-
Name
Triphenylmethyl chloride
- EINECS 200-986-4
- CAS No. 76-83-5
- Article Data132
- CAS DataBase
- Density 1.141 g/cm3
- Solubility chloroform: 0.1 g/mL
- Melting Point 109-112 °C
- Formula C19H15Cl
- Boiling Point 374.3 °C at 760 mmHg
- Molecular Weight 278.781
- Flash Point 177.9 °C
- Transport Information UN 3261 8/PG 3
- Appearance white to yellow solid
- Safety 26-36/37/39-45-61-27
- Risk Codes 34-50/53-14-36/37
-
Molecular Structure
-
Hazard Symbols
 C,
C, N
N
- Synonyms Methane,chlorotriphenyl- (8CI);1,1',1''-(Chloromethylidyne)tris[benzene];Chlorotriphenylmethane;
- PSA 0.00000
- LogP 5.21730
Synthetic route

| Conditions | Yield |
|---|---|
| With Vilsmeier reagent In 1,4-dioxane at 80℃; for 0.5h; | 100% |
| With hydrogenchloride; calcium chloride In water; toluene at 25℃; for 5h; Solvent; Reagent/catalyst; | 96% |
| With chloro-trimethyl-silane In dichloromethane; water at 0℃; for 0.666667h; | 90% |

| Conditions | Yield |
|---|---|
| With tetrachloromethane at 250℃; for 7h; Inert atmosphere; Autoclave; | 99% |
| With dihydrogen peroxide; lithium chloride In benzene at 50℃; for 5h; Irradiation; | 99.4% |
| With 2,2'-azobis(isobutyronitrile); benzyl(trimethyl)ammonium tetrachloroiodate In tetrachloromethane for 1h; Heating; | 90% |

| Conditions | Yield |
|---|---|
| at 250℃; under 52505.3 Torr; for 7h; Inert atmosphere; | 99% |
-
-
968-39-8
Triphenylmethylethylether

-
-
75-36-5
acetyl chloride

-
A
-
76-83-5
trityl chloride

-
B
-
141-78-6
ethyl acetate

| Conditions | Yield |
|---|---|
| at 13℃; for 70h; | A 97% B 82% |
| at 70℃; for 13h; | A 4.68 g B 1.25 g |


| Conditions | Yield |
|---|---|
| With thionyl chloride In n-heptane 1.) RT, 10 min, 2.) reflux, 1 d; | 96% |
-
-
968-39-8
Triphenylmethylethylether

-
-
98-88-4
benzoyl chloride

-
A
-
93-89-0
benzoic acid ethyl ester

-
B
-
76-83-5
trityl chloride

| Conditions | Yield |
|---|---|
| at 70 - 100℃; for 35h; | A 78% B 86% |
| at 70 - 100℃; for 35h; | A 0.81 g B 1.64 g |

-
-
24165-03-5
tritylsulfenyl chloride

-
-
76-83-5
trityl chloride

| Conditions | Yield |
|---|---|
| With 1H-imidazole; triethylamine In dichloromethane at 20℃; for 1h; | 83% |
| Heating; |

| Conditions | Yield |
|---|---|
| With iron(III) chloride at 15 - 150℃; under 2625.26 - 4500.45 Torr; for 5h; Temperature; Pressure; Autoclave; | 82% |
| Stage #1: tetrachloromethane With aluminum (III) chloride In Petroleum ether at 120 - 150℃; for 1h; Stage #2: benzene In Petroleum ether at 60℃; under 760.051 Torr; for 0.5h; Reagent/catalyst; Solvent; Temperature; Pressure; Microwave irradiation; | 80.1% |
| With iron(III) chloride |
-
-
498-66-8
norborn-2-ene

-
-
24165-03-5
tritylsulfenyl chloride

-
A
-
76-83-5
trityl chloride

-
-
80345-24-0
endo-2-chloro-exo-1-(triphenylmethyldithio)bicyclo<2.2.1>heptane

| Conditions | Yield |
|---|---|
| Ambient temperature; | A n/a B 82% |


| Conditions | Yield |
|---|---|
| With indium; ammonium chloride In methanol for 27h; Reflux; | A n/a B 81% |

| Conditions | Yield |
|---|---|
| With indium; ammonium chloride In methanol for 35h; Reagent/catalyst; Solvent; Temperature; Reflux; | A 80% B 65% |

| Conditions | Yield |
|---|---|
| With indium; ammonium chloride In methanol for 31h; Reflux; | A 75% B n/a |

| Conditions | Yield |
|---|---|
| With indium; ammonium chloride In methanol for 28h; Reflux; | A 71% B n/a |

| Conditions | Yield |
|---|---|
| With indium; ammonium chloride In methanol for 30h; Reflux; | A n/a B 70% |

| Conditions | Yield |
|---|---|
| With indium; ammonium chloride In methanol for 31h; Reflux; | A n/a B 70% |

| Conditions | Yield |
|---|---|
| In ethyl acetate for 13h; Heating; | A 26% B 23% C 69% |

-
-
80345-24-0
endo-2-chloro-exo-1-(triphenylmethyldithio)bicyclo<2.2.1>heptane

-
A
-
3695-77-0
triphenylmethanethiol

-
B
-
76-83-5
trityl chloride

| Conditions | Yield |
|---|---|
| In ethyl acetate for 40h; Heating; | A 21% B 19% C 68% |


| Conditions | Yield |
|---|---|
| With indium; ammonium chloride In methanol for 32h; Reflux; | A 68% B n/a |

| Conditions | Yield |
|---|---|
| With indium; ammonium chloride In methanol for 25h; Reflux; | A n/a B 68% |

| Conditions | Yield |
|---|---|
| In ethyl acetate for 10h; Heating; | A 27% B 19% C 67% |


| Conditions | Yield |
|---|---|
| In ethyl acetate for 8h; Heating; | A 24% B 20% C 65% |


| Conditions | Yield |
|---|---|
| In ethyl acetate for 15h; Heating; | A 28% B 21% C 65% |


| Conditions | Yield |
|---|---|
| With indium; ammonium chloride In methanol for 30h; Reflux; | A 62% B n/a |
-
-
92464-82-9
(2E)-3,7-dimethyl-1-triphenylmethoxy-2,6-octadiene

-
A
-
106-24-1
Geraniol

-
B
-
76-83-5
trityl chloride

| Conditions | Yield |
|---|---|
| With indium; ammonium chloride In methanol for 30h; Reflux; | A 62% B n/a |

| Conditions | Yield |
|---|---|
| In ethyl acetate for 42h; Heating; | A 25% B 21% C 60% |


| Conditions | Yield |
|---|---|
| With indium; ammonium chloride In methanol for 34h; Reflux; | A 60% B n/a |

| Conditions | Yield |
|---|---|
| With indium; ammonium chloride In methanol for 30h; Reflux; | A 58% B n/a |
-
-
595-91-5
triphenylacetic acid

-
-
13283-01-7
tungsten(VI) chloride

-
A
-
201230-82-2
carbon monoxide

-
C
-
76-83-5
trityl chloride

| Conditions | Yield |
|---|---|
| In dichloromethane at 20℃; for 18h; Inert atmosphere; Schlenk technique; | A 57% B 32% C n/a |

-
-
74500-56-4, 104784-02-3, 104871-06-9, 78666-39-4
1,4-pentanediol monotrityl ether

-
A
-
626-95-9
1,4-Pentanediol

-
B
-
76-83-5
trityl chloride

| Conditions | Yield |
|---|---|
| With indium; ammonium chloride In methanol for 28h; Reflux; | A 56% B n/a |
-
-
99-20-7
TREHALOSE

-
-
76-83-5
trityl chloride

-
-
50705-44-7, 108811-30-9
6-O-(triphenylmethyl)-α-D-glucopyranosyl-6'-O-(triphenylmethyl)-α-D-glucopyranoside

| Conditions | Yield |
|---|---|
| With pyridine at 20 - 40℃; for 20h; Inert atmosphere; | 100% |
| In pyridine | 88% |
| With pyridine at 40℃; for 36h; | 83% |

| Conditions | Yield |
|---|---|
| With pyridine; dmap for 48h; Ambient temperature; | 100% |
| With pyridine; dmap at 20℃; for 48h; | 100% |
| With pyridine for 1.5h; Reflux; | 93% |

| Conditions | Yield |
|---|---|
| With pyridine for 48h; Ambient temperature; | 100% |
| With pyridine at 70℃; for 24h; | 96% |
| With pyridine |

| Conditions | Yield |
|---|---|
| With triethylamine In dichloromethane at 20℃; for 2h; | 100% |
| With pyridine | |
| With pyridine |

| Conditions | Yield |
|---|---|
| With pyridine; dmap In dichloromethane at 20℃; for 16h; | 100% |
| With pyridine; dmap In dichloromethane at 20℃; for 18h; | 95% |
| With triethylamine In dichloromethane Ambient temperature; | 94% |

| Conditions | Yield |
|---|---|
| Stage #1: trityl chloride; aniline at 190℃; for 0.5h; Stage #2: With hydrogenchloride In methanol for 0.5h; Reflux; | 100% |
| at 50℃; for 1.5h; | 63% |
| With pyridine at 20℃; for 24h; Substitution; | 44% |

| Conditions | Yield |
|---|---|
| With water In tetrachloromethane | 100% |
| With water Kinetics; Rate constant; Mechanism; Two-phase systems; | |
| With water In acetone at 25℃; Kinetics; ΔS, ΔH (excit.); |
-
-
76-83-5
trityl chloride

-
-
20031-21-4
1,2-O-isopropylidene-α-D-xylose

-
-
20590-53-8
1,2-O-isopropylidene-5-O-trityl-α-D-xylofuranose

| Conditions | Yield |
|---|---|
| With pyridine at 20℃; for 12h; | 100% |
| With pyridine for 16h; | 95% |
| With triethylamine In dichloromethane for 3h; Inert atmosphere; | 95% |
-
-
76-83-5
trityl chloride

-
-
97-30-3
methyl-alpha-D-glucopyranoside

-
-
18311-26-7
methyl 6-O-trityl-α-D-glucopyranoside

| Conditions | Yield |
|---|---|
| With pyridine at 70℃; for 2h; | 100% |
| With pyridine at 90℃; for 72h; Inert atmosphere; Schlenk technique; | 100% |
| With 1,4-diaza-bicyclo[2.2.2]octane In dichloromethane at 30℃; for 3.5h; regioselective reaction; | 98% |
-
-
6145-69-3
3-(hexadecyloxy)-1,2-propanediol

-
-
76-83-5
trityl chloride

-
-
82002-20-8
1-O-hexadecyl-3-O-trityl-rac-glycerol

| Conditions | Yield |
|---|---|
| 100% | |
| With pyridine Ambient temperature; | 68% |
| In hexane Heating; | 56% |
-
-
305847-08-9
rac-1-monopalmitoylglycerol

-
-
76-83-5
trityl chloride

-
-
69256-58-2
1-palmitoyl-3-O-trityl-rac-glycerol

| Conditions | Yield |
|---|---|
| With pyridine at 60℃; for 3h; | 100% |
| With pyridine at 60℃; for 3h; | 100% |
| With pyridine In chloroform |
-
-
5680-80-8
methyl (2S)-2-amino-3-hydroxypropanoate hydrochloride

-
-
76-83-5
trityl chloride

-
-
13515-76-9, 116457-91-1, 4465-44-5
methyl N-trityl-L-serinate

| Conditions | Yield |
|---|---|
| With TEA In dichloromethane at 0℃; for 24h; | 100% |
| With triethylamine In dichloromethane at 0 - 20℃; | 98% |
| With triethylamine In dichloromethane at 0 - 20℃; | 98% |

| Conditions | Yield |
|---|---|
| With calcium for 0.5h; | 100% |
-
-
5382-16-1
4-HYDROXYPIPERIDINE

-
-
76-83-5
trityl chloride

-
-
227100-23-4
N-triphenylmethyl-4-hydroxypiperidine

| Conditions | Yield |
|---|---|
| With triethylamine In dichloromethane at 20℃; | 100% |
| With triethylamine In dichloromethane at 20℃; for 14h; | 82% |
| With triethylamine In dichloromethane at 0℃; for 1h; | |
| With potassium carbonate In N-methyl-acetamide |
-
-
69142-64-9
ethyl 6-aminopicolinate

-
-
76-83-5
trityl chloride

-
-
153140-21-7
Ethyl 6-tritylaminopicolinate

| Conditions | Yield |
|---|---|
| With triethylamine In chloroform for 5h; Ambient temperature; | 100% |
| With triethylamine In chloroform at 20℃; |
-
-
6926-51-8
5-(4-methoxyphenyl)-1H-tetrazole

-
-
76-83-5
trityl chloride

-
-
137898-60-3
5-(4-Methoxy-phenyl)-1-trityl-1H-tetrazole

| Conditions | Yield |
|---|---|
| With triethylamine In N,N-dimethyl-formamide at 70℃; for 3h; | 100% |
-
-
32233-40-2
(3aR,4S,5R,6aS)-5-hydroxy-4-(hydroxymethyl)hexahydro-2H-cyclopenta[b]furan-2-one

-
-
76-83-5
trityl chloride

-
-
62939-85-9
(-)-7α-hydroxy-6β-triphenylmethoxymethyl-cis-2-oxabicyclo<3.3.0>octan-3-one

| Conditions | Yield |
|---|---|
| With dmap; triethylamine In dichloromethane at 20℃; for 4h; Inert atmosphere; | 100% |
| With pyridine In dichloromethane | 93% |
| With pyridine at 20℃; for 48h; | 87% |
| In pyridine at 20℃; for 48h; Substitution; | 87% |
| With pyridine at 20℃; for 12h; Temperature; Large scale; |

| Conditions | Yield |
|---|---|
| With 1,8-diazabicyclo[5.4.0]undec-7-ene In dichloromethane for 48h; | 100% |
| Stage #1: (S)-Ethyl lactate; trityl chloride With zinc(II) chloride In acetonitrile at 20℃; for 0.0833333h; Stage #2: With triethylamine In acetonitrile at 20℃; for 0.166667h; Stage #3: With citric acid In water; acetonitrile at 20℃; for 0.0833333h; pH=5; | 83% |
| With 1,8-diazabicyclo[5.4.0]undec-7-ene In dichloromethane at 20℃; for 48h; Inert atmosphere; | 82% |
-
-
154-17-6
D-2-deoxyglucose

-
-
76-83-5
trityl chloride

-
-
84457-54-5
(3R,4S,5R)-3,4,5-Trihydroxy-6-trityloxy-hexanal

| Conditions | Yield |
|---|---|
| With pyridine for 15h; Ambient temperature; | 100% |

-
-
76-83-5
trityl chloride

| Conditions | Yield |
|---|---|
| With pyridine; tetrabutylammonium perchlorate In dichloromethane for 4.5h; Ambient temperature; | 100% |
-
-
103348-49-8
2-azido-sphingosine

-
-
76-83-5
trityl chloride

-
-
108283-57-4
(2S,3R,4E)-2-azido-1-(triphenylmethyl)-4-octadecene-1,3-diol

| Conditions | Yield |
|---|---|
| With N-ethyl-N,N-diisopropylamine In dichloromethane at 20℃; for 23h; | 100% |
| With N-ethyl-N,N-diisopropylamine In dichloromethane for 26h; | 91% |
| In tetrahydrofuran; pyridine; chloroform for 48h; Ambient temperature; | 90% |
-
-
39994-75-7
L-threonine methyl ester hydrochloride

-
-
76-83-5
trityl chloride

-
-
74481-55-3
methyl (2S,3R)-3-hydroxy-2-triphenylmethylaminobutanoate

| Conditions | Yield |
|---|---|
| With triethylamine In chloroform at 0℃; for 24h; | 100% |
| With triethylamine In chloroform at 0℃; for 20h; | 100% |
| With triethylamine In chloroform at 0℃; for 72h; | 91% |
| With triethylamine In dichloromethane at 20℃; for 19h; |
-
-
593-31-7, 6898-45-9, 10305-40-5, 13103-02-1, 15863-83-9, 34043-91-9, 34783-94-3, 88929-74-2, 88929-77-5, 106708-34-3, 106708-35-4
1-O-(Z)-9'-octadecenyl-sn-glycerol

-
-
76-83-5
trityl chloride

-
-
6068-24-2, 6110-56-1, 62777-23-5, 82950-68-3, 88929-78-6, 91050-73-6, 96765-83-2
1-O-(Z)-9'-octadecenyl-3-O-trityl-sn-glycerol

| Conditions | Yield |
|---|---|
| With pyridine for 48h; Ambient temperature; | 100% |

| Conditions | Yield |
|---|---|
| With potassium carbonate In dichloromethane | 100% |
| With potassium carbonate In dichloromethane at 20℃; for 4h; Inert atmosphere; | 90% |
| With triethylamine In dichloromethane at 20℃; | 83.3% |
-
-
76-83-5
trityl chloride

-
-
98123-61-6
2H-4-oxo-1,2,3,4,6,7,8,12b-octahydropyrazino<2,1-a><2>benzazepine

-
-
121654-85-1
2-(triphenylmethyl)-4-oxo-1,2,3,4,6,7,8,12b-octahydropyrazino<2,1-a><2>benzazepine

| Conditions | Yield |
|---|---|
| With triethylamine In chloroform 1) 0 deg C, 30 min, 2) r.t., 90 min; | 100% |
| With triethylamine In chloroform | 100% |
-
-
76-83-5
trityl chloride

| Conditions | Yield |
|---|---|
| With triethylamine 1.) -35 deg C to room temp., 1 h, 2.) room temp., 9 h; | 100% |
-
-
76-83-5
trityl chloride

-
-
68602-57-3
trifluoroacetyl triflate

-
-
115726-23-3
triphenylmethyl trifluoromethanesulfonate

| Conditions | Yield |
|---|---|
| In tetrachloromethane at 25℃; for 3h; | 100% |
-
-
76-83-5
trityl chloride

-
-
70346-51-9
(E)-methyl 3-(1H-imidazol-4-yl)acrylate

-
-
138408-36-3
trans-1-trityl-4-imidazoleacrylic acid methyl ester

| Conditions | Yield |
|---|---|
| With triethylamine In N,N-dimethyl-formamide for 0.5h; Ambient temperature; | 100% |
| With triethylamine In tetrahydrofuran at 0 - 20℃; | 97% |
| With triethylamine In N,N-dimethyl-formamide for 3h; Ambient temperature; | 70% |
-
-
76-83-5
trityl chloride

-
-
4152-09-4
N-benzylethylenediamine

-
-
141856-06-6
N-Benzyl-N'-(triphenylmethyl)ethane-1,2-diamine

| Conditions | Yield |
|---|---|
| With triethylamine In dichloromethane at 0 - 20℃; | 100% |
| With triethylamine In acetonitrile at 0 - 30℃; for 1h; Inert atmosphere; | 100% |
| With triethylamine In dichloromethane for 1h; 0 deg C to r.t.; | 83% |
Triphenylmethyl chloride Chemical Properties
Molecular Structure of Chlorotriphenylmethane (CAS NO.76-83-5):
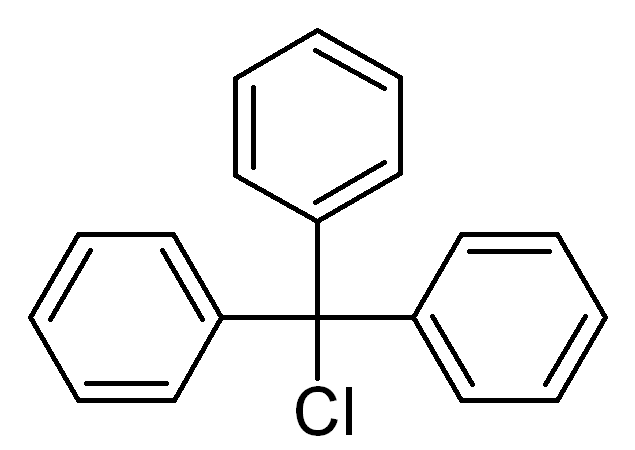
IUPAC Name: [chloro(diphenyl)methyl]benzene
Molecular formula: C19H15Cl
Molar mass: 278.78 g/mol
H bond acceptors: 0
H bond donors: 0
Freely Rotating Bonds: 3
Polar Surface Area: 0 Å2
Index of Refraction: 1.607
Molar Refractivity: 84.38 cm3
Molar Volume: 244.1 cm3
Surface Tension: 42.3 dyne/cm
Density: 1.141 g/cm3
Flash Point: 177.9 °C
Storage temp: Store under Nitrogen
Solubility: chloroform: 0.1 g/mL
Sensitive: Lachrymatory
BRN: 397363
Enthalpy of Vaporization: 59.73 kJ/mol
Boiling Point: 374.3 °C at 760 mmHg
Vapour Pressure: 1.82E-05 mmHg at 25°C
Melting point: 109-112°C
InChI
InChI=1/C19H15Cl/c20-19(16-10-4-1-5-11-16,17-12-6-2-7-13-17)18-14-8-3-9-15-18/h1-15H
Smiles
C(c1ccccc1)(c1ccccc1)(c1ccccc1)Cl
EINECS: 200-986-4
Product Categories: Amino Acid Derivatives; Starting Raw Materials & Intermediates; Organics; N-Protecting Reagents; Biochemistry; Nucleosides, Nucleotides & Related Reagents; Protecting Agents for Hydroxyl and Amino Groups; Protecting Agents, Phosphorylating Agents & Condensing Agents; Protection & Derivatization Reagents (for Synthesis); Synthetic Organic Chemistry; Regant series
Triphenylmethyl chloride Toxicity Data With Reference
| Organism | Test Type | Route | Reported Dose (Normalized Dose) | Effect | Source |
|---|---|---|---|---|---|
| mouse | LD50 | intravenous | 180mg/kg (180mg/kg) | U.S. Army Armament Research & Development Command, Chemical Systems Laboratory, NIOSH Exchange Chemicals. Vol. NX#04021, |
Triphenylmethyl chloride Safety Profile
Hazard Codes:  C,
C, N
N
Risk Statements: 34-50/53-14-36/37
R34:Causes burns.
R50/53:Very toxic to aquatic organisms, may cause long-term adverse effects in the aquatic environment.
R14 :Reacts violently with water.
R36/37:Irritating to eyes and respiratory system.
Safety Statements: 26-36/37/39-45-61-27
S26: In case of contact with eyes, rinse immediately with plenty of water and seek medical advice.
S36/37/39:Wear suitable protective clothing, gloves and eye/face protection.
S45:In case of accident or if you feel unwell, seek medical advice immediately (show the label whenever possible.)
S61:Avoid release to the environment. Refer to special instructions / safety data sheets.
S27:Take off immediately all contaminated clothing.
RIDADR: UN 3261 8/PG 3
WGK Germany: 3
RTECS: PA6450000
F: 10-19-21
Hazard Note: Irritant
HazardClass: 8
Triphenylmethyl chloride Specification
Chlorotriphenylmethane , with CAS number of 76-83-5, can be called [Chloro(diphenyl)methyl]benzene ; 1,1’,1’’-(chloromethylidyne)tris-benzen ; 1,1’,1’’(-Chloromethylidyne)trisben-zene ; chlorotriphenyl-methan ; Methane, chlorotriphenyl- ; alpha-chlorotriphenylmethane ; Benzene, 1,1',1''-(chloromethylidyne)tris- . It is a white solid . Sometimes it is used to introduce the trityl protecting group because it's an alkyl halide. Chlorotriphenylmethane (CAS NO.76-83-5) can be intermediates to produce drug especially for cephalosporins and be synthesized to idoxuridine for the anti-viral effect.
About|Contact|Cas|Product Name|Molecular|Country|Encyclopedia
Message|New Cas|MSDS|Service|Advertisement|CAS DataBase|Article Data|Manufacturers | Chemical Catalog
©2008 LookChem.com,License: ICP
NO.:Zhejiang16009103
complaints:service@lookchem.com Desktop View