Shandong Hanjiang Chemical Co., Ltd.
Hello, dear friend! I'm Hansen and Allen from China. Welcome to my lookchem mall! The following is a brief introduction of our company's products and services. If you are interested in our products, please contact us by emai
Cas:13209-41-1
Min.Order:1 Kilogram
Negotiable
Type:Lab/Research institutions
inquiryHenan Wentao Chemical Product Co., Ltd.
Henan Wentao Chemical Product Co.,Ltd is Located in Zhengzhou High-tech Development Zone with import and export license, We passed ISO 9001:2008 as well, Henan Wentao has developed more than 1000 compounds, which are widely used in the fields of prod
Cas:13209-41-1
Min.Order:0
Negotiable
Type:Lab/Research institutions
inquiryShaanxi Cuicheng Biomedical Technology Co., Ltd.
Why Choose Us: 1. Factory direct sales, so we can provide the competitive price and high quality product base on 8 years of production and R&D experience. 2. It is available in stock for quick shipment.Products could be packaged according to cu
Xiamen Jenny Chemical Technology Co., Ltd.
GMP standard, high purity, competitive price, in stock 1. Quick Response: within 6 hours after receiving your email. 2. Quality Guarantee: All products are strictly tested by our QC, confirmed by QA, and approved by a third-party lab in China, USA,
KAISA GROUP INC
1.Applied in food field.it can improve the immune system and prolong life. 2.Appliedin cosmetic field.it can improve the skin care. 3.Applied in pharmaceutical field.it can treat various dieases. 4.Our product quality assurance will make our customer
Cas:13209-41-1
Min.Order:1 Metric Ton
FOB Price: $7.0 / 8.0
Type:Trading Company
inquiryHangzhou Dingyan Chem Co., Ltd
R & D enterprises have their own stock in stock Package:1kg Application:pharmaceutical intermediates
Cas:13209-41-1
Min.Order:0
Negotiable
Type:Manufacturers
inquiryHenan Tianfu Chemical Co., Ltd.
1.Our services:A.Supply sampleB.The packing also can be according the customers` requirmentC.Any inquiries will be replied within 24 hoursD.we provide Commerical Invoice, Packing List, Bill of loading, COA , Health certificate and Origin certificate.
Cas:13209-41-1
Min.Order:0
Negotiable
Type:Lab/Research institutions
inquiryAntimex Chemical Limied
Ansciep Chemical is a professional enterprise manufacturing and distributing fine chemicals and speciality chemicals. We have been dedicated to heterocycle compounds and phenyl rings for tens of years. This is our mature product for export. Our quali
Cas:13209-41-1
Min.Order:0
Negotiable
Type:Lab/Research institutions
inquiryHANGZHOU TIANYE CHEMICALS CO., LTD.
We product this chemical more than 10 years . We are very experience to export it to many countries, Our superior & stable quality , competitive price gain warm reception from our customers. Application:Pharm intermediate
Wuhan Circle Star Chem-medical Technology co.,Ltd.
good quality, competitive price, thoughtful after sale serviceAppearance:white powder Storage:Keep it in dry,shady and cool place Package:25kg,50kg,180kg,200kg,250kg,1000kg,customization Application:Pharma;Industry;Agricultural;chemical reaserch Tran
Changchun Artel lmport and Export trade company
Supply top quality products with a reasonable price Application:api
Hangzhou Fandachem Co.,Ltd
17.alpha.,21-Dihydroxy-16.alpha.-methylpregna-1,4,9(11)-triene-3,20-dioneAppearance:white crystalline powder Storage:Store in dry, dark and ventilated place Package:25KG drum Application:pharmaceutical intermediate Transportation:by air, by sea, by e
Cas:13209-41-1
Min.Order:0
Negotiable
Type:Other
inquiryHenan Kanbei Chemical Co.,LTD
factory?direct?saleAppearance:White powder Storage:Sealed and preserved Package:200/Kilograms Application:healing drugs Transportation:By sea Port:Shanghai/tianjin
Cas:13209-41-1
Min.Order:0
Negotiable
Type:Lab/Research institutions
inquiryChemlyte Solutions
Stock products, own laboratory Package:Grams, Kilograms Application:For R&D Transportation:According to customer request Port:Shanghai
Shanghai Chinqesen Biotechnology Co., Ltd.
Good Quality Package:1kg/bag Application:Medical or chemical Transportation:Air/Train/Sea Port:Shenzhen
ZHEJIANG JIUZHOU CHEM CO.,LTD
factory?direct?saleAppearance:White powder Storage:Sealed and preserved Package:200/Kilograms Application:healing drugs Transportation:By sea Port:Shanghai/tianjin
Cas:13209-41-1
Min.Order:0
Negotiable
Type:Trading Company
inquiryLEAP CHEM Co., Ltd.
Best Seller, High Quality, Competitive Price, Fast Delivery, Quick ResponseAppearance:powder, or liquid Storage:Stored in room temperature, ventilated place Package:Bottle, barrel, cargo, container, etc. Application:Pharmaceuticals, intermediates, AP
Cas:13209-41-1
Min.Order:0
Negotiable
Type:Trading Company
inquiryJilin haofei import and export trade Co.,Ltd
Price, service, company and transport advantage: 1. Best service, place of origin China, high quality, and reasonable price. 2. It's customers' right to choose the package (EMS, DHL, FEDEX, UPS). 3. It's customers' right
suzhou BetterBioChem Co., Ltd.
fast delivery Hot sale 13209-41-1 Reliable quality Vamorolone Vamorolone (VBP15) is a first-in-class, orally active dissociative steroidal anti-inflammatory drug and membrane-stabilizer. Vamorolone improves muscular dystrophy without side effects. V
Cas:13209-41-1
Min.Order:1 Gram
Negotiable
Type:Lab/Research institutions
inquiryChungking Joyinchem Co., Ltd.
Joyinchem have been committed to chemical supply for several years and have built good cooperation records with multinational chemical corporations and importers from all over the world. Our services include:-Spot goods-Contract manufacturing-Custom
Cas:13209-41-1
Min.Order:0
Negotiable
Type:Lab/Research institutions
inquiryFinetech Industry Limited
FINETECH INDUSTRY LIMITED is a LONDON based CRO company providing drug discovery & development services to worldwide clients. FINETECH INDUSTRY LIMITED supplies the 16a-Methyl-9,11-dehydro Prednisolone, CAS:13209-41-1 with the most competitive price
Cas:13209-41-1
Min.Order:0
Negotiable
Type:Trading Company
inquiryNanjing Raymon Biotech Co., Ltd.
Pregna-1,4,9(11)-triene-3,20-dione,17,21-dihydroxy-16-methyl-, (16a)- Storage:keep in dry and cool condition Package:25kg or according to cutomer's demand Application:Chemical research/pharma intermediate Transportation:By Sea,by Air,By courier like
Cas:13209-41-1
Min.Order:0
Negotiable
Type:Trading Company
inquiryGuangzhou PI & PI Biotech Inc. Ltd.
Impurities from EP, CP, USP, JP, IP and independent R&D Package:10mg, 25mg, 50mg, 100mg, Other scale please send an equiry Application:For research and development purpose Port:Guangzhou
Cas:13209-41-1
Min.Order:0
Negotiable
Type:Lab/Research institutions
inquiryShenzhen Sumshine Biotech Co., Ltd.
1. Our staff are all biomedical related majors with rich experience in the pharmaceutical industry, and can provide you with more professional services.2. Our supplier has a good quality management system, and the quality of products is reliable and
Beyond Pharmaceutical Co., Ltd
Cas:13209-41-1
Min.Order:0
Negotiable
Type:Lab/Research institutions
inquiryChemical Co.Ltd
Pregna-1,4,9(11)-triene-3,20-dione,17,21-dihydroxy-16-methyl-, (16a)-Appearance:Off white to slight yellow solid Storage:Stored in shaded, cool and dry places Package:1L 5L 10L 25L bottle Application:pharma intermediate Transportation:Handle with car
Cas:13209-41-1
Min.Order:0
Negotiable
Type:Trading Company
inquiryNovaChemistry
High purity Application:Pharmaceutical intermediates, Material synthesis and analyzing the expectant ingredients
Synthetic route

-
-
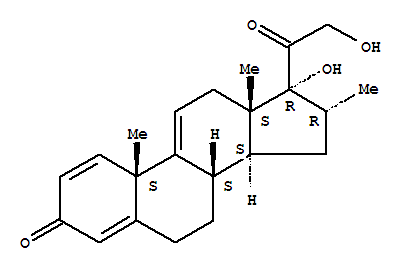
13209-41-1
(8S,10S,13S,14S,16R,17R)-17-hydroxy-17-(2-hydroxyacetyl)-10,13,16-trimethyl-7,8,12,14,15,16-hexahydro-6H-cyclopenta[a]phenanthren-3-one

| Conditions | Yield |
|---|---|
| With hydrogenchloride In pentan-1-ol | 80% |

-
-
13209-41-1
(8S,10S,13S,14S,16R,17R)-17-hydroxy-17-(2-hydroxyacetyl)-10,13,16-trimethyl-7,8,12,14,15,16-hexahydro-6H-cyclopenta[a]phenanthren-3-one

| Conditions | Yield |
|---|---|
| Multi-step reaction with 2 steps 1: potassium hydrogencarbonate; 3-chloro-benzenecarboperoxoic acid / dichloromethane / 0 °C 2: hydrogenchloride / pentan-1-ol View Scheme |
-
-
37413-91-5
2-((10S,13S,14S)-10,13-dimethyl-3-oxo-6,7,8,10,12,13,14,15-octahydro-3H-cyclopenta[a]phenanthren-17-yl)-2-oxoethyl acetate

-
-
13209-41-1
(8S,10S,13S,14S,16R,17R)-17-hydroxy-17-(2-hydroxyacetyl)-10,13,16-trimethyl-7,8,12,14,15,16-hexahydro-6H-cyclopenta[a]phenanthren-3-one

| Conditions | Yield |
|---|---|
| Multi-step reaction with 3 steps 1.1: dichloromethane; tetrahydrofuran / 3 h / 20 °C / Inert atmosphere 1.2: 3 h / 20 °C / Inert atmosphere 1.3: -50 °C / Inert atmosphere 2.1: acetic acid; peracetic acid / toluene / 0.5 h / -10 °C / Inert atmosphere 3.1: potassium carbonate; water / methanol / 0 - 20 °C View Scheme |
-
-
10106-41-9
17α,21-dihydroxy-16α-methyl-1,4,9(11)-pregnatriene-3,20-dione 21-acetate

-
-
13209-41-1
(8S,10S,13S,14S,16R,17R)-17-hydroxy-17-(2-hydroxyacetyl)-10,13,16-trimethyl-7,8,12,14,15,16-hexahydro-6H-cyclopenta[a]phenanthren-3-one

| Conditions | Yield |
|---|---|
| With water; potassium carbonate In methanol at 0 - 20℃; |
-
-
124-63-0
methanesulfonyl chloride

-
-
13209-41-1
(8S,10S,13S,14S,16R,17R)-17-hydroxy-17-(2-hydroxyacetyl)-10,13,16-trimethyl-7,8,12,14,15,16-hexahydro-6H-cyclopenta[a]phenanthren-3-one

-
-
23776-75-2
Methanesulfonic acid 2-((8S,10S,13S,14S,16R,17R)-17-hydroxy-10,13,16-trimethyl-3-oxo-6,7,8,10,12,13,14,15,16,17-decahydro-3H-cyclopenta[a]phenanthren-17-yl)-2-oxo-ethyl ester

| Conditions | Yield |
|---|---|
| With pyridine at 0 - 2℃; for 1.5h; | 85% |
-
-
50-00-0
formaldehyd

-
-
13209-41-1
(8S,10S,13S,14S,16R,17R)-17-hydroxy-17-(2-hydroxyacetyl)-10,13,16-trimethyl-7,8,12,14,15,16-hexahydro-6H-cyclopenta[a]phenanthren-3-one

-
-
14518-56-0
16α-methyl-17α,20;20,21-bismethylenedioxypregn-1,4,9(11)-triene-3-one

| Conditions | Yield |
|---|---|
| With hydrogenchloride In dichloromethane Ambient temperature; | 74% |
-
-
124-63-0
methanesulfonyl chloride

-
-
13209-41-1
(8S,10S,13S,14S,16R,17R)-17-hydroxy-17-(2-hydroxyacetyl)-10,13,16-trimethyl-7,8,12,14,15,16-hexahydro-6H-cyclopenta[a]phenanthren-3-one

| Conditions | Yield |
|---|---|
| With dmap In dichloromethane for 4h; Heating; | A 69% B 1.1% |

-
-
13209-41-1
(8S,10S,13S,14S,16R,17R)-17-hydroxy-17-(2-hydroxyacetyl)-10,13,16-trimethyl-7,8,12,14,15,16-hexahydro-6H-cyclopenta[a]phenanthren-3-one

| Conditions | Yield |
|---|---|
| With dmap; methanesulfonyl chloride In dichloromethane for 4h; Heating; | A 69% B 1.1% |
-
-
13209-41-1
(8S,10S,13S,14S,16R,17R)-17-hydroxy-17-(2-hydroxyacetyl)-10,13,16-trimethyl-7,8,12,14,15,16-hexahydro-6H-cyclopenta[a]phenanthren-3-one

| Conditions | Yield |
|---|---|
| With Vilsmeier reagent; water 1.) toluene, from -3 deg C to 2 deg C, 3 h, 2.) toluene, 5 deg C; Yield given. Multistep reaction; |
-
-
13209-41-1
(8S,10S,13S,14S,16R,17R)-17-hydroxy-17-(2-hydroxyacetyl)-10,13,16-trimethyl-7,8,12,14,15,16-hexahydro-6H-cyclopenta[a]phenanthren-3-one

-
-
83881-01-0
21-fluoro-17α-hydroxy-16α-methyl-1,4,9(11)-ppregnatriene-3,30-dione 17-(2'-furoate)

| Conditions | Yield |
|---|---|
| Multi-step reaction with 3 steps 1: 85 percent / pyridine / 1.5 h / 0 - 2 °C 2: 70 percent / KF / dimethylformamide / 4 h / 124 °C 3: 23 percent / 4-DMAP / CH2Cl2 / 27 h / 40 °C View Scheme | |
| Multi-step reaction with 3 steps 1: 85 percent / pyridine / 1.5 h / 0 - 2 °C 2: 70 percent / KF / dimethylformamide / 4 h / 124 °C 3: 58 percent / 4-DMAP / CH2Cl2 / 20 h / Ambient temperature View Scheme |
-
-
13209-41-1
(8S,10S,13S,14S,16R,17R)-17-hydroxy-17-(2-hydroxyacetyl)-10,13,16-trimethyl-7,8,12,14,15,16-hexahydro-6H-cyclopenta[a]phenanthren-3-one

-
-
83881-12-3
9α,11β-dichloro-21-fluoro-17α-hydroxy-16α-methyl-1,4-pregnadiene-3,20-dione 17-(2'-furoate)

| Conditions | Yield |
|---|---|
| Multi-step reaction with 4 steps 1: 85 percent / pyridine / 1.5 h / 0 - 2 °C 2: 70 percent / KF / dimethylformamide / 4 h / 124 °C 3: 23 percent / 4-DMAP / CH2Cl2 / 27 h / 40 °C 4: 72 percent / Cl2, pyridine hydrochloride / CCl4; CH2Cl2 / -35 °C View Scheme | |
| Multi-step reaction with 4 steps 1: 85 percent / pyridine / 1.5 h / 0 - 2 °C 2: 70 percent / KF / dimethylformamide / 4 h / 124 °C 3: 58 percent / 4-DMAP / CH2Cl2 / 20 h / Ambient temperature 4: 72 percent / Cl2, pyridine hydrochloride / CCl4; CH2Cl2 / -35 °C View Scheme |
-
-
13209-41-1
(8S,10S,13S,14S,16R,17R)-17-hydroxy-17-(2-hydroxyacetyl)-10,13,16-trimethyl-7,8,12,14,15,16-hexahydro-6H-cyclopenta[a]phenanthren-3-one

-
-
107742-74-5
C22H26O3

| Conditions | Yield |
|---|---|
| Multi-step reaction with 2 steps 1: 85 percent / pyridine / 1.5 h / 0 - 2 °C 2: 17 percent / KF / dimethylformamide / 4 h / 124 °C View Scheme |
-
-
13209-41-1
(8S,10S,13S,14S,16R,17R)-17-hydroxy-17-(2-hydroxyacetyl)-10,13,16-trimethyl-7,8,12,14,15,16-hexahydro-6H-cyclopenta[a]phenanthren-3-one

-
-
83881-00-9
21-fluoro-17α-hydroxy-16α-methyl-1,4,9(11)-ppregnatriene-3,30-dione

| Conditions | Yield |
|---|---|
| Multi-step reaction with 2 steps 1: 85 percent / pyridine / 1.5 h / 0 - 2 °C 2: 70 percent / KF / dimethylformamide / 4 h / 124 °C View Scheme |
-
-
13209-41-1
(8S,10S,13S,14S,16R,17R)-17-hydroxy-17-(2-hydroxyacetyl)-10,13,16-trimethyl-7,8,12,14,15,16-hexahydro-6H-cyclopenta[a]phenanthren-3-one

-
-
50-02-2
dexamethasone

| Conditions | Yield |
|---|---|
| Multi-step reaction with 4 steps 1: 74 percent / HCl / CH2Cl2 / Ambient temperature 2: 95 percent / 0.4M perchloric acid, N-bromoacetamide / dioxane / 15 h / Ambient temperature 3: 87 percent / potassium acetate / ethanol; dioxane / 19 h / Heating 4: 48percent hydrofluoric acid / ethanol; tetrahydrofuran / 20 h / 0 °C View Scheme |
-
-
13209-41-1
(8S,10S,13S,14S,16R,17R)-17-hydroxy-17-(2-hydroxyacetyl)-10,13,16-trimethyl-7,8,12,14,15,16-hexahydro-6H-cyclopenta[a]phenanthren-3-one

-
-
88509-06-2
C18H24O6

| Conditions | Yield |
|---|---|
| Multi-step reaction with 9 steps 1: 74 percent / HCl / CH2Cl2 / Ambient temperature 2: Zn / dimethylformamide; H2O / 145-147 deg C, 30 min; room temp., 2 h 3: 48 percent / Bu4NHSO4, NaOH / tetrahydrofuran; H2O / 5-10 deg C, 0.5 h, then room temp., 16 h 4: NH3, Li / tetrahydrofuran / 2 h / -78 °C 5: NH3, Li / tetrahydrofuran; ethanol 6: 1M oxalic acid / tetrahydrofuran; methanol / 2.5 h 7: 50 percent / pyridinium bromide perbromide / pyridine / room temp., 2 h 85 deg C, 1 h 8: 1.) O3; 2.) H2O2 / 1.) CH2Cl2, MeOH, acetic acid, -72 deg C, 3.5 h; 2.) water, room temp., 18 h 9: NaOAc / acetic anhydride / 2.5 h / 100 °C View Scheme |
-
-
13209-41-1
(8S,10S,13S,14S,16R,17R)-17-hydroxy-17-(2-hydroxyacetyl)-10,13,16-trimethyl-7,8,12,14,15,16-hexahydro-6H-cyclopenta[a]phenanthren-3-one

-
-
88509-05-1
des-A,B-9-keto-16α-methyl-17α,20;20,21-bismethylenedioxypregn-8α-propionic acid

| Conditions | Yield |
|---|---|
| Multi-step reaction with 8 steps 1: 74 percent / HCl / CH2Cl2 / Ambient temperature 2: Zn / dimethylformamide; H2O / 145-147 deg C, 30 min; room temp., 2 h 3: 48 percent / Bu4NHSO4, NaOH / tetrahydrofuran; H2O / 5-10 deg C, 0.5 h, then room temp., 16 h 4: NH3, Li / tetrahydrofuran / 2 h / -78 °C 5: NH3, Li / tetrahydrofuran; ethanol 6: 1M oxalic acid / tetrahydrofuran; methanol / 2.5 h 7: 50 percent / pyridinium bromide perbromide / pyridine / room temp., 2 h 85 deg C, 1 h 8: 1.) O3; 2.) H2O2 / 1.) CH2Cl2, MeOH, acetic acid, -72 deg C, 3.5 h; 2.) water, room temp., 18 h View Scheme |
-
-
13209-41-1
(8S,10S,13S,14S,16R,17R)-17-hydroxy-17-(2-hydroxyacetyl)-10,13,16-trimethyl-7,8,12,14,15,16-hexahydro-6H-cyclopenta[a]phenanthren-3-one

-
-
88509-03-9
16α-methyl-17α,20;20,21-bismethylenedioxy-19-norpregn-5(10)-en-3-one

| Conditions | Yield |
|---|---|
| Multi-step reaction with 6 steps 1: 74 percent / HCl / CH2Cl2 / Ambient temperature 2: Zn / dimethylformamide; H2O / 145-147 deg C, 30 min; room temp., 2 h 3: 48 percent / Bu4NHSO4, NaOH / tetrahydrofuran; H2O / 5-10 deg C, 0.5 h, then room temp., 16 h 4: NH3, Li / tetrahydrofuran / 2 h / -78 °C 5: NH3, Li / tetrahydrofuran; ethanol 6: 1M oxalic acid / tetrahydrofuran; methanol / 2.5 h View Scheme |
-
-
13209-41-1
(8S,10S,13S,14S,16R,17R)-17-hydroxy-17-(2-hydroxyacetyl)-10,13,16-trimethyl-7,8,12,14,15,16-hexahydro-6H-cyclopenta[a]phenanthren-3-one

-
-
88508-99-0
16α-methyl-17α,20;20,21-bismethylenedioxy-19-norpregn-1,3,5(10),9(11)-tetraen-3-ol

| Conditions | Yield |
|---|---|
| Multi-step reaction with 2 steps 1: 74 percent / HCl / CH2Cl2 / Ambient temperature 2: Zn / dimethylformamide; H2O / 145-147 deg C, 30 min; room temp., 2 h View Scheme |
-
-
13209-41-1
(8S,10S,13S,14S,16R,17R)-17-hydroxy-17-(2-hydroxyacetyl)-10,13,16-trimethyl-7,8,12,14,15,16-hexahydro-6H-cyclopenta[a]phenanthren-3-one

-
-
88509-01-7
16α-methyl-17α,20;20,21-bismethylenedioxy-19-norpregn-1,3,5(10)-trien-3-ol 3-methyl ether

| Conditions | Yield |
|---|---|
| Multi-step reaction with 4 steps 1: 74 percent / HCl / CH2Cl2 / Ambient temperature 2: Zn / dimethylformamide; H2O / 145-147 deg C, 30 min; room temp., 2 h 3: 48 percent / Bu4NHSO4, NaOH / tetrahydrofuran; H2O / 5-10 deg C, 0.5 h, then room temp., 16 h 4: NH3, Li / tetrahydrofuran / 2 h / -78 °C View Scheme |
-
-
13209-41-1
(8S,10S,13S,14S,16R,17R)-17-hydroxy-17-(2-hydroxyacetyl)-10,13,16-trimethyl-7,8,12,14,15,16-hexahydro-6H-cyclopenta[a]phenanthren-3-one

-
-
88509-02-8
16α-methyl-17α,20;20,21-bismethylenedioxy-19-norpregn-2,5(10)-dien-3-ol 3-methyl ether

| Conditions | Yield |
|---|---|
| Multi-step reaction with 5 steps 1: 74 percent / HCl / CH2Cl2 / Ambient temperature 2: Zn / dimethylformamide; H2O / 145-147 deg C, 30 min; room temp., 2 h 3: 48 percent / Bu4NHSO4, NaOH / tetrahydrofuran; H2O / 5-10 deg C, 0.5 h, then room temp., 16 h 4: NH3, Li / tetrahydrofuran / 2 h / -78 °C 5: NH3, Li / tetrahydrofuran; ethanol View Scheme |
-
-
13209-41-1
(8S,10S,13S,14S,16R,17R)-17-hydroxy-17-(2-hydroxyacetyl)-10,13,16-trimethyl-7,8,12,14,15,16-hexahydro-6H-cyclopenta[a]phenanthren-3-one

-
-
88509-04-0
16α-methyl-17α,20;20,21-bismethylenedioxy-19-norpregn-4,9(10)-diene-3-one

| Conditions | Yield |
|---|---|
| Multi-step reaction with 7 steps 1: 74 percent / HCl / CH2Cl2 / Ambient temperature 2: Zn / dimethylformamide; H2O / 145-147 deg C, 30 min; room temp., 2 h 3: 48 percent / Bu4NHSO4, NaOH / tetrahydrofuran; H2O / 5-10 deg C, 0.5 h, then room temp., 16 h 4: NH3, Li / tetrahydrofuran / 2 h / -78 °C 5: NH3, Li / tetrahydrofuran; ethanol 6: 1M oxalic acid / tetrahydrofuran; methanol / 2.5 h 7: 50 percent / pyridinium bromide perbromide / pyridine / room temp., 2 h 85 deg C, 1 h View Scheme |
-
-
13209-41-1
(8S,10S,13S,14S,16R,17R)-17-hydroxy-17-(2-hydroxyacetyl)-10,13,16-trimethyl-7,8,12,14,15,16-hexahydro-6H-cyclopenta[a]phenanthren-3-one

-
-
1966-25-2
16α-methyl-9β,11β-oxido-17α,20;20,21-bismethylenedioxy-pregna-1,4-diene-3-one

| Conditions | Yield |
|---|---|
| Multi-step reaction with 3 steps 1: 74 percent / HCl / CH2Cl2 / Ambient temperature 2: 95 percent / 0.4M perchloric acid, N-bromoacetamide / dioxane / 15 h / Ambient temperature 3: 87 percent / potassium acetate / ethanol; dioxane / 19 h / Heating View Scheme |
-
-
13209-41-1
(8S,10S,13S,14S,16R,17R)-17-hydroxy-17-(2-hydroxyacetyl)-10,13,16-trimethyl-7,8,12,14,15,16-hexahydro-6H-cyclopenta[a]phenanthren-3-one

-
-
88509-00-6
16α-methyl-17α,20;20,21-bismethylenedioxy-19-norpregn-1,3,5(10),9(11)-tetraen-3-ol 3-methyl ether

| Conditions | Yield |
|---|---|
| Multi-step reaction with 3 steps 1: 74 percent / HCl / CH2Cl2 / Ambient temperature 2: Zn / dimethylformamide; H2O / 145-147 deg C, 30 min; room temp., 2 h 3: 48 percent / Bu4NHSO4, NaOH / tetrahydrofuran; H2O / 5-10 deg C, 0.5 h, then room temp., 16 h View Scheme |
-
-
13209-41-1
(8S,10S,13S,14S,16R,17R)-17-hydroxy-17-(2-hydroxyacetyl)-10,13,16-trimethyl-7,8,12,14,15,16-hexahydro-6H-cyclopenta[a]phenanthren-3-one

-
-
88509-22-2
9α-bromo-11β-hydroxy-16α-methyl-17α,20;20,21-bismethylenedioxy-pregna-1,4-diene-3-one

| Conditions | Yield |
|---|---|
| Multi-step reaction with 2 steps 1: 74 percent / HCl / CH2Cl2 / Ambient temperature 2: 95 percent / 0.4M perchloric acid, N-bromoacetamide / dioxane / 15 h / Ambient temperature View Scheme |
-
-
13209-41-1
(8S,10S,13S,14S,16R,17R)-17-hydroxy-17-(2-hydroxyacetyl)-10,13,16-trimethyl-7,8,12,14,15,16-hexahydro-6H-cyclopenta[a]phenanthren-3-one

-
-
88509-20-0
C21(13)C3H30O5

| Conditions | Yield |
|---|---|
| Multi-step reaction with 15 steps 1: 74 percent / HCl / CH2Cl2 / Ambient temperature 2: Zn / dimethylformamide; H2O / 145-147 deg C, 30 min; room temp., 2 h 3: 48 percent / Bu4NHSO4, NaOH / tetrahydrofuran; H2O / 5-10 deg C, 0.5 h, then room temp., 16 h 4: NH3, Li / tetrahydrofuran / 2 h / -78 °C 5: NH3, Li / tetrahydrofuran; ethanol 6: 1M oxalic acid / tetrahydrofuran; methanol / 2.5 h 7: 50 percent / pyridinium bromide perbromide / pyridine / room temp., 2 h 85 deg C, 1 h 8: 1.) O3; 2.) H2O2 / 1.) CH2Cl2, MeOH, acetic acid, -72 deg C, 3.5 h; 2.) water, room temp., 18 h 9: NaOAc / acetic anhydride / 2.5 h / 100 °C 10: 1.) n-BuLi / 1.) THF, -78 deg C, 50 min; 2.) THF, -78 deg C, 4 h, then room temp., 15 h 11: sodium t-amyloxide / toluene / 0.67 h / Heating 12: 75 percent / glacial AcOH / H2O / 1.25 h / 65 °C 13: 82 percent / KO(t-butyl) / 2-methyl-propan-2-ol / 17 h / Ambient temperature 14: LDA / tetrahydrofuran 15: sodium metaperiodate / tetrahydrofuran; methanol; H2O / 2.5 h / Ambient temperature View Scheme |
-
-
13209-41-1
(8S,10S,13S,14S,16R,17R)-17-hydroxy-17-(2-hydroxyacetyl)-10,13,16-trimethyl-7,8,12,14,15,16-hexahydro-6H-cyclopenta[a]phenanthren-3-one

| Conditions | Yield |
|---|---|
| Multi-step reaction with 18 steps 1: 74 percent / HCl / CH2Cl2 / Ambient temperature 2: Zn / dimethylformamide; H2O / 145-147 deg C, 30 min; room temp., 2 h 3: 48 percent / Bu4NHSO4, NaOH / tetrahydrofuran; H2O / 5-10 deg C, 0.5 h, then room temp., 16 h 4: NH3, Li / tetrahydrofuran / 2 h / -78 °C 5: NH3, Li / tetrahydrofuran; ethanol 6: 1M oxalic acid / tetrahydrofuran; methanol / 2.5 h 7: 50 percent / pyridinium bromide perbromide / pyridine / room temp., 2 h 85 deg C, 1 h 8: 1.) O3; 2.) H2O2 / 1.) CH2Cl2, MeOH, acetic acid, -72 deg C, 3.5 h; 2.) water, room temp., 18 h 9: NaOAc / acetic anhydride / 2.5 h / 100 °C 10: 1.) n-BuLi / 1.) THF, -78 deg C, 50 min; 2.) THF, -78 deg C, 4 h, then room temp., 15 h 11: sodium t-amyloxide / toluene / 0.67 h / Heating 12: 75 percent / glacial AcOH / H2O / 1.25 h / 65 °C 13: 82 percent / KO(t-butyl) / 2-methyl-propan-2-ol / 17 h / Ambient temperature 14: LDA / tetrahydrofuran 15: sodium metaperiodate / tetrahydrofuran; methanol; H2O / 2.5 h / Ambient temperature 16: 0.4M perchloric acid, N-bromoacetamide / dioxane / 15 h / Ambient temperature 17: potassium acetate / ethanol; dioxane / 19 h / Heating 18: 73 percent / 48percent hydrofluoric acid / ethanol; tetrahydrofuran / 20 h / 0 °C View Scheme |
-
-
13209-41-1
(8S,10S,13S,14S,16R,17R)-17-hydroxy-17-(2-hydroxyacetyl)-10,13,16-trimethyl-7,8,12,14,15,16-hexahydro-6H-cyclopenta[a]phenanthren-3-one

| Conditions | Yield |
|---|---|
| Multi-step reaction with 17 steps 1: 74 percent / HCl / CH2Cl2 / Ambient temperature 2: Zn / dimethylformamide; H2O / 145-147 deg C, 30 min; room temp., 2 h 3: 48 percent / Bu4NHSO4, NaOH / tetrahydrofuran; H2O / 5-10 deg C, 0.5 h, then room temp., 16 h 4: NH3, Li / tetrahydrofuran / 2 h / -78 °C 5: NH3, Li / tetrahydrofuran; ethanol 6: 1M oxalic acid / tetrahydrofuran; methanol / 2.5 h 7: 50 percent / pyridinium bromide perbromide / pyridine / room temp., 2 h 85 deg C, 1 h 8: 1.) O3; 2.) H2O2 / 1.) CH2Cl2, MeOH, acetic acid, -72 deg C, 3.5 h; 2.) water, room temp., 18 h 9: NaOAc / acetic anhydride / 2.5 h / 100 °C 10: 1.) n-BuLi / 1.) THF, -78 deg C, 50 min; 2.) THF, -78 deg C, 4 h, then room temp., 15 h 11: sodium t-amyloxide / toluene / 0.67 h / Heating 12: 75 percent / glacial AcOH / H2O / 1.25 h / 65 °C 13: 82 percent / KO(t-butyl) / 2-methyl-propan-2-ol / 17 h / Ambient temperature 14: LDA / tetrahydrofuran 15: sodium metaperiodate / tetrahydrofuran; methanol; H2O / 2.5 h / Ambient temperature 16: 0.4M perchloric acid, N-bromoacetamide / dioxane / 15 h / Ambient temperature 17: potassium acetate / ethanol; dioxane / 19 h / Heating View Scheme |
-
-
13209-41-1
(8S,10S,13S,14S,16R,17R)-17-hydroxy-17-(2-hydroxyacetyl)-10,13,16-trimethyl-7,8,12,14,15,16-hexahydro-6H-cyclopenta[a]phenanthren-3-one

| Conditions | Yield |
|---|---|
| Multi-step reaction with 16 steps 1: 74 percent / HCl / CH2Cl2 / Ambient temperature 2: Zn / dimethylformamide; H2O / 145-147 deg C, 30 min; room temp., 2 h 3: 48 percent / Bu4NHSO4, NaOH / tetrahydrofuran; H2O / 5-10 deg C, 0.5 h, then room temp., 16 h 4: NH3, Li / tetrahydrofuran / 2 h / -78 °C 5: NH3, Li / tetrahydrofuran; ethanol 6: 1M oxalic acid / tetrahydrofuran; methanol / 2.5 h 7: 50 percent / pyridinium bromide perbromide / pyridine / room temp., 2 h 85 deg C, 1 h 8: 1.) O3; 2.) H2O2 / 1.) CH2Cl2, MeOH, acetic acid, -72 deg C, 3.5 h; 2.) water, room temp., 18 h 9: NaOAc / acetic anhydride / 2.5 h / 100 °C 10: 1.) n-BuLi / 1.) THF, -78 deg C, 50 min; 2.) THF, -78 deg C, 4 h, then room temp., 15 h 11: sodium t-amyloxide / toluene / 0.67 h / Heating 12: 75 percent / glacial AcOH / H2O / 1.25 h / 65 °C 13: 82 percent / KO(t-butyl) / 2-methyl-propan-2-ol / 17 h / Ambient temperature 14: LDA / tetrahydrofuran 15: sodium metaperiodate / tetrahydrofuran; methanol; H2O / 2.5 h / Ambient temperature 16: 0.4M perchloric acid, N-bromoacetamide / dioxane / 15 h / Ambient temperature View Scheme |
-
-
13209-41-1
(8S,10S,13S,14S,16R,17R)-17-hydroxy-17-(2-hydroxyacetyl)-10,13,16-trimethyl-7,8,12,14,15,16-hexahydro-6H-cyclopenta[a]phenanthren-3-one

| Conditions | Yield |
|---|---|
| Multi-step reaction with 10 steps 1: 74 percent / HCl / CH2Cl2 / Ambient temperature 2: Zn / dimethylformamide; H2O / 145-147 deg C, 30 min; room temp., 2 h 3: 48 percent / Bu4NHSO4, NaOH / tetrahydrofuran; H2O / 5-10 deg C, 0.5 h, then room temp., 16 h 4: NH3, Li / tetrahydrofuran / 2 h / -78 °C 5: NH3, Li / tetrahydrofuran; ethanol 6: 1M oxalic acid / tetrahydrofuran; methanol / 2.5 h 7: 50 percent / pyridinium bromide perbromide / pyridine / room temp., 2 h 85 deg C, 1 h 8: 1.) O3; 2.) H2O2 / 1.) CH2Cl2, MeOH, acetic acid, -72 deg C, 3.5 h; 2.) water, room temp., 18 h 9: NaOAc / acetic anhydride / 2.5 h / 100 °C 10: 1.) n-BuLi / 1.) THF, -78 deg C, 50 min; 2.) THF, -78 deg C, 4 h, then room temp., 15 h View Scheme |
-
-
13209-41-1
(8S,10S,13S,14S,16R,17R)-17-hydroxy-17-(2-hydroxyacetyl)-10,13,16-trimethyl-7,8,12,14,15,16-hexahydro-6H-cyclopenta[a]phenanthren-3-one

| Conditions | Yield |
|---|---|
| Multi-step reaction with 15 steps 1: 74 percent / HCl / CH2Cl2 / Ambient temperature 2: Zn / dimethylformamide; H2O / 145-147 deg C, 30 min; room temp., 2 h 3: 48 percent / Bu4NHSO4, NaOH / tetrahydrofuran; H2O / 5-10 deg C, 0.5 h, then room temp., 16 h 4: NH3, Li / tetrahydrofuran / 2 h / -78 °C 5: NH3, Li / tetrahydrofuran; ethanol 6: 1M oxalic acid / tetrahydrofuran; methanol / 2.5 h 7: 50 percent / pyridinium bromide perbromide / pyridine / room temp., 2 h 85 deg C, 1 h 8: 1.) O3; 2.) H2O2 / 1.) CH2Cl2, MeOH, acetic acid, -72 deg C, 3.5 h; 2.) water, room temp., 18 h 9: NaOAc / acetic anhydride / 2.5 h / 100 °C 10: n-BuLi / tetrahydrofuran / -78 °C 11: 25 percent / sodium t-amyloxide / toluene / Heating 12: glacial AcOH / H2O / 1.25 h / 65 °C 13: 76 percent / KO(t-butyl) / 2-methyl-propan-2-ol / 17 h / Ambient temperature 14: LDA / tetrahydrofuran 15: sodium metaperiodate / tetrahydrofuran; methanol; H2O / 2.5 h / Ambient temperature View Scheme |
-
-
13209-41-1
(8S,10S,13S,14S,16R,17R)-17-hydroxy-17-(2-hydroxyacetyl)-10,13,16-trimethyl-7,8,12,14,15,16-hexahydro-6H-cyclopenta[a]phenanthren-3-one

| Conditions | Yield |
|---|---|
| Multi-step reaction with 18 steps 1: 74 percent / HCl / CH2Cl2 / Ambient temperature 2: Zn / dimethylformamide; H2O / 145-147 deg C, 30 min; room temp., 2 h 3: 48 percent / Bu4NHSO4, NaOH / tetrahydrofuran; H2O / 5-10 deg C, 0.5 h, then room temp., 16 h 4: NH3, Li / tetrahydrofuran / 2 h / -78 °C 5: NH3, Li / tetrahydrofuran; ethanol 6: 1M oxalic acid / tetrahydrofuran; methanol / 2.5 h 7: 50 percent / pyridinium bromide perbromide / pyridine / room temp., 2 h 85 deg C, 1 h 8: 1.) O3; 2.) H2O2 / 1.) CH2Cl2, MeOH, acetic acid, -72 deg C, 3.5 h; 2.) water, room temp., 18 h 9: NaOAc / acetic anhydride / 2.5 h / 100 °C 10: n-BuLi / tetrahydrofuran / -78 °C 11: 25 percent / sodium t-amyloxide / toluene / Heating 12: glacial AcOH / H2O / 1.25 h / 65 °C 13: 76 percent / KO(t-butyl) / 2-methyl-propan-2-ol / 17 h / Ambient temperature 14: LDA / tetrahydrofuran 15: sodium metaperiodate / tetrahydrofuran; methanol; H2O / 2.5 h / Ambient temperature 16: 0.4M perchloric acid, N-bromoacetamide / dioxane / 15 h / Ambient temperature 17: potassium acetate / ethanol; dioxane / 19 h / Heating 18: 48percent hydrofluoric acid / ethanol; tetrahydrofuran / 20 h / 0 °C View Scheme |
-
-
13209-41-1
(8S,10S,13S,14S,16R,17R)-17-hydroxy-17-(2-hydroxyacetyl)-10,13,16-trimethyl-7,8,12,14,15,16-hexahydro-6H-cyclopenta[a]phenanthren-3-one

| Conditions | Yield |
|---|---|
| Multi-step reaction with 17 steps 1: 74 percent / HCl / CH2Cl2 / Ambient temperature 2: Zn / dimethylformamide; H2O / 145-147 deg C, 30 min; room temp., 2 h 3: 48 percent / Bu4NHSO4, NaOH / tetrahydrofuran; H2O / 5-10 deg C, 0.5 h, then room temp., 16 h 4: NH3, Li / tetrahydrofuran / 2 h / -78 °C 5: NH3, Li / tetrahydrofuran; ethanol 6: 1M oxalic acid / tetrahydrofuran; methanol / 2.5 h 7: 50 percent / pyridinium bromide perbromide / pyridine / room temp., 2 h 85 deg C, 1 h 8: 1.) O3; 2.) H2O2 / 1.) CH2Cl2, MeOH, acetic acid, -72 deg C, 3.5 h; 2.) water, room temp., 18 h 9: NaOAc / acetic anhydride / 2.5 h / 100 °C 10: n-BuLi / tetrahydrofuran / -78 °C 11: 25 percent / sodium t-amyloxide / toluene / Heating 12: glacial AcOH / H2O / 1.25 h / 65 °C 13: 76 percent / KO(t-butyl) / 2-methyl-propan-2-ol / 17 h / Ambient temperature 14: LDA / tetrahydrofuran 15: sodium metaperiodate / tetrahydrofuran; methanol; H2O / 2.5 h / Ambient temperature 16: 0.4M perchloric acid, N-bromoacetamide / dioxane / 15 h / Ambient temperature 17: potassium acetate / ethanol; dioxane / 19 h / Heating View Scheme |
-
-
13209-41-1
(8S,10S,13S,14S,16R,17R)-17-hydroxy-17-(2-hydroxyacetyl)-10,13,16-trimethyl-7,8,12,14,15,16-hexahydro-6H-cyclopenta[a]phenanthren-3-one

| Conditions | Yield |
|---|---|
| Multi-step reaction with 13 steps 1: 74 percent / HCl / CH2Cl2 / Ambient temperature 2: Zn / dimethylformamide; H2O / 145-147 deg C, 30 min; room temp., 2 h 3: 48 percent / Bu4NHSO4, NaOH / tetrahydrofuran; H2O / 5-10 deg C, 0.5 h, then room temp., 16 h 4: NH3, Li / tetrahydrofuran / 2 h / -78 °C 5: NH3, Li / tetrahydrofuran; ethanol 6: 1M oxalic acid / tetrahydrofuran; methanol / 2.5 h 7: 50 percent / pyridinium bromide perbromide / pyridine / room temp., 2 h 85 deg C, 1 h 8: 1.) O3; 2.) H2O2 / 1.) CH2Cl2, MeOH, acetic acid, -72 deg C, 3.5 h; 2.) water, room temp., 18 h 9: NaOAc / acetic anhydride / 2.5 h / 100 °C 10: n-BuLi / tetrahydrofuran / -78 °C 11: 25 percent / sodium t-amyloxide / toluene / Heating 12: glacial AcOH / H2O / 1.25 h / 65 °C 13: 76 percent / KO(t-butyl) / 2-methyl-propan-2-ol / 17 h / Ambient temperature View Scheme |
Related products
Hot Products
About|Contact|Cas|Product Name|Molecular|Country|Encyclopedia
Message|New Cas|MSDS|Service|Advertisement|CAS DataBase|Article Data|Manufacturers | Chemical Catalog
©2008 LookChem.com,License: ICP
NO.:Zhejiang16009103
complaints:service@lookchem.com Desktop View