-
Name
Tetraethylammonium bromide
- EINECS 200-769-4
- CAS No. 71-91-0
- Article Data22
- CAS DataBase
- Density 1.397 g/cm3
- Solubility 2795 g/L (25 °C) in water
- Melting Point 285 °C (dec.)(lit.)
- Formula C8H20BrN
- Boiling Point
- Molecular Weight 210.157
- Flash Point
- Transport Information
- Appearance white to light yellow crystalline solid
- Safety 26-36-37/39
- Risk Codes 36/37/38
-
Molecular Structure
-
Hazard Symbols
 Xi
Xi
- Synonyms Ammonium,tetraethyl-, bromide (8CI);Ethanaminium, N,N,N-triethyl-, bromide (9CI);Ethanaminium,N,N,N-triethyl-, bromide (1:1);Beparon;Bromethyl;Etambro;Etamon;Ethylon;Etylon;Sympatektoman;TEA bromide;TEAB;TMD 10;Teamon;Tetranium;Tetrylammonium bromide;
- PSA 0.00000
- LogP -1.11320
Synthetic route
-
-
2509-16-2
trans-2-bromobutenedioic acid dimethyl ester

-
-
121-44-8
triethylamine

-
A
-
71-91-0
tetraethylammonium bromide

-
B
-
996-85-0, 98033-35-3
dimethyl diethylaminofumarate

| Conditions | Yield |
|---|---|
| In acetonitrile for 7h; Heating; | A 98.6% B 87% |


| Conditions | Yield |
|---|---|
| In acetonitrile at 60℃; for 72h; | 98% |

| Conditions | Yield |
|---|---|
| With P(C6H5)3 In methanol Stirring of a suspn. of Mo-compd. and PPh3 (MeOH, 1 h).; Filtn. of pptd. green solid, washing with hot MeOH and benzene, drying (vac.), elem. anal.; | A 60% B n/a C >99 |

-
-
91-22-5
quinoline

-
-
120782-58-3
Et4N{Rh(1,5-cyclooctadiene)Br2}

-
A
-
120782-61-8
{Rh(Br)(1,5-cyclooctadiene)(quinoline)}

-
B
-
71-91-0
tetraethylammonium bromide

| Conditions | Yield |
|---|---|
| In dichloromethane addn. of methanol, elimination of CH2Cl2, pptn. filtered off, washed with methanol, air-dried; elem. anal.; | A 53% B n/a |


| Conditions | Yield |
|---|---|
| With C2H4(P2(C6H5)4) In methanol byproducts: (dppe)S2; Boiling of Mo-compd. with dppe (MeOH, 1 h).; Recrystn. of bright green ppt. from a CH2Cl2/hexane mixt., elem. anal.; | A 48% B n/a |

| Conditions | Yield |
|---|---|
| With Petroleum ether durch Zerlegung des entstandenen Aethylsulfats mit wss. Ba(OH)2; |
-
-
540-49-8
cis+trans-dibromoethylene

-
-
121-44-8
triethylamine

-
A
-
71-91-0
tetraethylammonium bromide

-
B
-
109-89-7
diethylamine

-
-
121-44-8
triethylamine

-
-
108-88-3
toluene

-
-
535-11-5, 41978-69-2
Ethyl 2-bromopropionate

-
A
-
82614-48-0
ethyl 2-diethylaminopropanoate

-
B
-
71-91-0
tetraethylammonium bromide

-
C
-
636-70-4
triethylamine hydrobromide

| Conditions | Yield |
|---|---|
| at 50℃; |

-
-
121-44-8
triethylamine

-
-
535-11-5, 41978-69-2
Ethyl 2-bromopropionate

-
A
-
82614-48-0
ethyl 2-diethylaminopropanoate

-
B
-
71-91-0
tetraethylammonium bromide

-
C
-
636-70-4
triethylamine hydrobromide

| Conditions | Yield |
|---|---|
| at 107℃; |

-
-
20688-29-3
dimethyl 2-bromofumarate

-
-
121-44-8
triethylamine

-
A
-
71-91-0
tetraethylammonium bromide

-
B
-
996-85-0, 98033-35-3
dimethyl diethylaminofumarate

| Conditions | Yield |
|---|---|
| In acetonitrile for 7h; Heating; |

-
-
51575-86-1
dimethyl 2,3-dibromobutane-1,4-dicarboxylate

-
-
121-44-8
triethylamine

-
A
-
71-91-0
tetraethylammonium bromide

-
B
-
996-85-0, 98033-35-3
dimethyl diethylaminofumarate

| Conditions | Yield |
|---|---|
| In acetonitrile for 7h; Heating; |

-
-
121-44-8
triethylamine

-
-
533-68-6
2-bromobutyric acid ethyl ester

-
B
-
79433-52-6
butyric acid pin-2-en-10-yl ester

-
C
-
71-91-0
tetraethylammonium bromide

| Conditions | Yield |
|---|---|
| at 100℃; |

-
-
121-44-8
triethylamine

-
-
535-11-5, 41978-69-2
Ethyl 2-bromopropionate

-
A
-
849585-22-4
LACTIC ACID

-
B
-
71-91-0
tetraethylammonium bromide

| Conditions | Yield |
|---|---|
| nachfolgende Verseifung; |

-
-
110-85-0
piperazine

-
-
77-98-5
tetraethylammonium hydroxide

-
-
79-08-3
bromoacetic acid

-
B
-
71-91-0
tetraethylammonium bromide

| Conditions | Yield |
|---|---|
| In water at 80℃; Title compound not separated from byproducts; |


| Conditions | Yield |
|---|---|
| In acetonitrile for 8h; Heating; Title compound not separated from byproducts; | A 216 mg B n/a |


-
B
-
71-91-0
tetraethylammonium bromide

| Conditions | Yield |
|---|---|
| In trifluoroacetic acid heating Re-compd. with mixt. of trifluoroaceti acid and trifluoroacetic anhydride (1:1) (Ar atmosphere); washing (solvents), drying (vac., KOH); elem. anal.; | A 75-85 B n/a |
-
-
120782-58-3
Et4N{Rh(1,5-cyclooctadiene)Br2}

-
A
-
120782-68-5
Rh(Br)(1,5-cyclooctadiene)(ethylenediamine)

-
B
-
71-91-0
tetraethylammonium bromide

| Conditions | Yield |
|---|---|
| With ethylenediamine In dichloromethane pptn. filtered off, washed with CH2Cl2, air-dried; elem. anal.; |
-
-
120782-58-3
Et4N{Rh(1,5-cyclooctadiene)Br2}

-
A
-
33136-93-5
Rh(Br)(1,5-cyclooctadiene)(triphenylphosphine)

-
B
-
71-91-0
tetraethylammonium bromide

| Conditions | Yield |
|---|---|
| With P(C6H5)3 In methanol; dichloromethane CH2Cl2/MeOH 1:1; elimination of CH2Cl2, filtered off, washed with methanol, air-dried; |
-
-
1392488-19-5
C6H5O(1-)*C6H6O*C8H20N(1+)

-
-
100-39-0
benzyl bromide

-
A
-
946-80-5
(benzyloxy)benzene

-
B
-
71-91-0
tetraethylammonium bromide

-
C
-
108-95-2
phenol

| Conditions | Yield |
|---|---|
| In acetonitrile at 20℃; |


| Conditions | Yield |
|---|---|
| In [D3]acetonitrile at 24.84℃; Equilibrium constant; |
-
-
71-91-0
tetraethylammonium bromide

| Conditions | Yield |
|---|---|
| With sodium hydride 1.) THF, 2.) CH2Cl2; | 100% |
-
-
71-91-0
tetraethylammonium bromide

| Conditions | Yield |
|---|---|
| With sodium hydride 1.) THF, 1 h, 2.) CH2Cl2; | 100% |
-
-
1232003-43-8
1-benzyloxymethyl-9-(4'-β-(dibenzylphosphono)methoxy-2',3'-dideoxy-2',3'-didehydro-β-D-erythrofuranosyl)hypoxanthine

-
-
71-91-0
tetraethylammonium bromide

| Conditions | Yield |
|---|---|
| With 5% Pd(II)/C(eggshell); hydrogen In methanol under 760.051 Torr; | 100% |
-
-
1232003-54-1
C32H33N4O9P

-
-
71-91-0
tetraethylammonium bromide

| Conditions | Yield |
|---|---|
| With 5% Pd(II)/C(eggshell); hydrogen In methanol under 760.051 Torr; | 100% |

| Conditions | Yield |
|---|---|
| Stage #1: 2-pyridylphenyl-biphenyl-2,2'-silane; methyllithium In tetrahydrofuran; diethyl ether at -78 - 20℃; for 0.5h; Stage #2: tetraethylammonium bromide In tetrahydrofuran; diethyl ether at 20℃; | 100% |

-
-
71-91-0
tetraethylammonium bromide

-
-
77-78-1
dimethyl sulfate

-
-
20648-49-1
tetraethylammonium methyl sulfate

| Conditions | Yield |
|---|---|
| In acetonitrile at 20℃; for 0.583333h; | 100% |
-
-
71-91-0
tetraethylammonium bromide

-
-
333-27-7
methyl trifluoromethanesulfonate

-
A
-
74-83-9
methyl bromide

-
B
-
35895-69-3
tetraethylammonium trifluoromethanesulphonate

| Conditions | Yield |
|---|---|
| In acetonitrile at 20℃; for 0.583333h; Temperature; | A n/a B 100% |

-
-
71-91-0
tetraethylammonium bromide

-
-
80-48-8
methyl p-toluene sulfonate

-
-
733-44-8
tetraethylammonium tosylate

| Conditions | Yield |
|---|---|
| In acetonitrile at 20℃; for 0.583333h; | 100% |
-
-
66-27-3
Methyl methanesulfonate

-
-
71-91-0
tetraethylammonium bromide

-
-
16909-18-5
tetraethylammonium methanesulfonate

| Conditions | Yield |
|---|---|
| In acetonitrile at 20℃; for 0.583333h; | 100% |

| Conditions | Yield |
|---|---|
| In toluene; acetonitrile under dry N2, finely divided metal and o-quinone refluxed in toluene (24 h), filtration, addn. of Et4NBr in minimum amount of CH3CN (light-brown solid deposit), stirred (2 h, room temp.), addn. of diethyl ether, pptn.; filtration, evapn.; elem. anal.; | 99% |

-
-
58904-63-5
[Cs][Re(CO)3(η(5)-7,8-C2B9H11)]

-
-
71-91-0
tetraethylammonium bromide

| Conditions | Yield |
|---|---|
| In dichloromethane | 99% |

-
-
71-91-0
tetraethylammonium bromide

| Conditions | Yield |
|---|---|
| In water (H3O)2((Mn(H2O)1.5)3(Re6Se8(CN)6)2)*19H2O was stirred in hot aq. soln. Et4NBr for 2 h; ppt. was filtered, washed with water and air-dried; elem. anal.; | 99% |

-
-
71-91-0
tetraethylammonium bromide

| Conditions | Yield |
|---|---|
| In dichloromethane byproducts: NaBr, water; N2-atmosphere; equimolar amts., room temp.; filtration, solvent removal from filtrate; can be recrystallized (CH2Cl2/hexane); elem. anal.; | 99% |

-
-
71-91-0
tetraethylammonium bromide

| Conditions | Yield |
|---|---|
| In dichloromethane react. mixt. was filtered through Celite, solvent was removed in vacuo; | 99% |

| Conditions | Yield |
|---|---|
| In ammonia aq. NH3; Cu salt and ethylenediamine in aq.NH3 added to soln. of Re complex and Et4NBr in aq.NH3, kept in a closed vessel at room temp. for 2 weeks; filtered, dried, elem. anal.; | 99% |


-
-
1042436-03-2
4,6-bis(4-methoxyphenyl)-thieno[3,4-d]-1,3-dithiol-2-one

-
-
71-91-0
tetraethylammonium bromide

| Conditions | Yield |
|---|---|
| With CH3CH2ONa In tetrahydrofuran (Ar); addn. of EtONa to a soln. of ligand in THF, stirring for 40 min, addn. of ammonium salt, then metal salt, react. for 24 h; filtration, washing with water; elem. anal.; | 99% |


| Conditions | Yield |
|---|---|
| With KNCS In water aq. soln. of Ni compd. and aq. soln. of bromide and K salt were combined; kept for 1 wk; elem. anal.; | 99% |

| Conditions | Yield |
|---|---|
| Stage #1: C8H20N(1+)*C41H47B11N5O5(1-) With ammonium bromide In ethylenediamine at 80℃; for 22h; Microwave irradiation; Stage #2: tetraethylammonium bromide In water | 99% |
-
-
70091-69-9
3,3,3',3'-tetrakis(trifluoromethyl)-1,1'(3H,3'H)-spirobi<2,1-benzoxasilole>

-
-
71-91-0
tetraethylammonium bromide

-
-
1822-00-0
trimethylsilylmethyllithium

| Conditions | Yield |
|---|---|
| Stage #1: 3,3,3',3'-tetrakis(trifluoromethyl)-1,1'(3H,3'H)-spirobi<2,1-benzoxasilole>; trimethylsilylmethyllithium In diethyl ether at -78 - 20℃; for 3h; Stage #2: tetraethylammonium bromide In dichloromethane for 1h; | 99% |

-
-
14786-84-6
2,3,5,6-tetramethylthiophenol

-
-
71-91-0
tetraethylammonium bromide

-
-
7705-08-0
iron(III) chloride

| Conditions | Yield |
|---|---|
| With Li In methanol N2-atmosphere; addn. of Li-wire to thiol, treatment with FeCl3 and Et4NBr; | 98% |
| With Li In ethanol N2-atmosphere; addn. of Li-wire to thiol, treatment with FeCl3 and Et4NBr; | 98% |


-
-
12082-03-0
tricarbonyl(η6-chlorobenzene)chromium

-
-
71-91-0
tetraethylammonium bromide

-
A
-
697302-50-4, 697302-51-5, 12082-08-5, 697302-53-7
(benzene)tricarbonylchromium

| Conditions | Yield |
|---|---|
| In not given reaction of K2(Cr(CO)5) with (η6-ClC6H5)Cr(CO)3, aq. workup; 1H NMR, IR; | A 98% B 0% |


-
-
12082-03-0
tricarbonyl(η6-chlorobenzene)chromium

-
-
71-91-0
tetraethylammonium bromide

-
A
-
697302-50-4, 697302-51-5, 12082-08-5, 697302-53-7
(benzene)tricarbonylchromium

| Conditions | Yield |
|---|---|
| In not given reaction of K2(W(CO)5) with (η6-ClC6H5)Cr(CO)3, aq. workup; 1H NMR, IR; | A 98% B 0% C n/a |

-
-
82807-97-4
[closo-3-triphenylphosphine-3,3-nitrato-3,1,2-RhC2B9H11]

-
-
71-91-0
tetraethylammonium bromide

| Conditions | Yield |
|---|---|
| With PPh3 In dichloromethane under Ar, Rh complex dissolved in CH2Cl2 at room temp., PPh3 added withstirring, after 5 min (C2H5)4NBr added, mixt. stirred for 45 min; chromd., treated with heptane, evapd., washed with petroleum ether, dried by suction; | 98% |

-
-
71-91-0
tetraethylammonium bromide

| Conditions | Yield |
|---|---|
| In water (N2), heating (sealed Pyrex tube, 110°C, 2 d); dissolving educts excess and KCl (water, methanol), washing (anhydrous eher); semiquantitative microprobe analysis; | 98% |

-
-
25637-16-5
4-bromotetrahydro-2H-pyran

-
-
70091-69-9
3,3,3',3'-tetrakis(trifluoromethyl)-1,1'(3H,3'H)-spirobi<2,1-benzoxasilole>

-
-
71-91-0
tetraethylammonium bromide

| Conditions | Yield |
|---|---|
| Stage #1: 4-bromotetrahydro-2H-pyran With iodine; magnesium In tetrahydrofuran at 60℃; Inert atmosphere; Stage #2: 3,3,3',3'-tetrakis(trifluoromethyl)-1,1'(3H,3'H)-spirobi<2,1-benzoxasilole> In tetrahydrofuran Inert atmosphere; Reflux; Stage #3: tetraethylammonium bromide In dichloromethane at 20℃; for 1h; | 98% |

-
-
14040-11-0
tungsten hexacarbonyl

-
-
71-91-0
tetraethylammonium bromide

-
-
14780-94-0
tetraethylammonium bromopentacarbonyltungstate

| Conditions | Yield |
|---|---|
| In 1,4-dioxane heating at reflux, 2 h; after cooling, addn. of petroleum ether, washing (petroleum ether), heated (60°C, under vac. for 2 h to remove unreacted W(CO)6), uptake in THF (under N2), mixture is filtered and stripped; | 97% |
| In 1,4-dioxane for 2h; Reflux; | 86% |
| In diethylene glycol Anhydrous Et4NBr was heated with an excess of M(CO)6 in dimethyldigol at 120°C under dry N2;; soln. was filtered hot under N2, light petroleum added and cooling at -5°C for ca. 8 h; crystals was washed and dried;; | 90-95 |
| In diethylene glycol byproducts: CO; NEt4Br (18 mmol) was heated with an excess of metal hexacarbonyl (23 mmol, purified by sublimation) in O(CH2CH2OMe)2 at ca. 120°C under dry N2;; filtration while hot under N2; addn. of equal amt. of light petroleum (b.p. 40-60°C) and cooling to -5°C for ca. 6 h; the solventwas decanted, the ppt. washed with light petroleum; heating at 60°C (0.001 mm Hg) for ca. 2 h;; | 90-95 |
| In 2-methoxy-ethanol byproducts: CO; N2 atmosphere; refluxing (2 h); cooling, solvent removal (vac.), recrystn. (CH2Cl2/hexane); |

| Conditions | Yield |
|---|---|
| In acetonitrile (Et4N)2W3S7Br6 and KSCN boiled for 1 h in Ar, Et4NBr in water added; ppt. filtered, washed with water, ethanol, benzene and ether; elem. anal.; | 97% |

-
-
14040-11-0
tungsten hexacarbonyl

-
-
71-91-0
tetraethylammonium bromide

| Conditions | Yield |
|---|---|
| Stage #1: tungsten hexacarbonyl; potassium hydridotris(3,4,5-trimethyl-1H-pyrazol-1-yl)borate In N,N-dimethyl-formamide at 130℃; for 2.5h; Inert atmosphere; Schlenk technique; Glovebox; Stage #2: tetraethylammonium bromide In water; N,N-dimethyl-formamide at 60℃; Inert atmosphere; Saturated gas; Glovebox; | 97% |

Tetraethylammonium bromide Chemical Properties
Molecule structure of Tetraethylammonium bromide (CAS NO.71-91-0):
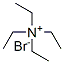
IUPAC Name: Tetraethylazanium bromide
Molecular Formula: C8H20BrN
Molecualr Weight: 210.16 g/mol
Melting Point: 285 °C (dec.)(lit.)
Density: 1.397 g/cm3
Water Solubility: 2795 g/L (25 °C)
Sensitive: hygroscopic
H-Bond Acceptor: 1
Rotatable Bond Count: 4
Exact Mass: 209.077912
MonoIsotopic Mass: 209.077912
Canonical SMILES: CC[N+](CC)(CC)CC.[Br-]
InChI: InChI=1S/C8H20N.BrH/c1-5-9(6-2,7-3)8-4;/h5-8H2,1-4H3;1H/q+1;/p-1
InChIKey: HWCKGOZZJDHMNC-UHFFFAOYSA-M
EINECS: 200-769-4
Product Categories: quarternary ammonium salts; Ammonium Bromides (Quaternary); Quaternary Ammonium Compounds; Ammonium Salts; Greener Alternatives: Catalysis; Phase Transfer Catalysts; Ion-pair Reagents
Tetraethylammonium bromide Uses
Tetraethylammonium bromide (CAS NO.71-91-0) is used as reagents of polarographic analysis and the intermediates of pesticide.
Tetraethylammonium bromide Toxicity Data With Reference
| Organism | Test Type | Route | Reported Dose (Normalized Dose) | Effect | Source |
|---|---|---|---|---|---|
| dog | LD50 | intravenous | 55mg/kg (55mg/kg) | LUNGS, THORAX, OR RESPIRATION: OTHER CHANGES | Journal of Pharmacology and Experimental Therapeutics. Vol. 97, Pg. 48, 1949. |
| mouse | LD50 | intraperitoneal | 50mg/kg (50mg/kg) | National Technical Information Service. Vol. AD277-689, | |
| mouse | LD50 | intravenous | 14200ug/kg (14.2mg/kg) | Journal de Physiologie Vol. 49, Pg. 220, 1957. | |
| mouse | LD50 | oral | > 2gm/kg (2000mg/kg) | LUNGS, THORAX, OR RESPIRATION: OTHER CHANGES | Journal of Pharmacology and Experimental Therapeutics. Vol. 97, Pg. 48, 1949. |
| rabbit | LD50 | intravenous | 72mg/kg (72mg/kg) | LUNGS, THORAX, OR RESPIRATION: OTHER CHANGES | Journal of Pharmacology and Experimental Therapeutics. Vol. 97, Pg. 48, 1949. |
| rat | LD50 | intraperitoneal | 108mg/kg (108mg/kg) | Pharmacology: International Journal of Experimental and Clinical Pharmacology. Vol. 21, Pg. 256, 1980. | |
| rat | LD50 | intravenous | 63mg/kg (63mg/kg) | LUNGS, THORAX, OR RESPIRATION: OTHER CHANGES | Journal of Pharmacology and Experimental Therapeutics. Vol. 97, Pg. 48, 1949. |
| rat | LD50 | subcutaneous | 200mg/kg (200mg/kg) | Proceedings of the Society for Experimental Biology and Medicine. Vol. 78, Pg. 708, 1951. |
Tetraethylammonium bromide Safety Profile
Hazard Codes:  Xi
Xi
Risk Statements: 36/37/38
R36/37/38:Irritating to eyes, respiratory system and skin.
Safety Statements: 26-36-37/39
S26: In case of contact with eyes, rinse immediately with plenty of water and seek medical advice.
S36:Wear suitable protective clothing.
S37/39:Wear suitable gloves and eye/face protection.
WGK Germany: 3
RTECS: BS5950000
F: 3
HS Code: 29239000
Tetraethylammonium bromide Specification
Tetraethylammonium bromide (CAS NO.71-91-0) is also named as AI3-19150 ; Beparon ; Bromethyl ; Bromure de tetraethylammonium ; Bromure de tetraethylammonium [French] ; Bromure de tetrylammonium ; Bromure de tetrylammonium [INN-French] ; Bromuro de tetrilamonio ; Bromuro de tetrilamonio [INN-Spanish] ; Etambro ; Etamon ; Ethylon ; Etylon ; NSC 36724 ; Sympatektoman ; TEA bromide ; TEAB ; TMD 10 ; Teamon ; Tetraethyl ammonium bromide ; Tetranium ; Tetrylammonii bromidum ; Tetrylammonii bromidum [INN-Latin] ; Tetrylammonium bromide ; USAF DO-32 . Tetraethylammonium bromide (CAS NO.71-91-0) is white to light yellow crystalline solid. It is a quaternary ammonium salt. It is a common source for the tetraethylammonium cation.
Related Products
- Tetraethylammonium
- Tetraethylammonium acetate
- Tetraethylammonium borohydride
- Tetraethylammonium bromide
- Tetraethylammonium fluoride dihydrate
- Tetraethylammonium fluoride hydrate
- Tetraethylammonium formate
- Tetraethylammonium hexafluorophosphate
- Tetraethylammonium hydrogensulfate
- Tetraethylammonium hydroxide
- 71913-10-5
- 71914-80-2
- 71916-82-0
- 71916-91-1
- 719-22-2
- 71924-62-4
- 71925-11-6
- 71925-33-2
- 71926-19-7
- 719268-92-5
Hot Products
About|Contact|Cas|Product Name|Molecular|Country|Encyclopedia
Message|New Cas|MSDS|Service|Advertisement|CAS DataBase|Article Data|Manufacturers | Chemical Catalog
©2008 LookChem.com,License: ICP
NO.:Zhejiang16009103
complaints:service@lookchem.com Desktop View