-
Name
Tetraethylammonium
- EINECS
- CAS No. 66-40-0
- Density 0.7930 (rough estimate)
- Solubility
- Melting Point 223 °C (decomp)
- Formula C8H20N
- Boiling Point 231°C (rough estimate)
- Molecular Weight 130.254
- Flash Point
- Transport Information
- Appearance
- Safety Poison by intravenous and intraperitoneal routes. When heated to decomposition it emits toxic fumes of NOx and NH3.
- Risk Codes
-
Molecular Structure
- Hazard Symbols
- Synonyms [3H]-Tetraethylammonium cation;teteraethylammonium;N,N,N-Triethylethanaminium;AMMONIUM,TETRAETHYL;Tetramon;tetraethylazanium;TETRAETHYLAMMONIUM ION;[14C]-Tetraethylammonium cation;Tetraethylammonium;Tetrylammonium;Tetraetilammonio [Italian];
- PSA 0.00000
- LogP 1.88280
Tetraethylammonium Chemical Properties
Molecule structure of Tetraethylammonium (CAS NO.66-40-0) :
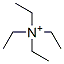
IUPAC Name: tetraethylazanium
Molecular Weight: 130.2511 g/mol
Molecular Formula: C8H20N+
XLogP3-AA: 1.7
Rotatable Bond Count: 4
Exact Mass: 130.159575
MonoIsotopic Mass: 130.159575
Heavy Atom Count: 9
Formal Charge: 1
Complexity: 47.5
Canonical SMILES: CC[N+](CC)(CC)CC
InChI: InChI=1S/C8H20N/c1-5-9(6-2,7-3)8-4/h5-8H2,1-4H3/q+1
InChIKey: CBXCPBUEXACCNR-UHFFFAOYSA-N
Tetraethylammonium Uses
Tetraethylammonium (CAS NO.66-40-0) can be used to alter a compound's solubility by displacing hard acids with this comparatively softer acid. For example, addition of tetraethylammonium bromide to an aqueous solution of a sodium salt may cause it to precipitate from water as the tetraethylammonium salt. The tetraethylammonium salt may also be more lipophilic, dissolving in organic solvents such as chloroform.
In biology, the tetraethylammonium cation is a potassium-selective ion channel blocker. It is used in neurophysiology experiments to block the voltage activated potassium channels involved in the trailing part of the transmission of an action potential along a neuron. Tetraethylammonium is also known to reverse the action of drugs such as tubocurarine, a non-depolarizing blocker. Tetraethylammonium evokes more release of the neurotransmitter, and thus it will reverse the competitive antagonistic block of any drugs belonging to the curare family. Tetraethylammonium salts are used in supercapacitors as electrochemically active species.
Tetraethylammonium Toxicity Data With Reference
| 1. | ipr-rat LD50:115 mg/kg | FRPSAX Farmaco, Edizione Scientifica. 10 (1955),1027. | ||
| 2. | ivn-rat LD50:63 mg/kg | FRPSAX Farmaco, Edizione Scientifica. 10 (1955),1027. | ||
| 3. | ipr-mus LD50:60 mg/kg | FRPSAX Farmaco, Edizione Scientifica. 10 (1955),1027. | ||
| 4. | ivn-mus LD50:36 mg/kg | JMPCAS Journal of Medicinal and Pharmaceutical Chemistry. 3 (1961),167. | ||
| 5. | ivn-dog LD50:55 mg/kg | FRPSAX Farmaco, Edizione Scientifica. 10 (1955),1027. | ||
| 6. | ivn-rbt LD50:72 mg/kg | FRPSAX Farmaco, Edizione Scientifica. 10 (1955),1027. |
Tetraethylammonium Safety Profile
Poison by intravenous and intraperitoneal routes. When heated to decomposition it emits toxic fumes of NOx and NH3.
Tetraethylammonium Specification
Tetraethylammonium (CAS NO.66-40-0) is also called 4-04-00-00331 (Beilstein Handbook Reference) ; BRN 1738225 ; N,N,N-Triethylethanaminium ; Tetraetilammonio ; Tetraetilammonio [Italian] ; Tetramon . Tetraethylammonium (CAS NO.66-40-0) is a quaternary ammonium cation consisting of four ethyl groups attached to a central nitrogen atom.
Related Products
- Tetraethylammonium
- Tetraethylammonium acetate
- Tetraethylammonium borohydride
- Tetraethylammonium bromide
- Tetraethylammonium fluoride dihydrate
- Tetraethylammonium fluoride hydrate
- Tetraethylammonium formate
- Tetraethylammonium hexafluorophosphate
- Tetraethylammonium hydrogensulfate
- Tetraethylammonium hydroxide
- 6640-07-9
- 6640-22-8
- 6640-23-9
- 6640-24-0
- 6640-25-1
- 66402-68-4
- 6640-27-3
- 66403-32-5
- 664-03-9
- 6640-47-7
Hot Products
About|Contact|Cas|Product Name|Molecular|Country|Encyclopedia
Message|New Cas|MSDS|Service|Advertisement|CAS DataBase|Article Data|Manufacturers | Chemical Catalog
©2008 LookChem.com,License: ICP
NO.:Zhejiang16009103
complaints:service@lookchem.com Desktop View