---|---|
| In ethanol; dichloromethane soln. of Me4NCl in EtOH added to a soln. of (n-Bu4N)2(Os(18)O2(CN)4) in CH2Cl2; pptd. Os complex recrystd. from CH3CN; | A 70% B n/a |


| Conditions | Yield |
|---|---|
| With Zn In tetrahydrofuran mixture of Mo-compound, Zn metal dust and trimethylphosphine was stirred at room temp. in THF under Ar for 36 h; filtn., the ppt. was treated with acetone, filtn., the filtrate was layered with hexane, crystn. after several days of standing; | A 63% B n/a |

-
-
76634-95-2
3-chloro-2-(trimethylsilyloxy)prop-1-ene

-
B
-
1112-67-0
tetrabutyl-ammonium chloride

| Conditions | Yield |
|---|---|
| In dichloromethane byproducts: CO; Stirring of mixt. at room temp. for 2 h, during this period, one molar equiv. of CO to TBAFe evolved; Evapn. in vac., chromy. (silica gel, pentane).; | A 57% B n/a |


| Conditions | Yield |
|---|---|
| With hydrogenchloride In methanol; water | 57% |
| With [(Cy3P)2Pd(Cl)Ph]; water In tetrahydrofuran at 20℃; Equilibrium constant; Inert atmosphere; Sealed vial; |

| Conditions | Yield |
|---|---|
| In ethanol; dichloromethane soln. of Me4NCl in EtOH added to a soln. of (n-Bu4N)2(OsO2((13)CN)4) in CH2Cl2; pptd. Os complex isolated; | A 50% B n/a |

-
-
111697-70-2
1,3-dichloro-2-trimethylsiloxy-1-propene

-
B
-
1112-67-0
tetrabutyl-ammonium chloride

| Conditions | Yield |
|---|---|
| In dichloromethane byproducts: CO; Stirring of mixt. at room temp. for 2 h.; Evapn. in vac., chromy. (silica gel, pentane).; | A 37% B n/a |

-
-
1923-70-2
tetrabutylammonium perchlorate

-
-
554-68-7
triethylamine hydrochloride

-
A
-
1112-67-0
tetrabutyl-ammonium chloride

-
B
-
14999-75-8
Triethylammonium perchlorate

| Conditions | Yield |
|---|---|
| In chloroform at 25℃; Equilibrium constant; |


| Conditions | Yield |
|---|---|
| With octachlorocyclotetraphosphazene In chloroform at 24.9℃; Rate constant; var. solvents; |

| Conditions | Yield |
|---|---|
| With 2,2,4,4,6,6-hexachloro-1,3,5-triaza-2,4,6-triphosphorine In chloroform at 24.9℃; Rate constant; var. solvents; |
-
-
3002-49-1
tetrabutylammonium 2,4-dinitrophenolate

-
A
-
1112-67-0
tetrabutyl-ammonium chloride

-
B
-
157033-41-5
2,2,4,4,6-Pentachloro-6-(2,4-dinitro-phenoxy)-2λ5,4λ5,6λ5-[1,3,5,2,4,6]triazatriphosphinine

| Conditions | Yield |
|---|---|
| With 2,2,4,4,6,6-hexachloro-1,3,5-triaza-2,4,6-triphosphorine; 4-nitro-phenol; buffer pH 9.18 In chlorobenzene at 24.9℃; Rate constant; Equilibrium constant; other proton donors, var. pH; |

| Conditions | Yield |
|---|---|
| lithium fluoride at 20℃; Rate constant; Equilibrium constant; other salts as catalysts; |

-
A
-
14618-10-1
5-Nitrobenz<1,6-d>-3H-1,2-oxathiole S,S-dioxide

-
B
-
1112-67-0
tetrabutyl-ammonium chloride

| Conditions | Yield |
|---|---|
| In nitrobenzene Equilibrium constant; other solvent; |

-
A
-
1013937-44-4
N,N''-cis-1,2-cyclohexanediylbis[N'-phenylthiourea]

-
B
-
1112-67-0
tetrabutyl-ammonium chloride

| Conditions | Yield |
|---|---|
| In dimethylsulfoxide-d6 Equilibrium constant; Further Variations:; Solvents; |

| Conditions | Yield |
|---|---|
| In dimethylsulfoxide-d6 Equilibrium constant; |

| Conditions | Yield |
|---|---|
| In dimethylsulfoxide-d6 Equilibrium constant; |

-
A
-
1013937-46-6
C31H26F6N4S2

-
B
-
1112-67-0
tetrabutyl-ammonium chloride

| Conditions | Yield |
|---|---|
| In dimethylsulfoxide-d6 Equilibrium constant; |

-
A
-
1013937-48-8
C36H48N6O4S2

-
B
-
1112-67-0
tetrabutyl-ammonium chloride

| Conditions | Yield |
|---|---|
| In dimethylsulfoxide-d6 Equilibrium constant; |

-
-
1643-19-2
tetrabutylammomium bromide

-
A
-
1112-67-0
tetrabutyl-ammonium chloride

-
B
-
7558-02-3
potassium bromide

| Conditions | Yield |
|---|---|
| In toluene Kinetics; no ion exchange between solid KCl and Bu4NBr in toluene (50-105°C); | A 0% B 0% |

-
-
116882-28-1
carbonato{2,2'-bipyridyl}{1,2-bis(diphenylphosphino)ethano}osmium(II)

-
-
1112-67-0
tetrabutyl-ammonium chloride

| Conditions | Yield |
|---|---|
| With hydrogenchloride In acetone | >99 |

-
-
125876-20-2
tetra-n-butylammonium tetrachlorobis(tetrahydrofuran)titanate(III) tetrahydrofuran

-
B
-
1112-67-0
tetrabutyl-ammonium chloride

| Conditions | Yield |
|---|---|
| In tetrahydrofuran byproducts: HCl, C4H7OCl; Irradiation (UV/VIS); N2 atmosphere; influence of light (1 month); |

-
A
-
1195974-31-2
1-(2-((S)-2-(3-((1R,2R)-2-(2,5-dimethyl-1H-pyrrol-1-yl)cyclohexyl)thioureido)-3,3-dimethylbutoxy)-5-(trifluoromethyl)phenyl)-3-ethylurea

-
B
-
1112-67-0
tetrabutyl-ammonium chloride

| Conditions | Yield |
|---|---|
| In chloroform-d1 at -15℃; Equilibrium constant; |

-
A
-
1195974-41-4
1-((S)-3,3-dimethyl-1-(4-(trifluoromethyl)phenoxy)butan-2-yl)-3-((1R,2R)-2-(2,5-dimethyl-1Hpyrrol-1-yl)cyclohexyl)thiourea

-
B
-
1112-67-0
tetrabutyl-ammonium chloride

| Conditions | Yield |
|---|---|
| In chloroform-d1 at -15℃; Equilibrium constant; |
-
-
1242065-15-1
C16H36N(1+)*C30H33N3O12*Cl(1-)

-
A
-
1242065-09-3
C30H33N3O12

-
B
-
1112-67-0
tetrabutyl-ammonium chloride

| Conditions | Yield |
|---|---|
| In [D3]acetonitrile at 23℃; Equilibrium constant; |
-
-
1186217-02-6
C16H36N(1+)*C72H72N4O9*Cl(1-)

-
A
-
1186216-97-6
C72H72N4O9

-
B
-
1112-67-0
tetrabutyl-ammonium chloride

| Conditions | Yield |
|---|---|
| In dimethylsulfoxide-d6; [D3]acetonitrile at 24.84℃; Equilibrium constant; |
-
-
1186217-03-7
C16H36N(1+)*C36H48N4O9*Cl(1-)

-
A
-
1186216-98-7
C36H48N4O9

-
B
-
1112-67-0
tetrabutyl-ammonium chloride

| Conditions | Yield |
|---|---|
| In dimethylsulfoxide-d6; [D3]acetonitrile at 24.84℃; Equilibrium constant; |

-
A
-
1112-67-0
tetrabutyl-ammonium chloride

-
B
-
124764-08-5
N,N',N''-(nitrilotris(ethane-2,1-diyl))triacetamide

| Conditions | Yield |
|---|---|
| In dimethylsulfoxide-d6; [D3]acetonitrile at 24.84℃; Equilibrium constant; |
-
-
1186217-04-8
C16H36N(1+)*C21H42N4O3*Cl(1-)

-
A
-
1186217-01-5
C21H42N4O3

-
B
-
1112-67-0
tetrabutyl-ammonium chloride

| Conditions | Yield |
|---|---|
| In dimethylsulfoxide-d6; [D3]acetonitrile at 24.84℃; Equilibrium constant; |
-
-
1186217-05-9
C16H36N(1+)*C27H54N4O3*Cl(1-)

-
A
-
1112-67-0
tetrabutyl-ammonium chloride

-
B
-
239116-99-5
heptanoic acid {2-[bis-(2-heptanoylamino-ethyl)-amino]-ethyl}-amide

| Conditions | Yield |
|---|---|
| In dimethylsulfoxide-d6; [D3]acetonitrile at 24.84℃; Equilibrium constant; |
-
-
1112-67-0
tetrabutyl-ammonium chloride

-
-
148305-65-1, 148305-68-4, 148305-70-8
tetra-n-butyl-ammonium dihydrogentrifluoride

| Conditions | Yield |
|---|---|
| With potassium hydrogen bifluoride In dichloromethane for 0.5h; Ambient temperature; | 100% |
-
-
1112-67-0
tetrabutyl-ammonium chloride

-
-
930-69-8
sodium thiophenolate

-
-
4670-62-6
tetra-N-butylammonium benzenethiolate

| Conditions | Yield |
|---|---|
| In tetrahydrofuran for 4h; Inert atmosphere; | 100% |
-
-
1112-67-0
tetrabutyl-ammonium chloride

-
-
286471-91-8
phosphonocrotonate

| Conditions | Yield |
|---|---|
| In diethyl ether; water pH=7; Addition; | 100% |
-
-
1112-67-0
tetrabutyl-ammonium chloride

-
-
7782-50-5
chlorine

-
-
17769-64-1
tetra-n-butylammonium tetrachloroaurate(III)

| Conditions | Yield |
|---|---|
| In ethanol bubbling of Cl2 into suspn. of gold in soln. of Bu4NBr (in dark, with stirring), warming 60 - 70°C; decantation and cooling to 0°C, crystn.; elem. anal.; | 100% |

-
-
1112-67-0
tetrabutyl-ammonium chloride

-
-
70197-13-6
methyltrioxorhenium(VII)

| Conditions | Yield |
|---|---|
| In diethyl ether addn. of NBu4Cl to a soln. of MeReO3 at room temp.; after 10 min solvent removed, washed the residue with ether, drying in vac.; elem. anal.; | 100% |
-
-
671821-32-2
(C2H5)3NH(1+)*(CH3N(CH2CH2NC6F5)2)MoCl3(1-)=((C2H5)3NH)((CH3N(CH2CH2NC6F5)2)MoCl3)

-
-
1112-67-0
tetrabutyl-ammonium chloride

-
-
282726-71-0
(C4H9)4N(1+)*(CH3N(CH2CH2NC6F5)2)MoCl3(1-)=((C4H9)4N)((CH3N(CH2CH2NC6F5)2)MoCl3)

| Conditions | Yield |
|---|---|
| In tetrahydrofuran | 100% |
| In tetrahydrofuran byproducts: Et3NHCl; under N2; Bu4NCl (1.1 equiv.) added to soln. of Mo complex in THF; stirred for 12 h; filtered through Celite; volatiles removed from filtrate in vac.; dissolved in toluene; filtered; vol. of filtrate reduced in vac.; added dropwise to rapidly stirred pentane; ppt. collected on frit; washed with pentane; dried in vac.; elem. anal.; | 98% |
-
-
202645-79-2
iodophthalocyaninato(2-)thallium(III)

-
-
1112-67-0
tetrabutyl-ammonium chloride

| Conditions | Yield |
|---|---|
| In dichloromethane stirring (1 h, room temp.); filtn., addn. of Et2O, crystn. (24 h), washing (acetone/Et2O 1:3, Et2O),drying (vac.); | 100% |
-
-
71870-09-2
(tetrahydrofuran)(carbonyl)phthalocyaninato(2-)osmium(II)

-
-
1112-67-0
tetrabutyl-ammonium chloride

-
-
186336-60-7
N(C4H9)4(1+)*[Os(CO)(Cl)(C6H4C2N2)4](1-)=[N(C4H9)4][Os(CO)Cl(C6H4C2N2)4]

| Conditions | Yield |
|---|---|
| In tetrahydrofuran 1 h, 293+/-2 K; addn. of n-pentane, washing (H2O), drying (vac.); elem. anal.; | 100% |

| Conditions | Yield |
|---|---|
| In water byproducts: KCl; | 100% |


-
-
1112-67-0
tetrabutyl-ammonium chloride

| Conditions | Yield |
|---|---|
| In acetone refluxing (5 min), pptn.; centrifugation, washing (acetone/Et2O 1/3), drying (vac., over KOH); elem. anal.; | 100% |
-
-
575456-04-1
Fe(3,6,13,17-tetraethyl-2,7,12,18-tetramethylhemiporphycene)I

-
-
1112-67-0
tetrabutyl-ammonium chloride

-
-
575456-02-9
Fe(3,6,13,17-tetraethyl-2,7,12,18-tetramethylhemiporphycene)Cl

| Conditions | Yield |
|---|---|
| In not given 1 equivalent of nBu4NCl added; | 100% |
-
-
1112-67-0
tetrabutyl-ammonium chloride

-
-
127-08-2
potassium acetate

-
-
10534-59-5
tetrabutylammonium acetate

| Conditions | Yield |
|---|---|
| In methanol | 100% |
| In methanol for 24h; Inert atmosphere; | |
| In methanol for 24h; Inert atmosphere; |
-
-
1112-67-0
tetrabutyl-ammonium chloride

-
-
2388-10-5
lithium isopropoxide

-
-
851848-96-9
C16H36N(1+)*C3H7O(1-)

| Conditions | Yield |
|---|---|
| In dichloromethane at 20℃; for 18h; Inert atmosphere; | 100% |

| Conditions | Yield |
|---|---|
| In diethyl ether; water | 100% |


-
-
1112-67-0
tetrabutyl-ammonium chloride

| Conditions | Yield |
|---|---|
| In acetone | 100% |

-
-
1112-67-0
tetrabutyl-ammonium chloride

| Conditions | Yield |
|---|---|
| In acetone | 100% |

-
-
1112-67-0
tetrabutyl-ammonium chloride

| Conditions | Yield |
|---|---|
| In acetone | 100% |

-
-
1112-67-0
tetrabutyl-ammonium chloride

| Conditions | Yield |
|---|---|
| In acetone | 100% |

-
-
1112-67-0
tetrabutyl-ammonium chloride

| Conditions | Yield |
|---|---|
| In acetone | 100% |

-
-
1112-67-0
tetrabutyl-ammonium chloride

| Conditions | Yield |
|---|---|
| In acetone | 100% |

-
-
1112-67-0
tetrabutyl-ammonium chloride

| Conditions | Yield |
|---|---|
| In acetone | 100% |

-
-
1112-67-0
tetrabutyl-ammonium chloride

| Conditions | Yield |
|---|---|
| In acetone | 100% |

-
-
1112-67-0
tetrabutyl-ammonium chloride

| Conditions | Yield |
|---|---|
| In acetone | 100% |

-
-
1112-67-0
tetrabutyl-ammonium chloride

| Conditions | Yield |
|---|---|
| In acetone | 100% |

| Conditions | Yield |
|---|---|
| In acetone | 100% |


| Conditions | Yield |
|---|---|
| In acetone | 100% |


| Conditions | Yield |
|---|---|
| In acetone | 100% |


-
-
1112-67-0
tetrabutyl-ammonium chloride

| Conditions | Yield |
|---|---|
| In acetone | 100% |

-
-
1112-67-0
tetrabutyl-ammonium chloride

| Conditions | Yield |
|---|---|
| Stage #1: 2,5,8-tri(4-pyridyl)1,3-diazaphenalene With sodium methylate In methanol at 20℃; for 0.5h; Inert atmosphere; Stage #2: tetrabutyl-ammonium chloride In methanol at 20℃; for 0.5h; Inert atmosphere; | 100% |

-
-
1112-67-0
tetrabutyl-ammonium chloride

| Conditions | Yield |
|---|---|
| In acetone Inert atmosphere; | 100% |
Tetrabutylammoniumchloride Specification
The 1-Butanaminium, N,N,N-tributyl-, chloride (1:1), with the CAS registry number 1112-67-0 and EINECS registry number 214-195-7, has the systematic name of N,N,N-tributylbutan-1-aminium chloride. And the molecular formula of this chemical is C16H36ClN. It is a kind of light sensitive white crystals, granules or powder, and should be stored under Nitrogen. In addition, it is used as Ion-pair chromatography reagent and phase transfer catalyst.
The physical properties of 1-Butanaminium, N,N,N-tributyl-, chloride (1:1) are as following: (1)ACD/LogP: -1.72; (2)# of Rule of 5 Violations: 0; (3)ACD/LogD (pH 5.5): -1.72; (4)ACD/LogD (pH 7.4): -1.72; (5)ACD/BCF (pH 5.5): 1; (6)ACD/BCF (pH 7.4): 1; (7)ACD/KOC (pH 5.5): 2.78; (8)ACD/KOC (pH 7.4): 2.78; (9)#H bond acceptors: 1; (10)#H bond donors: 0; (11)#Freely Rotating Bonds: 12; (12)Polar Surface Area: 0 Å2.
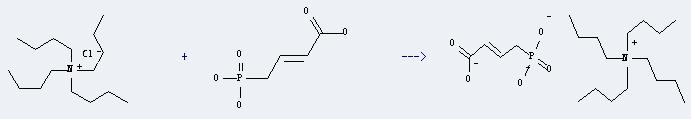
Uses of 1-Butanaminium, N,N,N-tributyl-, chloride (1:1): It can react with 4-phosphono-but-2-enoic acid to produce phosphono crotonic acid tri(tetrabutylammonium) salt. This reaction will need solvent diethyl ether and H2O, and PH 7. And the yield is about 100%.
You should be cautious while dealing with this chemical. It irritates eyes, respiratory system and skin. Therefore, you had better take the following instructions: Wear suitable protective clothing, gloves and eye/face protection, and in case of contacting with eyes, rinse immediately with plenty of water and seek medical advice
You can still convert the following datas into molecular structure:
(1)SMILES: [Cl-].CCCC[N+](CCCC)(CCCC)CCCC
(2)InChI: InChI=1/C16H36N.ClH/c1-5-9-13-17(14-10-6-2,15-11-7-3)16-12-8-4;/h5-16H2,1-4H3;1H/q+1;/p-1
(3)InChIKey: NHGXDBSUJJNIRV-REWHXWOFAB
Related Products
- Tetrabutylammoniumchloride
- 111-27-3
- 111274-98-7
- 111-28-4
- 1112850-40-4
- 11128-96-4
- 11128-98-6
- 1112-91-0
- 11129-12-7
- 11129-15-0
- 11129-18-3
Hot Products
About|Contact|Cas|Product Name|Molecular|Country|Encyclopedia
Message|New Cas|MSDS|Service|Advertisement|CAS DataBase|Article Data|Manufacturers | Chemical Catalog
©2008 LookChem.com,License: ICP
NO.:Zhejiang16009103
complaints:service@lookchem.com Desktop View