Hangzhou Hysen Pharma co.,Ltd.
We have years of experience to produce Dapagliflozin .We can provide high quality,cheap price,spot supply and good service. If you have any questions,pls do not hesitate to contact us. Hangzhou Hysen pharma CO.,LTD. is a professional Pharmaceutical
Cas:104121-92-8
Min.Order:10 Gram
Negotiable
Type:Lab/Research institutions
inquiryChemvon Biotechnology Co. Ltd.
Eldecalcitolcasno.:104121-92-8 Package:According to your demand Transportation:as requested
Kono Chem Co.,Ltd
Founded in 2014, Kono Chem Co., Ltd is an export-oriented production enterprise supported by the State Department of Commerce. Kono Chem covers an area of 312,000 square meters, with scientific and technological personnel accounting for more than 35%
Cas:104121-92-8
Min.Order:1 Kilogram
FOB Price: $195.0 / 200.0
Type:Other
inquiryDayang Chem (Hangzhou) Co.,Ltd.
DayangChem exported this product to many countries and regions at best price. If you are looking for the material's manufacturer or supplier in China, DayangChem is your best choice. Pls contact with us freely for getting detailed product spe
Cas:104121-92-8
Min.Order:1 Kilogram
FOB Price: $3.0
Type:Lab/Research institutions
inquiryChemwill Asia Co., Ltd.
Our main production base is located in Xuzhou industry park. We are certified both to the ISO 9001 and ISO 14001 Standards, have a safety management system in place.Our R&D team masters core technology for process-design of target building block
Cas:104121-92-8
Min.Order:5 Kiloliter
FOB Price: $1.2 / 5.0
Type:Manufacturers
inquiryHenan Tianfu Chemical Co., Ltd.
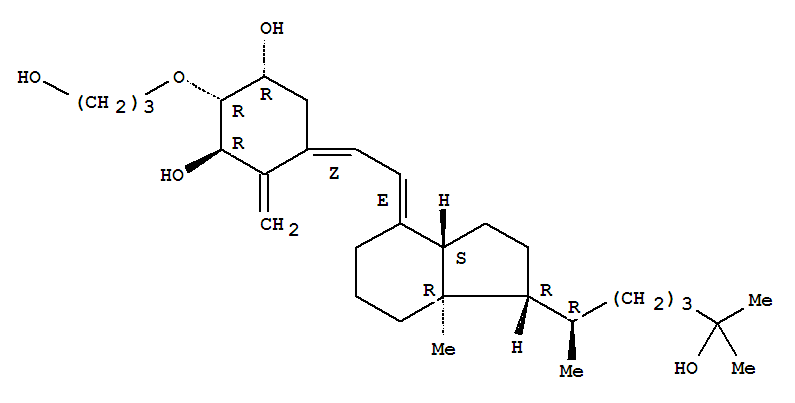
Product Name: 2-(3-hydroxypropoxy)-1,25-dihydroxyvitamin D3 Synonyms: 2-(3-hydroxypropoxy)-1,25-dihydroxyvitamin D3;Eldecalcitol;ED-71;Eldecalcitol(ED-71);2.beta.-(3-Hydroxypropoxy)-1.alpha.,25-dihydroxyvitaMin D3;(1R,Z)-5-((E)-2-((1R,
Cas:104121-92-8
Min.Order:1 Gram
FOB Price: $8900.0
Type:Lab/Research institutions
inquiryZhuozhou Wenxi import and Export Co., Ltd
WITH US,YOUR MONEY IN SAFE,YOUR BUSINESS IN SAFE 1)Quick Response Within 12 hours; 2)Quality Guarantee: All products are strictly tested by our QC, confirmed by QA and approved by third party lab in China, USA, Canada, Germany, UK, Italy, France et
Cas:104121-92-8
Min.Order:10 Gram
FOB Price: $146.0 / 176.0
Type:Trading Company
inquiryLeader Biochemical Group
About Product Details
Cas:104121-92-8
Min.Order:1 Gram
FOB Price: $3.0 / 5.0
Type:Lab/Research institutions
inquiryHebei Sankai Chemical Technology Co., Ltd
1. Product advantages High purity, all above 98.5%, no impurities after dissolution We will test each batch to ensure quality OEM and private brand services designed for free Various cap colors available We can also provide MT1 peptide powd
Cas:104121-92-8
Min.Order:1 Kilogram
FOB Price: $100.0 / 102.0
Type:Trading Company
inquiryHebei Nengqian Chemical Import and Export Co., LTD
Our advantages: 1. All inquiries will be replied within 12 hours. 2. Dedication to quality, supply & service. 3. Strictly on selecting raw materials. 4. Reasonable & competitive price, fast lead time. 5. Sample is available for your eva
Cas:104121-92-8
Min.Order:1 Kilogram
FOB Price: $9.0 / 99.0
Type:Trading Company
inquiryShanghai Upbio Tech Co.,Ltd
1.No Less 8 years exporting experience. Clients can 100% received goods 2.Lower Price with higher quality 3,Free sample 4,We are sincerely responsible for the "product quality" and "After Service" Upbio is Specialized
Cas:104121-92-8
Min.Order:1 Kilogram
Negotiable
Type:Lab/Research institutions
inquiryQingdao Beluga Import and Export Co., LTD
2-(3-hydroxypropoxy)-1,25-dihydroxyvitamin D3 CAS:104121-92-8 Qingdao Belugas Import and Export Co., Ltd. is a scientific and technological company integrating research and development, production and trade of chemical intermediates, specializing in
Cas:104121-92-8
Min.Order:1 Gram
Negotiable
Type:Lab/Research institutions
inquiryShandong Hanjiang Chemical Co., Ltd.
Hello, dear friend! I'm Hansen and Allen from China. Welcome to my lookchem mall! The following is a brief introduction of our company's products and services. If you are interested in our products, please contact us by emai
Cas:104121-92-8
Min.Order:1 Kilogram
Negotiable
Type:Lab/Research institutions
inquiryHangzhou JINLAN Pharm-Drugs Technology Co., Ltd
We can provide GMP validation service that complies with SFDA, FDA, WHO and EU EMPA.Excellent registration team could help us easlily to register our products in different countries.If you and your customer are interested in some products or need C
Cas:104121-92-8
Min.Order:1 Gram
Negotiable
Type:Trading Company
inquiryHenan Wentao Chemical Product Co., Ltd.
Henan Wentao Chemical Product Co.,Ltd is Located in Zhengzhou High-tech Development Zone with import and export license, We passed ISO 9001:2008 as well, Henan Wentao has developed more than 1000 compounds, which are widely used in the fields of prod
Cas:104121-92-8
Min.Order:0
Negotiable
Type:Lab/Research institutions
inquiryTriumph International Development Limilted
Triumph has the complete production of G- KG - MT service chain,we can make the new technology into productivity quickly in the research and development of new products. Main Business Custom Synthesis: Trading(Raw Mater
Cas:104121-92-8
Min.Order:0 Metric Ton
FOB Price: $18.0 / 20.0
Type:Lab/Research institutions
inquiryTaizhou Crene Biotechnology co.ltd
Our company provides one-stop services of research - development - production for a variety of special prouducts. Not only do we make effective use of our strong technological strength, but also establish of cooperative relations with several well-
Cas:104121-92-8
Min.Order:100 Milligram
Negotiable
Type:Lab/Research institutions
inquiryWuhan Han Sheng New Material Technology Co.,Ltd
Our Advantage: high quality with competitive price High quality standard: BP/USP/EP Enterprise standard All purity customized Fast and safe delivery We have reliable forwarder who can help us deliver our goods more fast and safe. We
Cas:104121-92-8
Min.Order:10 Kilogram
Negotiable
Type:Trading Company
inquiryTianjin Kind Pharma Co., Ltd.
Factory direct sales, accept customization. Eldecalcitol is a derivative of vitamin D3 which is the vitamin that mediates intestinal calcium absorbtion, bone calcium metabolism and probably, muscle activity. Kind Pharma has its own laboratory, we c
Afine Chemicals Limited
Company Introduction 1. Established in 2005, with two independent business divisions: Fine chemicals division; Pharmaceutical division. 2. Main product: Optical brightener Textile auxiliary Dye stuff Pigments
Hangzhou Lingrui Chemical Co.,Ltd.
advantage: 1. The best price, satisfactory quality; 2. customers have the right to choose the delivery of parcels (EMS, DHL, FedEx, UPS); 3. customers have the right to choose from the recent effective packaging methods of their products packaging
Cas:104121-92-8
Min.Order:1 Gram
Negotiable
Type:Other
inquiryHangzhou J&H Chemical Co., Ltd.
J&H CHEM R&D center can offer custom synthesis according to the contract research and development services for the fine chemicals, pharmaceutical, biotechnique and some of the other chemicals. J&H CHEM has some Manufacturing base in Jia
Cas:104121-92-8
Min.Order:0
Negotiable
Type:Lab/Research institutions
inquiryShanghai Massive Chemical Technology Co., Ltd.
Massive Chemical is certified with ISO9001 and ISO14001 manufacturer for this product. We will offer all documents as requirement for the materials which includes, Certificate of Analysis, Material Safety Data Sheet, and Method of Analysis and
Cas:104121-92-8
Min.Order:1 Gram
FOB Price: $1.0
Type:Lab/Research institutions
inquiryZibo Hangyu Biotechnology Development Co., Ltd
Zibo Hangyu Biotechnology Development Co., Ltd is a leading manufacturer and supplier of chemicals in China. We develop produce and distribute high quality pharmaceuticals, intermediates, special chemicals and OLED intermediates and other fine chemi
Cas:104121-92-8
Min.Order:10 Gram
FOB Price: $100.0
Type:Lab/Research institutions
inquirySiwei Development Group Ltd.
Product name: Eldecalcitol CAS No.:104121-92-8 Molecule Formula:C30H50O5 Molecule Weight:490.72 Purity: 99.0% Package: 25kg/drum Description:White powder Manufacture Standards:Enterprise Standard TESTING ITEMS SPE
Cas:104121-92-8
Min.Order:1 Kilogram
Negotiable
Type:Lab/Research institutions
inquiryZibo Dorne chemical technology co. LTD
Product Details Grade: pharmaceutical grade Purity:99%+ ProductionCapacity: 1000 Kilogram/Month Scope of use: For scientific research only(The product must be used legally) Our Advantage 1. Best quality with competitive price. 2. Quick shipping,
Cas:104121-92-8
Min.Order:1 Gram
Negotiable
Type:Lab/Research institutions
inquiryXiamen Jenny Chemical Technology Co., Ltd.
GMP standard, high purity, competitive price, in stock 1. Quick Response: within 6 hours after receiving your email. 2. Quality Guarantee: All products are strictly tested by our QC, confirmed by QA, and approved by a third-party lab in China, USA,
Cas:104121-92-8
Min.Order:1 Milligram
Negotiable
Type:Trading Company
inquiryEAST CHEMSOURCES LIMITED
factory?direct?saleAppearance:White Powder Storage:Store In Dry, Cool And Ventilated Place Package:25kg/drum, also according to the clients requirement Application:It is widely used as a thickener, emulsifier and stabilizer Transportation:By Sea Or B
Cas:104121-92-8
Min.Order:1 Kilogram
FOB Price: $18.0 / 20.0
Type:Trading Company
inquiryShandong Mopai Biotechnology Co., LTD
Shandong Mopai Biotechnology Co., LTD is a leading manufacturer and supplier of chemicals in China. We develop produce and distribute high quality pharmaceuticals, intermediates, special chemicals and OLED intermediates and other fine chemicals. W
Cas:104121-92-8
Min.Order:1 Gram
Negotiable
Type:Lab/Research institutions
inquiryKAISA GROUP INC
1.Applied in food field.it can improve the immune system and prolong life. 2.Appliedin cosmetic field.it can improve the skin care. 3.Applied in pharmaceutical field.it can treat various dieases. 4.Our product quality assurance will make our customer
Cas:104121-92-8
Min.Order:1 Metric Ton
FOB Price: $1.5
Type:Trading Company
inquirySynthetic route
-
-
342645-84-5
(1β,2α,3α,5Z,7E)-1,3-bis[(1,1-dimethylethyl)dimethylsilyloxy]-2-[3-[(1,1-dimethylethyl)dimethylsilyloxy]propoxy]-25-trimethylsilyloxy-9,10-secocholesta-5,7,10(19)-triene

-
-
104121-92-8
Eldecalcitol

| Conditions | Yield |
|---|---|
| With pyridine; hydrogen fluoride In tetrahydrofuran at 20℃; | 96% |

-
-
104121-92-8
Eldecalcitol

| Conditions | Yield |
|---|---|
| With tetrabutyl ammonium fluoride In tetrahydrofuran at 25℃; | 89% |

-
-
104121-92-8
Eldecalcitol

| Conditions | Yield |
|---|---|
| With toluene-4-sulfonic acid In methanol; dichloromethane at 20℃; for 5h; Inert atmosphere; | 80% |

-
-
104121-92-8
Eldecalcitol

| Conditions | Yield |
|---|---|
| With methanol at 20℃; for 66h; Inert atmosphere; stereoselective reaction; | 68% |

-
-
104121-92-8
Eldecalcitol

| Conditions | Yield |
|---|---|
| With tetrabutyl ammonium fluoride In tetrahydrofuran at 20℃; for 96h; | 61% |
-
-
933779-95-4
(5Z,7E)-(1R,2R,3R)-1,3-bis(tert-butyldimethylsilyloxy)-2-(3-tert-butyldimethylsilyloxypropoxy)-25-triethylsilyloxy-9,10-secocholesta-5,7,10(19)-triene

-
-
104121-92-8
Eldecalcitol

| Conditions | Yield |
|---|---|
| With tetrabutyl ammonium fluoride In tetrahydrofuran; toluene at 105℃; for 2h; | 60% |

-
-
104121-92-8
Eldecalcitol

| Conditions | Yield |
|---|---|
| Stage #1: (5R,6R)-6-((R)-1-(methoxymethoxy)allyl)-5-(prop-2-yn-1-yl)-2,4,7,11,13-pentaoxatetradecane; C21H37BrO2 With tetrakis(triphenylphosphine) palladium(0); triethylamine In toluene at 110℃; for 2h; Stage #2: With camphor-10-sulfonic acid In methanol at 20℃; for 72h; | 36% |
-
-
144848-24-8
25-[(triethylsilyl)oxy]de-A,B-cholestan-8-one

-
-
200636-54-0
[3R-(1Z,3β,4α,5α)]-[2-[3,5-bis[(1,1-dimethylethyl)dimethylsilyloxy]-4-[3-[(1,1-dimethylethyl)dimethylsilyloxy]propoxy]-2-methylenecyclohexylidene]ethyl]diphenylphosphine oxide

-
-
104121-92-8
Eldecalcitol

| Conditions | Yield |
|---|---|
| Multistep reaction; |
-
-
299410-93-8
methyl 4,6-O-benzylidene-3-O-(3-hydroxypropyl)-α-D-altropyranoside

-
-
104121-92-8
Eldecalcitol

| Conditions | Yield |
|---|---|
| Multi-step reaction with 9 steps 1.1: 2.1 g / Et3N; DMAP / CH2Cl2 / 3 h / 20 °C 2.1: 91 percent / BaCO3; NBS / CCl4 / Heating 3.1: 86 percent / Zn; NaBH3CN / propan-1-ol; H2O / 95 °C 4.1: pyridine / 20 °C 5.1: 1.4 g / LiHMDS / tetrahydrofuran / 1 h / -78 - 0 °C 6.1: n-BuLi / tetrahydrofuran; hexane / -78 °C 6.2: BF3*OEt2 / tetrahydrofuran; hexane / -78 - 20 °C 6.3: 90 percent / K2CO3 / methanol / 20 °C 7.1: 100 percent / 2,6-lutidine / CH2Cl2 / 0 °C 8.1: 52 percent / Et3N; Pd(PPh3)4 / toluene / Heating 9.1: 61 percent / TBAF / tetrahydrofuran / 96 h / 20 °C View Scheme |
-
-
299410-95-0
methyl 4,6-O-benzylidene-3-O-[3-{(tert-butyldimethylsilyl)oxy}propyl]-α-D-altropyranoside

-
-
104121-92-8
Eldecalcitol

| Conditions | Yield |
|---|---|
| Multi-step reaction with 8 steps 1.1: 91 percent / BaCO3; NBS / CCl4 / Heating 2.1: 86 percent / Zn; NaBH3CN / propan-1-ol; H2O / 95 °C 3.1: pyridine / 20 °C 4.1: 1.4 g / LiHMDS / tetrahydrofuran / 1 h / -78 - 0 °C 5.1: n-BuLi / tetrahydrofuran; hexane / -78 °C 5.2: BF3*OEt2 / tetrahydrofuran; hexane / -78 - 20 °C 5.3: 90 percent / K2CO3 / methanol / 20 °C 6.1: 100 percent / 2,6-lutidine / CH2Cl2 / 0 °C 7.1: 52 percent / Et3N; Pd(PPh3)4 / toluene / Heating 8.1: 61 percent / TBAF / tetrahydrofuran / 96 h / 20 °C View Scheme |
-
-
299410-97-2
methyl 4-O-benzoyl-6-bromo-3-O-[3-{(tert-butyldimethylsilyl)oxy}propyl]-6-deoxy-α-D-altropyranoside

-
-
104121-92-8
Eldecalcitol

| Conditions | Yield |
|---|---|
| Multi-step reaction with 7 steps 1.1: 86 percent / Zn; NaBH3CN / propan-1-ol; H2O / 95 °C 2.1: pyridine / 20 °C 3.1: 1.4 g / LiHMDS / tetrahydrofuran / 1 h / -78 - 0 °C 4.1: n-BuLi / tetrahydrofuran; hexane / -78 °C 4.2: BF3*OEt2 / tetrahydrofuran; hexane / -78 - 20 °C 4.3: 90 percent / K2CO3 / methanol / 20 °C 5.1: 100 percent / 2,6-lutidine / CH2Cl2 / 0 °C 6.1: 52 percent / Et3N; Pd(PPh3)4 / toluene / Heating 7.1: 61 percent / TBAF / tetrahydrofuran / 96 h / 20 °C View Scheme |
-
-
299411-15-7
(3R,4S,5R)-3,5-bis(tert-butyldimethylsilyloxy)-4-[3-(tert-butyldimethylsilyloxy)propoxy]oct-1-en-7-yne

-
-
104121-92-8
Eldecalcitol

| Conditions | Yield |
|---|---|
| Multi-step reaction with 2 steps 1: 52 percent / Et3N; Pd(PPh3)4 / toluene / Heating 2: 61 percent / TBAF / tetrahydrofuran / 96 h / 20 °C View Scheme |
-
-
3150-15-0, 3150-16-1, 3257-59-8, 14187-71-4, 15384-57-3, 19465-13-5, 32976-15-1, 52885-37-7, 53270-02-3, 53270-03-4, 65391-11-9, 65391-12-0, 66537-92-6, 67226-04-4, 71184-14-0, 86420-78-2, 86420-79-3, 91177-51-4, 91177-52-5, 117708-93-7, 120408-72-2, 2880-96-8
methyl 2,3-anhydro-4,6-O-benzilidene-α-D-mannopyranoside

-
-
104121-92-8
Eldecalcitol

| Conditions | Yield |
|---|---|
| Multi-step reaction with 10 steps 1.1: KOt-Bu / 110 °C 2.1: 2.1 g / Et3N; DMAP / CH2Cl2 / 3 h / 20 °C 3.1: 91 percent / BaCO3; NBS / CCl4 / Heating 4.1: 86 percent / Zn; NaBH3CN / propan-1-ol; H2O / 95 °C 5.1: pyridine / 20 °C 6.1: 1.4 g / LiHMDS / tetrahydrofuran / 1 h / -78 - 0 °C 7.1: n-BuLi / tetrahydrofuran; hexane / -78 °C 7.2: BF3*OEt2 / tetrahydrofuran; hexane / -78 - 20 °C 7.3: 90 percent / K2CO3 / methanol / 20 °C 8.1: 100 percent / 2,6-lutidine / CH2Cl2 / 0 °C 9.1: 52 percent / Et3N; Pd(PPh3)4 / toluene / Heating 10.1: 61 percent / TBAF / tetrahydrofuran / 96 h / 20 °C View Scheme |
-
-
794516-13-5
(3R,4S,5R)-4-[3-(tert-butyldimethylsilyloxy)propoxy]oct-1-en-7-yne-3,5-diol

-
-
104121-92-8
Eldecalcitol

| Conditions | Yield |
|---|---|
| Multi-step reaction with 3 steps 1: 100 percent / 2,6-lutidine / CH2Cl2 / 0 °C 2: 52 percent / Et3N; Pd(PPh3)4 / toluene / Heating 3: 61 percent / TBAF / tetrahydrofuran / 96 h / 20 °C View Scheme |
-
-
794516-11-3
(3R,4R,5R)-3-benzoyloxy-4-[3-(tert-butyldimethylsilyloxy)propoxy]-5,6-epoxyhex-1-ene

-
-
104121-92-8
Eldecalcitol

| Conditions | Yield |
|---|---|
| Multi-step reaction with 4 steps 1.1: n-BuLi / tetrahydrofuran; hexane / -78 °C 1.2: BF3*OEt2 / tetrahydrofuran; hexane / -78 - 20 °C 1.3: 90 percent / K2CO3 / methanol / 20 °C 2.1: 100 percent / 2,6-lutidine / CH2Cl2 / 0 °C 3.1: 52 percent / Et3N; Pd(PPh3)4 / toluene / Heating 4.1: 61 percent / TBAF / tetrahydrofuran / 96 h / 20 °C View Scheme |
-
-
794516-09-9
(2R,3S,4R)-4-benzoyloxy-3-[3-(tert-butyldimethylsilyloxy)propoxy]hex-5-ene-1,2-diol

-
-
104121-92-8
Eldecalcitol

| Conditions | Yield |
|---|---|
| Multi-step reaction with 6 steps 1.1: pyridine / 20 °C 2.1: 1.4 g / LiHMDS / tetrahydrofuran / 1 h / -78 - 0 °C 3.1: n-BuLi / tetrahydrofuran; hexane / -78 °C 3.2: BF3*OEt2 / tetrahydrofuran; hexane / -78 - 20 °C 3.3: 90 percent / K2CO3 / methanol / 20 °C 4.1: 100 percent / 2,6-lutidine / CH2Cl2 / 0 °C 5.1: 52 percent / Et3N; Pd(PPh3)4 / toluene / Heating 6.1: 61 percent / TBAF / tetrahydrofuran / 96 h / 20 °C View Scheme |

| Conditions | Yield |
|---|---|
| Multi-step reaction with 5 steps 1.1: 1.4 g / LiHMDS / tetrahydrofuran / 1 h / -78 - 0 °C 2.1: n-BuLi / tetrahydrofuran; hexane / -78 °C 2.2: BF3*OEt2 / tetrahydrofuran; hexane / -78 - 20 °C 2.3: 90 percent / K2CO3 / methanol / 20 °C 3.1: 100 percent / 2,6-lutidine / CH2Cl2 / 0 °C 4.1: 52 percent / Et3N; Pd(PPh3)4 / toluene / Heating 5.1: 61 percent / TBAF / tetrahydrofuran / 96 h / 20 °C View Scheme |

| Conditions | Yield |
|---|---|
| Multi-step reaction with 7 steps 1.1: 57 percent / NCS; Me2S / CH2Cl2 / 0.67 h 2.1: 92 percent / imidazole / dimethylformamide / 0.25 h / 20 °C 3.1: n-BuLi / hexane; tetrahydrofuran / 0.33 h / -50 °C 4.1: 86 mg / aq. H2O2 / CHCl3 / 0.02 h 5.1: 66 percent / n-butyllithium / tetrahydrofuran; toluene / 0.5 h / -50 °C 6.1: Mg / tetrahydrofuran / 0.5 h / 20 °C 6.2: 89 percent / CuBr*Me2S / tetrahydrofuran / 0.5 h / -10 °C 7.1: 96 percent / HF/pyridine / tetrahydrofuran / 20 °C View Scheme |
-
-
342645-07-2
(1α,2β,3β,5Z,7E,20S)-1,3-bis[(1,1-dimethylethyl)dimethyl-silyloxy]-2-[3-[(1,1-dimethylethyl)dimethylsilyloxy]propoxy]-9,10-secopregna-5,7,10(19)-triene-20-methyl 4-methylbenzenesulfonate

-
-
104121-92-8
Eldecalcitol

| Conditions | Yield |
|---|---|
| Multi-step reaction with 2 steps 1.1: Mg / tetrahydrofuran / 0.5 h / 20 °C 1.2: 89 percent / CuBr*Me2S / tetrahydrofuran / 0.5 h / -10 °C 2.1: 96 percent / HF/pyridine / tetrahydrofuran / 20 °C View Scheme |
-
-
66774-80-9
(S)-2-((3R,3aR,7S,7aR)-octahydro-7-hydroxy-3a-methyl-1H-inden-3-yl)propyl 4-methylbenzenesulfonate

-
-
104121-92-8
Eldecalcitol

| Conditions | Yield |
|---|---|
| Multi-step reaction with 4 steps 1.1: 97 percent / pyridinium p-toluenesulfonate; pyridinium dichromate / CH2Cl2 / 6 h / 20 °C 2.1: 66 percent / n-butyllithium / tetrahydrofuran; toluene / 0.5 h / -50 °C 3.1: Mg / tetrahydrofuran / 0.5 h / 20 °C 3.2: 89 percent / CuBr*Me2S / tetrahydrofuran / 0.5 h / -10 °C 4.1: 96 percent / HF/pyridine / tetrahydrofuran / 20 °C View Scheme | |
| Multi-step reaction with 4 steps 1.1: magnesium / tetrahydrofuran / 20 °C / Inert atmosphere 1.2: 18 h / -20 - 0 °C / Inert atmosphere 2.1: tetrabutylammomium bromide; 2,2,6,6-Tetramethyl-1-piperidinyloxy free radical; oxone / dichloromethane / 6 h / 20 °C / Inert atmosphere 3.1: n-butyllithium / tetrahydrofuran; hexane / 0.17 h / -78 °C / Inert atmosphere 3.2: 4 h / -78 °C / Inert atmosphere 4.1: methanol / 66 h / 20 °C / Inert atmosphere View Scheme |
-
-
200636-50-6
[3R-(1Z,3β,4α,5α)]-2-[3,5-bis[(1,1-dimethylethyl)dimethylsilyloxy]-4-[3-[(1,1-dimethylethyl)dimethylsilyloxy]propoxy]-2-methylenecyclohexylidene]-1-chloroethane

-
-
104121-92-8
Eldecalcitol

| Conditions | Yield |
|---|---|
| Multi-step reaction with 5 steps 1.1: n-BuLi / hexane; tetrahydrofuran / 0.33 h / -50 °C 2.1: 86 mg / aq. H2O2 / CHCl3 / 0.02 h 3.1: 66 percent / n-butyllithium / tetrahydrofuran; toluene / 0.5 h / -50 °C 4.1: Mg / tetrahydrofuran / 0.5 h / 20 °C 4.2: 89 percent / CuBr*Me2S / tetrahydrofuran / 0.5 h / -10 °C 5.1: 96 percent / HF/pyridine / tetrahydrofuran / 20 °C View Scheme | |
| Multi-step reaction with 3 steps 2: H2O2 View Scheme |
-
-
200636-54-0
[3R-(1Z,3β,4α,5α)]-[2-[3,5-bis[(1,1-dimethylethyl)dimethylsilyloxy]-4-[3-[(1,1-dimethylethyl)dimethylsilyloxy]propoxy]-2-methylenecyclohexylidene]ethyl]diphenylphosphine oxide

-
-
104121-92-8
Eldecalcitol

| Conditions | Yield |
|---|---|
| Multi-step reaction with 3 steps 1.1: 66 percent / n-butyllithium / tetrahydrofuran; toluene / 0.5 h / -50 °C 2.1: Mg / tetrahydrofuran / 0.5 h / 20 °C 2.2: 89 percent / CuBr*Me2S / tetrahydrofuran / 0.5 h / -10 °C 3.1: 96 percent / HF/pyridine / tetrahydrofuran / 20 °C View Scheme |

-
-
104121-92-8
Eldecalcitol

| Conditions | Yield |
|---|---|
| Multi-step reaction with 4 steps 1.1: 86 mg / aq. H2O2 / CHCl3 / 0.02 h 2.1: 66 percent / n-butyllithium / tetrahydrofuran; toluene / 0.5 h / -50 °C 3.1: Mg / tetrahydrofuran / 0.5 h / 20 °C 3.2: 89 percent / CuBr*Me2S / tetrahydrofuran / 0.5 h / -10 °C 4.1: 96 percent / HF/pyridine / tetrahydrofuran / 20 °C View Scheme | |
| Multi-step reaction with 2 steps 1: H2O2 View Scheme |
-
-
244278-87-3
[3R-(1Z,3β,4α,5α)]-2-[5-(1,1-dimethylethyl)dimethylsilyloxy-3,4-isopropylidenedioxy-2-methylenecyclohexylidene]ethanol

-
-
104121-92-8
Eldecalcitol

| Conditions | Yield |
|---|---|
| Multi-step reaction with 12 steps 1.1: 83 percent / pyridine; DMAP / CH2Cl2 / 1 h / 0 °C 2.1: 82 percent / CSA; (+)-10-camphorsulfonic acid / methanol / 3 h / 20 °C 3.1: 96 percent / imidazole / dimethylformamide / 3 h / 0 °C 4.1: 88 percent / Me4NOH; aq. NaOH / toluene / 72 h / 20 °C 5.1: 91 percent / LiAlH4 / tetrahydrofuran / 0.25 h / 0 °C 6.1: 57 percent / NCS; Me2S / CH2Cl2 / 0.67 h 7.1: 92 percent / imidazole / dimethylformamide / 0.25 h / 20 °C 8.1: n-BuLi / hexane; tetrahydrofuran / 0.33 h / -50 °C 9.1: 86 mg / aq. H2O2 / CHCl3 / 0.02 h 10.1: 66 percent / n-butyllithium / tetrahydrofuran; toluene / 0.5 h / -50 °C 11.1: Mg / tetrahydrofuran / 0.5 h / 20 °C 11.2: 89 percent / CuBr*Me2S / tetrahydrofuran / 0.5 h / -10 °C 12.1: 96 percent / HF/pyridine / tetrahydrofuran / 20 °C View Scheme | |
| Multi-step reaction with 14 steps 1.1: pyridine; dmap / dichloromethane / Inert atmosphere 2.1: water; trifluoroacetic acid / dichloromethane / 1 h / -10 °C / Inert atmosphere 3.1: 1H-imidazole / dichloromethane / 11 h / 0 °C / Inert atmosphere 4.1: pyridinium p-toluenesulfonate / dichloromethane / 36 h / 20 °C / Inert atmosphere 5.1: tetrabutyl ammonium fluoride / tetrahydrofuran / 5 h / 70 °C / Inert atmosphere 6.1: N-ethyl-N,N-diisopropylamine / dichloromethane / 6 h / Reflux; Inert atmosphere 7.1: acetic acid; water / tetrahydrofuran / 14 h / 40 °C / Inert atmosphere 8.1: water; tetramethyl ammoniumhydroxide; sodium hydroxide / toluene / 20 h / 20 °C / Inert atmosphere 9.1: lithium aluminium tetrahydride / tetrahydrofuran / 0 °C / Inert atmosphere 10.1: N-chloro-succinimide; dimethylsulfide / dichloromethane / 0.5 h / 0 °C / Inert atmosphere 11.1: N-ethyl-N,N-diisopropylamine / dichloromethane / 3 h / 20 °C / Inert atmosphere 12.1: lithium hexamethyldisilazane / tetrahydrofuran; dichloromethane / 3 h / -78 - 20 °C / Inert atmosphere 12.2: 13 h / 20 °C / Inert atmosphere 13.1: n-butyllithium / tetrahydrofuran; hexane / 0.17 h / -78 °C / Inert atmosphere 13.2: 4 h / -78 °C / Inert atmosphere 14.1: methanol / 66 h / 20 °C / Inert atmosphere View Scheme |
-
-
377091-68-4
[3R-(1Z,3β,4α,5α)]-2-[3,4,5-trihydroxy-2-methylenecyclohexylidene]ethyl trimethylacetate

-
-
104121-92-8
Eldecalcitol

| Conditions | Yield |
|---|---|
| Multi-step reaction with 10 steps 1.1: 96 percent / imidazole / dimethylformamide / 3 h / 0 °C 2.1: 88 percent / Me4NOH; aq. NaOH / toluene / 72 h / 20 °C 3.1: 91 percent / LiAlH4 / tetrahydrofuran / 0.25 h / 0 °C 4.1: 57 percent / NCS; Me2S / CH2Cl2 / 0.67 h 5.1: 92 percent / imidazole / dimethylformamide / 0.25 h / 20 °C 6.1: n-BuLi / hexane; tetrahydrofuran / 0.33 h / -50 °C 7.1: 86 mg / aq. H2O2 / CHCl3 / 0.02 h 8.1: 66 percent / n-butyllithium / tetrahydrofuran; toluene / 0.5 h / -50 °C 9.1: Mg / tetrahydrofuran / 0.5 h / 20 °C 9.2: 89 percent / CuBr*Me2S / tetrahydrofuran / 0.5 h / -10 °C 10.1: 96 percent / HF/pyridine / tetrahydrofuran / 20 °C View Scheme |
-
-
342644-83-1
[3R-(1Z,3β,4α,5α)]-2-[5-(1,1-dimethylethyl)dimethylsilyloxy-3,4-isopropylidenedioxy-2-methylenecyclohexylidene]ethyl trimethylacetate

-
-
104121-92-8
Eldecalcitol

| Conditions | Yield |
|---|---|
| Multi-step reaction with 11 steps 1.1: 82 percent / CSA; (+)-10-camphorsulfonic acid / methanol / 3 h / 20 °C 2.1: 96 percent / imidazole / dimethylformamide / 3 h / 0 °C 3.1: 88 percent / Me4NOH; aq. NaOH / toluene / 72 h / 20 °C 4.1: 91 percent / LiAlH4 / tetrahydrofuran / 0.25 h / 0 °C 5.1: 57 percent / NCS; Me2S / CH2Cl2 / 0.67 h 6.1: 92 percent / imidazole / dimethylformamide / 0.25 h / 20 °C 7.1: n-BuLi / hexane; tetrahydrofuran / 0.33 h / -50 °C 8.1: 86 mg / aq. H2O2 / CHCl3 / 0.02 h 9.1: 66 percent / n-butyllithium / tetrahydrofuran; toluene / 0.5 h / -50 °C 10.1: Mg / tetrahydrofuran / 0.5 h / 20 °C 10.2: 89 percent / CuBr*Me2S / tetrahydrofuran / 0.5 h / -10 °C 11.1: 96 percent / HF/pyridine / tetrahydrofuran / 20 °C View Scheme | |
| Multi-step reaction with 13 steps 1.1: water; trifluoroacetic acid / dichloromethane / 1 h / -10 °C / Inert atmosphere 2.1: 1H-imidazole / dichloromethane / 11 h / 0 °C / Inert atmosphere 3.1: pyridinium p-toluenesulfonate / dichloromethane / 36 h / 20 °C / Inert atmosphere 4.1: tetrabutyl ammonium fluoride / tetrahydrofuran / 5 h / 70 °C / Inert atmosphere 5.1: N-ethyl-N,N-diisopropylamine / dichloromethane / 6 h / Reflux; Inert atmosphere 6.1: acetic acid; water / tetrahydrofuran / 14 h / 40 °C / Inert atmosphere 7.1: water; tetramethyl ammoniumhydroxide; sodium hydroxide / toluene / 20 h / 20 °C / Inert atmosphere 8.1: lithium aluminium tetrahydride / tetrahydrofuran / 0 °C / Inert atmosphere 9.1: N-chloro-succinimide; dimethylsulfide / dichloromethane / 0.5 h / 0 °C / Inert atmosphere 10.1: N-ethyl-N,N-diisopropylamine / dichloromethane / 3 h / 20 °C / Inert atmosphere 11.1: lithium hexamethyldisilazane / tetrahydrofuran; dichloromethane / 3 h / -78 - 20 °C / Inert atmosphere 11.2: 13 h / 20 °C / Inert atmosphere 12.1: n-butyllithium / tetrahydrofuran; hexane / 0.17 h / -78 °C / Inert atmosphere 12.2: 4 h / -78 °C / Inert atmosphere 13.1: methanol / 66 h / 20 °C / Inert atmosphere View Scheme |
-
-
342645-84-5
(1β,2α,3α,5Z,7E)-1,3-bis[(1,1-dimethylethyl)dimethylsilyloxy]-2-[3-[(1,1-dimethylethyl)dimethylsilyloxy]propoxy]-25-trimethylsilyloxy-9,10-secocholesta-5,7,10(19)-triene

-
-
104121-92-8
Eldecalcitol

| Conditions | Yield |
|---|---|
| With pyridine; hydrogen fluoride In tetrahydrofuran at 20℃; | 96% |

-
-
104121-92-8
Eldecalcitol

| Conditions | Yield |
|---|---|
| With tetrabutyl ammonium fluoride In tetrahydrofuran at 25℃; | 89% |

-
-
104121-92-8
Eldecalcitol

| Conditions | Yield |
|---|---|
| With toluene-4-sulfonic acid In methanol; dichloromethane at 20℃; for 5h; Inert atmosphere; | 80% |

-
-
104121-92-8
Eldecalcitol

| Conditions | Yield |
|---|---|
| With methanol at 20℃; for 66h; Inert atmosphere; stereoselective reaction; | 68% |

-
-
104121-92-8
Eldecalcitol

| Conditions | Yield |
|---|---|
| With tetrabutyl ammonium fluoride In tetrahydrofuran at 20℃; for 96h; | 61% |
-
-
933779-95-4
(5Z,7E)-(1R,2R,3R)-1,3-bis(tert-butyldimethylsilyloxy)-2-(3-tert-butyldimethylsilyloxypropoxy)-25-triethylsilyloxy-9,10-secocholesta-5,7,10(19)-triene

-
-
104121-92-8
Eldecalcitol

| Conditions | Yield |
|---|---|
| With tetrabutyl ammonium fluoride In tetrahydrofuran; toluene at 105℃; for 2h; | 60% |

-
-
104121-92-8
Eldecalcitol

| Conditions | Yield |
|---|---|
| Stage #1: (5R,6R)-6-((R)-1-(methoxymethoxy)allyl)-5-(prop-2-yn-1-yl)-2,4,7,11,13-pentaoxatetradecane; C21H37BrO2 With tetrakis(triphenylphosphine) palladium(0); triethylamine In toluene at 110℃; for 2h; Stage #2: With camphor-10-sulfonic acid In methanol at 20℃; for 72h; | 36% |
-
-
144848-24-8
25-[(triethylsilyl)oxy]de-A,B-cholestan-8-one

-
-
200636-54-0
[3R-(1Z,3β,4α,5α)]-[2-[3,5-bis[(1,1-dimethylethyl)dimethylsilyloxy]-4-[3-[(1,1-dimethylethyl)dimethylsilyloxy]propoxy]-2-methylenecyclohexylidene]ethyl]diphenylphosphine oxide

-
-
104121-92-8
Eldecalcitol

| Conditions | Yield |
|---|---|
| Multistep reaction; |
-
-
299410-93-8
methyl 4,6-O-benzylidene-3-O-(3-hydroxypropyl)-α-D-altropyranoside

-
-
104121-92-8
Eldecalcitol

| Conditions | Yield |
|---|---|
| Multi-step reaction with 9 steps 1.1: 2.1 g / Et3N; DMAP / CH2Cl2 / 3 h / 20 °C 2.1: 91 percent / BaCO3; NBS / CCl4 / Heating 3.1: 86 percent / Zn; NaBH3CN / propan-1-ol; H2O / 95 °C 4.1: pyridine / 20 °C 5.1: 1.4 g / LiHMDS / tetrahydrofuran / 1 h / -78 - 0 °C 6.1: n-BuLi / tetrahydrofuran; hexane / -78 °C 6.2: BF3*OEt2 / tetrahydrofuran; hexane / -78 - 20 °C 6.3: 90 percent / K2CO3 / methanol / 20 °C 7.1: 100 percent / 2,6-lutidine / CH2Cl2 / 0 °C 8.1: 52 percent / Et3N; Pd(PPh3)4 / toluene / Heating 9.1: 61 percent / TBAF / tetrahydrofuran / 96 h / 20 °C View Scheme |
-
-
299410-95-0
methyl 4,6-O-benzylidene-3-O-[3-{(tert-butyldimethylsilyl)oxy}propyl]-α-D-altropyranoside

-
-
104121-92-8
Eldecalcitol

| Conditions | Yield |
|---|---|
| Multi-step reaction with 8 steps 1.1: 91 percent / BaCO3; NBS / CCl4 / Heating 2.1: 86 percent / Zn; NaBH3CN / propan-1-ol; H2O / 95 °C 3.1: pyridine / 20 °C 4.1: 1.4 g / LiHMDS / tetrahydrofuran / 1 h / -78 - 0 °C 5.1: n-BuLi / tetrahydrofuran; hexane / -78 °C 5.2: BF3*OEt2 / tetrahydrofuran; hexane / -78 - 20 °C 5.3: 90 percent / K2CO3 / methanol / 20 °C 6.1: 100 percent / 2,6-lutidine / CH2Cl2 / 0 °C 7.1: 52 percent / Et3N; Pd(PPh3)4 / toluene / Heating 8.1: 61 percent / TBAF / tetrahydrofuran / 96 h / 20 °C View Scheme |
-
-
299410-97-2
methyl 4-O-benzoyl-6-bromo-3-O-[3-{(tert-butyldimethylsilyl)oxy}propyl]-6-deoxy-α-D-altropyranoside

-
-
104121-92-8
Eldecalcitol

| Conditions | Yield |
|---|---|
| Multi-step reaction with 7 steps 1.1: 86 percent / Zn; NaBH3CN / propan-1-ol; H2O / 95 °C 2.1: pyridine / 20 °C 3.1: 1.4 g / LiHMDS / tetrahydrofuran / 1 h / -78 - 0 °C 4.1: n-BuLi / tetrahydrofuran; hexane / -78 °C 4.2: BF3*OEt2 / tetrahydrofuran; hexane / -78 - 20 °C 4.3: 90 percent / K2CO3 / methanol / 20 °C 5.1: 100 percent / 2,6-lutidine / CH2Cl2 / 0 °C 6.1: 52 percent / Et3N; Pd(PPh3)4 / toluene / Heating 7.1: 61 percent / TBAF / tetrahydrofuran / 96 h / 20 °C View Scheme |
-
-
299411-15-7
(3R,4S,5R)-3,5-bis(tert-butyldimethylsilyloxy)-4-[3-(tert-butyldimethylsilyloxy)propoxy]oct-1-en-7-yne

-
-
104121-92-8
Eldecalcitol

| Conditions | Yield |
|---|---|
| Multi-step reaction with 2 steps 1: 52 percent / Et3N; Pd(PPh3)4 / toluene / Heating 2: 61 percent / TBAF / tetrahydrofuran / 96 h / 20 °C View Scheme |
-
-
3150-15-0, 3150-16-1, 3257-59-8, 14187-71-4, 15384-57-3, 19465-13-5, 32976-15-1, 52885-37-7, 53270-02-3, 53270-03-4, 65391-11-9, 65391-12-0, 66537-92-6, 67226-04-4, 71184-14-0, 86420-78-2, 86420-79-3, 91177-51-4, 91177-52-5, 117708-93-7, 120408-72-2, 2880-96-8
methyl 2,3-anhydro-4,6-O-benzilidene-α-D-mannopyranoside

-
-
104121-92-8
Eldecalcitol

| Conditions | Yield |
|---|---|
| Multi-step reaction with 10 steps 1.1: KOt-Bu / 110 °C 2.1: 2.1 g / Et3N; DMAP / CH2Cl2 / 3 h / 20 °C 3.1: 91 percent / BaCO3; NBS / CCl4 / Heating 4.1: 86 percent / Zn; NaBH3CN / propan-1-ol; H2O / 95 °C 5.1: pyridine / 20 °C 6.1: 1.4 g / LiHMDS / tetrahydrofuran / 1 h / -78 - 0 °C 7.1: n-BuLi / tetrahydrofuran; hexane / -78 °C 7.2: BF3*OEt2 / tetrahydrofuran; hexane / -78 - 20 °C 7.3: 90 percent / K2CO3 / methanol / 20 °C 8.1: 100 percent / 2,6-lutidine / CH2Cl2 / 0 °C 9.1: 52 percent / Et3N; Pd(PPh3)4 / toluene / Heating 10.1: 61 percent / TBAF / tetrahydrofuran / 96 h / 20 °C View Scheme |
-
-
794516-13-5
(3R,4S,5R)-4-[3-(tert-butyldimethylsilyloxy)propoxy]oct-1-en-7-yne-3,5-diol

-
-
104121-92-8
Eldecalcitol

| Conditions | Yield |
|---|---|
| Multi-step reaction with 3 steps 1: 100 percent / 2,6-lutidine / CH2Cl2 / 0 °C 2: 52 percent / Et3N; Pd(PPh3)4 / toluene / Heating 3: 61 percent / TBAF / tetrahydrofuran / 96 h / 20 °C View Scheme |
-
-
794516-11-3
(3R,4R,5R)-3-benzoyloxy-4-[3-(tert-butyldimethylsilyloxy)propoxy]-5,6-epoxyhex-1-ene

-
-
104121-92-8
Eldecalcitol

| Conditions | Yield |
|---|---|
| Multi-step reaction with 4 steps 1.1: n-BuLi / tetrahydrofuran; hexane / -78 °C 1.2: BF3*OEt2 / tetrahydrofuran; hexane / -78 - 20 °C 1.3: 90 percent / K2CO3 / methanol / 20 °C 2.1: 100 percent / 2,6-lutidine / CH2Cl2 / 0 °C 3.1: 52 percent / Et3N; Pd(PPh3)4 / toluene / Heating 4.1: 61 percent / TBAF / tetrahydrofuran / 96 h / 20 °C View Scheme |
-
-
794516-09-9
(2R,3S,4R)-4-benzoyloxy-3-[3-(tert-butyldimethylsilyloxy)propoxy]hex-5-ene-1,2-diol

-
-
104121-92-8
Eldecalcitol

| Conditions | Yield |
|---|---|
| Multi-step reaction with 6 steps 1.1: pyridine / 20 °C 2.1: 1.4 g / LiHMDS / tetrahydrofuran / 1 h / -78 - 0 °C 3.1: n-BuLi / tetrahydrofuran; hexane / -78 °C 3.2: BF3*OEt2 / tetrahydrofuran; hexane / -78 - 20 °C 3.3: 90 percent / K2CO3 / methanol / 20 °C 4.1: 100 percent / 2,6-lutidine / CH2Cl2 / 0 °C 5.1: 52 percent / Et3N; Pd(PPh3)4 / toluene / Heating 6.1: 61 percent / TBAF / tetrahydrofuran / 96 h / 20 °C View Scheme |

-
-
104121-92-8
Eldecalcitol

| Conditions | Yield |
|---|---|
| Multi-step reaction with 5 steps 1.1: 1.4 g / LiHMDS / tetrahydrofuran / 1 h / -78 - 0 °C 2.1: n-BuLi / tetrahydrofuran; hexane / -78 °C 2.2: BF3*OEt2 / tetrahydrofuran; hexane / -78 - 20 °C 2.3: 90 percent / K2CO3 / methanol / 20 °C 3.1: 100 percent / 2,6-lutidine / CH2Cl2 / 0 °C 4.1: 52 percent / Et3N; Pd(PPh3)4 / toluene / Heating 5.1: 61 percent / TBAF / tetrahydrofuran / 96 h / 20 °C View Scheme |
-
-
342644-91-1
[3R-(1Z,3β,4α,5α)]-2-[3,5-bis[(1,1-dimethylethyl)dimethylsilyloxy]-4-(3-hydroxypropoxy)-2-methylenecyclohexylidene]ethanol

-
-
104121-92-8
Eldecalcitol

| Conditions | Yield |
|---|---|
| Multi-step reaction with 7 steps 1.1: 57 percent / NCS; Me2S / CH2Cl2 / 0.67 h 2.1: 92 percent / imidazole / dimethylformamide / 0.25 h / 20 °C 3.1: n-BuLi / hexane; tetrahydrofuran / 0.33 h / -50 °C 4.1: 86 mg / aq. H2O2 / CHCl3 / 0.02 h 5.1: 66 percent / n-butyllithium / tetrahydrofuran; toluene / 0.5 h / -50 °C 6.1: Mg / tetrahydrofuran / 0.5 h / 20 °C 6.2: 89 percent / CuBr*Me2S / tetrahydrofuran / 0.5 h / -10 °C 7.1: 96 percent / HF/pyridine / tetrahydrofuran / 20 °C View Scheme |
-
-
342645-07-2
(1α,2β,3β,5Z,7E,20S)-1,3-bis[(1,1-dimethylethyl)dimethyl-silyloxy]-2-[3-[(1,1-dimethylethyl)dimethylsilyloxy]propoxy]-9,10-secopregna-5,7,10(19)-triene-20-methyl 4-methylbenzenesulfonate

-
-
104121-92-8
Eldecalcitol

| Conditions | Yield |
|---|---|
| Multi-step reaction with 2 steps 1.1: Mg / tetrahydrofuran / 0.5 h / 20 °C 1.2: 89 percent / CuBr*Me2S / tetrahydrofuran / 0.5 h / -10 °C 2.1: 96 percent / HF/pyridine / tetrahydrofuran / 20 °C View Scheme |
-
-
66774-80-9
(S)-2-((3R,3aR,7S,7aR)-octahydro-7-hydroxy-3a-methyl-1H-inden-3-yl)propyl 4-methylbenzenesulfonate

-
-
104121-92-8
Eldecalcitol

| Conditions | Yield |
|---|---|
| Multi-step reaction with 4 steps 1.1: 97 percent / pyridinium p-toluenesulfonate; pyridinium dichromate / CH2Cl2 / 6 h / 20 °C 2.1: 66 percent / n-butyllithium / tetrahydrofuran; toluene / 0.5 h / -50 °C 3.1: Mg / tetrahydrofuran / 0.5 h / 20 °C 3.2: 89 percent / CuBr*Me2S / tetrahydrofuran / 0.5 h / -10 °C 4.1: 96 percent / HF/pyridine / tetrahydrofuran / 20 °C View Scheme | |
| Multi-step reaction with 4 steps 1.1: magnesium / tetrahydrofuran / 20 °C / Inert atmosphere 1.2: 18 h / -20 - 0 °C / Inert atmosphere 2.1: tetrabutylammomium bromide; 2,2,6,6-Tetramethyl-1-piperidinyloxy free radical; oxone / dichloromethane / 6 h / 20 °C / Inert atmosphere 3.1: n-butyllithium / tetrahydrofuran; hexane / 0.17 h / -78 °C / Inert atmosphere 3.2: 4 h / -78 °C / Inert atmosphere 4.1: methanol / 66 h / 20 °C / Inert atmosphere View Scheme |
-
-
200636-50-6
[3R-(1Z,3β,4α,5α)]-2-[3,5-bis[(1,1-dimethylethyl)dimethylsilyloxy]-4-[3-[(1,1-dimethylethyl)dimethylsilyloxy]propoxy]-2-methylenecyclohexylidene]-1-chloroethane

-
-
104121-92-8
Eldecalcitol

| Conditions | Yield |
|---|---|
| Multi-step reaction with 5 steps 1.1: n-BuLi / hexane; tetrahydrofuran / 0.33 h / -50 °C 2.1: 86 mg / aq. H2O2 / CHCl3 / 0.02 h 3.1: 66 percent / n-butyllithium / tetrahydrofuran; toluene / 0.5 h / -50 °C 4.1: Mg / tetrahydrofuran / 0.5 h / 20 °C 4.2: 89 percent / CuBr*Me2S / tetrahydrofuran / 0.5 h / -10 °C 5.1: 96 percent / HF/pyridine / tetrahydrofuran / 20 °C View Scheme | |
| Multi-step reaction with 3 steps 2: H2O2 View Scheme |
-
-
200636-54-0
[3R-(1Z,3β,4α,5α)]-[2-[3,5-bis[(1,1-dimethylethyl)dimethylsilyloxy]-4-[3-[(1,1-dimethylethyl)dimethylsilyloxy]propoxy]-2-methylenecyclohexylidene]ethyl]diphenylphosphine oxide

-
-
104121-92-8
Eldecalcitol

| Conditions | Yield |
|---|---|
| Multi-step reaction with 3 steps 1.1: 66 percent / n-butyllithium / tetrahydrofuran; toluene / 0.5 h / -50 °C 2.1: Mg / tetrahydrofuran / 0.5 h / 20 °C 2.2: 89 percent / CuBr*Me2S / tetrahydrofuran / 0.5 h / -10 °C 3.1: 96 percent / HF/pyridine / tetrahydrofuran / 20 °C View Scheme |

-
-
104121-92-8
Eldecalcitol

| Conditions | Yield |
|---|---|
| Multi-step reaction with 4 steps 1.1: 86 mg / aq. H2O2 / CHCl3 / 0.02 h 2.1: 66 percent / n-butyllithium / tetrahydrofuran; toluene / 0.5 h / -50 °C 3.1: Mg / tetrahydrofuran / 0.5 h / 20 °C 3.2: 89 percent / CuBr*Me2S / tetrahydrofuran / 0.5 h / -10 °C 4.1: 96 percent / HF/pyridine / tetrahydrofuran / 20 °C View Scheme | |
| Multi-step reaction with 2 steps 1: H2O2 View Scheme |
-
-
244278-87-3
[3R-(1Z,3β,4α,5α)]-2-[5-(1,1-dimethylethyl)dimethylsilyloxy-3,4-isopropylidenedioxy-2-methylenecyclohexylidene]ethanol

-
-
104121-92-8
Eldecalcitol

| Conditions | Yield |
|---|---|
| Multi-step reaction with 12 steps 1.1: 83 percent / pyridine; DMAP / CH2Cl2 / 1 h / 0 °C 2.1: 82 percent / CSA; (+)-10-camphorsulfonic acid / methanol / 3 h / 20 °C 3.1: 96 percent / imidazole / dimethylformamide / 3 h / 0 °C 4.1: 88 percent / Me4NOH; aq. NaOH / toluene / 72 h / 20 °C 5.1: 91 percent / LiAlH4 / tetrahydrofuran / 0.25 h / 0 °C 6.1: 57 percent / NCS; Me2S / CH2Cl2 / 0.67 h 7.1: 92 percent / imidazole / dimethylformamide / 0.25 h / 20 °C 8.1: n-BuLi / hexane; tetrahydrofuran / 0.33 h / -50 °C 9.1: 86 mg / aq. H2O2 / CHCl3 / 0.02 h 10.1: 66 percent / n-butyllithium / tetrahydrofuran; toluene / 0.5 h / -50 °C 11.1: Mg / tetrahydrofuran / 0.5 h / 20 °C 11.2: 89 percent / CuBr*Me2S / tetrahydrofuran / 0.5 h / -10 °C 12.1: 96 percent / HF/pyridine / tetrahydrofuran / 20 °C View Scheme | |
| Multi-step reaction with 14 steps 1.1: pyridine; dmap / dichloromethane / Inert atmosphere 2.1: water; trifluoroacetic acid / dichloromethane / 1 h / -10 °C / Inert atmosphere 3.1: 1H-imidazole / dichloromethane / 11 h / 0 °C / Inert atmosphere 4.1: pyridinium p-toluenesulfonate / dichloromethane / 36 h / 20 °C / Inert atmosphere 5.1: tetrabutyl ammonium fluoride / tetrahydrofuran / 5 h / 70 °C / Inert atmosphere 6.1: N-ethyl-N,N-diisopropylamine / dichloromethane / 6 h / Reflux; Inert atmosphere 7.1: acetic acid; water / tetrahydrofuran / 14 h / 40 °C / Inert atmosphere 8.1: water; tetramethyl ammoniumhydroxide; sodium hydroxide / toluene / 20 h / 20 °C / Inert atmosphere 9.1: lithium aluminium tetrahydride / tetrahydrofuran / 0 °C / Inert atmosphere 10.1: N-chloro-succinimide; dimethylsulfide / dichloromethane / 0.5 h / 0 °C / Inert atmosphere 11.1: N-ethyl-N,N-diisopropylamine / dichloromethane / 3 h / 20 °C / Inert atmosphere 12.1: lithium hexamethyldisilazane / tetrahydrofuran; dichloromethane / 3 h / -78 - 20 °C / Inert atmosphere 12.2: 13 h / 20 °C / Inert atmosphere 13.1: n-butyllithium / tetrahydrofuran; hexane / 0.17 h / -78 °C / Inert atmosphere 13.2: 4 h / -78 °C / Inert atmosphere 14.1: methanol / 66 h / 20 °C / Inert atmosphere View Scheme |
-
-
377091-68-4
[3R-(1Z,3β,4α,5α)]-2-[3,4,5-trihydroxy-2-methylenecyclohexylidene]ethyl trimethylacetate

-
-
104121-92-8
Eldecalcitol

| Conditions | Yield |
|---|---|
| Multi-step reaction with 10 steps 1.1: 96 percent / imidazole / dimethylformamide / 3 h / 0 °C 2.1: 88 percent / Me4NOH; aq. NaOH / toluene / 72 h / 20 °C 3.1: 91 percent / LiAlH4 / tetrahydrofuran / 0.25 h / 0 °C 4.1: 57 percent / NCS; Me2S / CH2Cl2 / 0.67 h 5.1: 92 percent / imidazole / dimethylformamide / 0.25 h / 20 °C 6.1: n-BuLi / hexane; tetrahydrofuran / 0.33 h / -50 °C 7.1: 86 mg / aq. H2O2 / CHCl3 / 0.02 h 8.1: 66 percent / n-butyllithium / tetrahydrofuran; toluene / 0.5 h / -50 °C 9.1: Mg / tetrahydrofuran / 0.5 h / 20 °C 9.2: 89 percent / CuBr*Me2S / tetrahydrofuran / 0.5 h / -10 °C 10.1: 96 percent / HF/pyridine / tetrahydrofuran / 20 °C View Scheme |
-
-
342644-83-1
[3R-(1Z,3β,4α,5α)]-2-[5-(1,1-dimethylethyl)dimethylsilyloxy-3,4-isopropylidenedioxy-2-methylenecyclohexylidene]ethyl trimethylacetate

-
-
104121-92-8
Eldecalcitol

| Conditions | Yield |
|---|---|
| Multi-step reaction with 11 steps 1.1: 82 percent / CSA; (+)-10-camphorsulfonic acid / methanol / 3 h / 20 °C 2.1: 96 percent / imidazole / dimethylformamide / 3 h / 0 °C 3.1: 88 percent / Me4NOH; aq. NaOH / toluene / 72 h / 20 °C 4.1: 91 percent / LiAlH4 / tetrahydrofuran / 0.25 h / 0 °C 5.1: 57 percent / NCS; Me2S / CH2Cl2 / 0.67 h 6.1: 92 percent / imidazole / dimethylformamide / 0.25 h / 20 °C 7.1: n-BuLi / hexane; tetrahydrofuran / 0.33 h / -50 °C 8.1: 86 mg / aq. H2O2 / CHCl3 / 0.02 h 9.1: 66 percent / n-butyllithium / tetrahydrofuran; toluene / 0.5 h / -50 °C 10.1: Mg / tetrahydrofuran / 0.5 h / 20 °C 10.2: 89 percent / CuBr*Me2S / tetrahydrofuran / 0.5 h / -10 °C 11.1: 96 percent / HF/pyridine / tetrahydrofuran / 20 °C View Scheme | |
| Multi-step reaction with 13 steps 1.1: water; trifluoroacetic acid / dichloromethane / 1 h / -10 °C / Inert atmosphere 2.1: 1H-imidazole / dichloromethane / 11 h / 0 °C / Inert atmosphere 3.1: pyridinium p-toluenesulfonate / dichloromethane / 36 h / 20 °C / Inert atmosphere 4.1: tetrabutyl ammonium fluoride / tetrahydrofuran / 5 h / 70 °C / Inert atmosphere 5.1: N-ethyl-N,N-diisopropylamine / dichloromethane / 6 h / Reflux; Inert atmosphere 6.1: acetic acid; water / tetrahydrofuran / 14 h / 40 °C / Inert atmosphere 7.1: water; tetramethyl ammoniumhydroxide; sodium hydroxide / toluene / 20 h / 20 °C / Inert atmosphere 8.1: lithium aluminium tetrahydride / tetrahydrofuran / 0 °C / Inert atmosphere 9.1: N-chloro-succinimide; dimethylsulfide / dichloromethane / 0.5 h / 0 °C / Inert atmosphere 10.1: N-ethyl-N,N-diisopropylamine / dichloromethane / 3 h / 20 °C / Inert atmosphere 11.1: lithium hexamethyldisilazane / tetrahydrofuran; dichloromethane / 3 h / -78 - 20 °C / Inert atmosphere 11.2: 13 h / 20 °C / Inert atmosphere 12.1: n-butyllithium / tetrahydrofuran; hexane / 0.17 h / -78 °C / Inert atmosphere 12.2: 4 h / -78 °C / Inert atmosphere 13.1: methanol / 66 h / 20 °C / Inert atmosphere View Scheme |
-
-
342644-90-0
[3R-(1Z,3β,4α,5α)]-2-[3,5-bis[(1,1-dimethylethyl)dimethylsilyloxy]-4-hydroxy-2-methylenecyclohexylidene]ethyl trimethylacetate

-
-
104121-92-8
Eldecalcitol

| Conditions | Yield |
|---|---|
| Multi-step reaction with 9 steps 1.1: 88 percent / Me4NOH; aq. NaOH / toluene / 72 h / 20 °C 2.1: 91 percent / LiAlH4 / tetrahydrofuran / 0.25 h / 0 °C 3.1: 57 percent / NCS; Me2S / CH2Cl2 / 0.67 h 4.1: 92 percent / imidazole / dimethylformamide / 0.25 h / 20 °C 5.1: n-BuLi / hexane; tetrahydrofuran / 0.33 h / -50 °C 6.1: 86 mg / aq. H2O2 / CHCl3 / 0.02 h 7.1: 66 percent / n-butyllithium / tetrahydrofuran; toluene / 0.5 h / -50 °C 8.1: Mg / tetrahydrofuran / 0.5 h / 20 °C 8.2: 89 percent / CuBr*Me2S / tetrahydrofuran / 0.5 h / -10 °C 9.1: 96 percent / HF/pyridine / tetrahydrofuran / 20 °C View Scheme | |
| Multi-step reaction with 11 steps 1.1: pyridinium p-toluenesulfonate / dichloromethane / 36 h / 20 °C / Inert atmosphere 2.1: tetrabutyl ammonium fluoride / tetrahydrofuran / 5 h / 70 °C / Inert atmosphere 3.1: N-ethyl-N,N-diisopropylamine / dichloromethane / 6 h / Reflux; Inert atmosphere 4.1: acetic acid; water / tetrahydrofuran / 14 h / 40 °C / Inert atmosphere 5.1: water; tetramethyl ammoniumhydroxide; sodium hydroxide / toluene / 20 h / 20 °C / Inert atmosphere 6.1: lithium aluminium tetrahydride / tetrahydrofuran / 0 °C / Inert atmosphere 7.1: N-chloro-succinimide; dimethylsulfide / dichloromethane / 0.5 h / 0 °C / Inert atmosphere 8.1: N-ethyl-N,N-diisopropylamine / dichloromethane / 3 h / 20 °C / Inert atmosphere 9.1: lithium hexamethyldisilazane / tetrahydrofuran; dichloromethane / 3 h / -78 - 20 °C / Inert atmosphere 9.2: 13 h / 20 °C / Inert atmosphere 10.1: n-butyllithium / tetrahydrofuran; hexane / 0.17 h / -78 °C / Inert atmosphere 10.2: 4 h / -78 °C / Inert atmosphere 11.1: methanol / 66 h / 20 °C / Inert atmosphere View Scheme |
-
-
342645-82-3
[3R-(1Z,3β,4α,5α)]-2-[3,5-bis[(1,1-dimethylethyl)dimethylsilyloxy]-4-(3-hydroxypropoxy)-2-methylenecyclohexylidene]-1-chloroethane

-
-
104121-92-8
Eldecalcitol

| Conditions | Yield |
|---|---|
| Multi-step reaction with 6 steps 1.1: 92 percent / imidazole / dimethylformamide / 0.25 h / 20 °C 2.1: n-BuLi / hexane; tetrahydrofuran / 0.33 h / -50 °C 3.1: 86 mg / aq. H2O2 / CHCl3 / 0.02 h 4.1: 66 percent / n-butyllithium / tetrahydrofuran; toluene / 0.5 h / -50 °C 5.1: Mg / tetrahydrofuran / 0.5 h / 20 °C 5.2: 89 percent / CuBr*Me2S / tetrahydrofuran / 0.5 h / -10 °C 6.1: 96 percent / HF/pyridine / tetrahydrofuran / 20 °C View Scheme |
-
-
342645-81-2
[3R-(1Z,3β,4α,5α)]-2-[3,5-bis[(1,1-dimethylethyl)dimethylsilyloxy]-4-[2-(ethoxycarbonyl)ethoxy]-2-methylenecyclohexylidene]ethyl trimethylacetate

-
-
104121-92-8
Eldecalcitol

| Conditions | Yield |
|---|---|
| Multi-step reaction with 8 steps 1.1: 91 percent / LiAlH4 / tetrahydrofuran / 0.25 h / 0 °C 2.1: 57 percent / NCS; Me2S / CH2Cl2 / 0.67 h 3.1: 92 percent / imidazole / dimethylformamide / 0.25 h / 20 °C 4.1: n-BuLi / hexane; tetrahydrofuran / 0.33 h / -50 °C 5.1: 86 mg / aq. H2O2 / CHCl3 / 0.02 h 6.1: 66 percent / n-butyllithium / tetrahydrofuran; toluene / 0.5 h / -50 °C 7.1: Mg / tetrahydrofuran / 0.5 h / 20 °C 7.2: 89 percent / CuBr*Me2S / tetrahydrofuran / 0.5 h / -10 °C 8.1: 96 percent / HF/pyridine / tetrahydrofuran / 20 °C View Scheme |
-
-
342645-83-4
(S)-2-[(1R,3aR,7aR)-octahydro-7a-methyl-4-oxo-4H-inden-1-yl]propyl 4-methylbenzenesulfonate

-
-
104121-92-8
Eldecalcitol

| Conditions | Yield |
|---|---|
| Multi-step reaction with 3 steps 1.1: 66 percent / n-butyllithium / tetrahydrofuran; toluene / 0.5 h / -50 °C 2.1: Mg / tetrahydrofuran / 0.5 h / 20 °C 2.2: 89 percent / CuBr*Me2S / tetrahydrofuran / 0.5 h / -10 °C 3.1: 96 percent / HF/pyridine / tetrahydrofuran / 20 °C View Scheme |
Related products
Hot Products
About|Contact|Cas|Product Name|Molecular|Country|Encyclopedia
Message|New Cas|MSDS|Service|Advertisement|CAS DataBase|Article Data|Manufacturers | Chemical Catalog
©2008 LookChem.com,License: ICP
NO.:Zhejiang16009103
complaints:service@lookchem.com Desktop View