Dayang Chem (Hangzhou) Co.,Ltd.
Dayangchem's R&D center can offer custom synthesis according to the contract research and development services for the fine chemicals, pharmaceutical, biotechnique and some of the other chemicals. DayangChem can provide different quantities
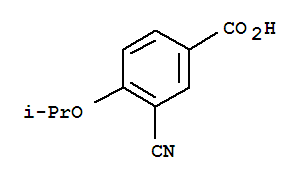
Cas:258273-31-3
Min.Order:1 Kilogram
FOB Price: $3.0
Type:Lab/Research institutions
inquiryHangzhou Dingyan Chem Co., Ltd
Items Standard Result Assay (Ursolic acid) 98%min 98.22% ----------------------------------------------------------------
Cas:258273-31-3
Min.Order:1 Gram
FOB Price: $100.0 / 500.0
Type:Trading Company
inquiryWuhan Fortuna Chemical Co.,Ltd
Factory supply 3-cyano-4-isopropoxybenzoic acid CAS 258273-31-3 with fast delivery Company profile Wuhan Fortuna Chemical Co.,Ltd established in 2006, is a big integrative chemical enterprise being engaged in Pharmaceutical & its intermediat
Cas:258273-31-3
Min.Order:10 Kilogram
FOB Price: $10.0
Type:Trading Company
inquiryHangzhou Think Chemical Co. Ltd
3-Cyano-4-Isopropoxybenzoic Acid[CAS:258273-31-3] Name: Ozanimod Synonyms: Benzonitrile, 5-[3-[(1S)-2,3-dihydro-1-[(2-hydroxyethyl)aMino]-1H-inden-4-yl]-1,2,4-oxadiazol-5-yl]-2-(1-Methylethoxy)- (fr
Cas:258273-31-3
Min.Order:1 Kilogram
FOB Price: $2.0
Type:Other
inquiryXi'an Xszo Chem Co., Ltd.
1. Factory price and high quality must be guaranteed, base on 8 years of production and R&D experience2. Free samples will be provided,ensure specifications and quality are right for customer3. Customers will receive the most professional technical s
Cas:258273-31-3
Min.Order:1 Gram
FOB Price: $0.1
Type:Manufacturers
inquiryChemwill Asia Co., Ltd.
Our main production base is located in Xuzhou industry park. We are certified both to the ISO 9001 and ISO 14001 Standards, have a safety management system in place.Our R&D team masters core technology for process-design of target building block
Cas:258273-31-3
Min.Order:5 Kiloliter
FOB Price: $1.2 / 5.0
Type:Manufacturers
inquiryHebei Nengqian Chemical Import and Export Co., LTD
Our advantages: 1, High quality with competitive price: 1) Standard:BP/USP/EP/Enterprise standard 2) All Purity≥99% 3) We are manufacturer and can provide high quality products with factory price. 2, Fast and safe delivery 1) Parcel can be
Cas:258273-31-3
Min.Order:1 Kilogram
FOB Price: $30.0 / 40.0
Type:Trading Company
inquiryHenan Tianfu Chemical Co., Ltd.
Our company was built in 2009 with an ISO certificate.In the past 6 years, we have grown up as a famous fine chemicals supplier in China and we had established stable business relationships with Samsung,LG,Merck,Thermo Fisher Scientific and so on.O
Cas:258273-31-3
Min.Order:1 Metric Ton
FOB Price: $500.0
Type:Lab/Research institutions
inquiryLIDE PHARMACEUTICALS LIMITED
Advantage : LIDE PHARMACEUTICALS LTD. is a mid-small manufacturing-type enterprise, engaged in pharmaceutical intermediates of R&D, custom-made and production, and also involving trading chemicals for export. We have established the
Cas:258273-31-3
Min.Order:1 Kilogram
Negotiable
Type:Lab/Research institutions
inquiryBaoji Guokang Healthchem co.,ltd
Our company has been in existence for 10 years since its establishment. We have our own unique team. The company integrates independent research and development, production and sales. We have established famous brands at home and abroad. At prese
Cas:258273-31-3
Min.Order:1 Kilogram
FOB Price: $154.0 / 165.0
Type:Trading Company
inquiryHenan Wentao Chemical Product Co., Ltd.
Henan Wentao Chemical Product Co.,Ltd is Located in Zhengzhou High-tech Development Zone with import and export license, We passed ISO 9001:2008 as well, Henan Wentao has developed more than 1000 compounds, which are widely used in the fields of prod
Cas:258273-31-3
Min.Order:0
Negotiable
Type:Lab/Research institutions
inquiryTriumph International Development Limilted
Appearance:White solid Storage:In stock Package:As customer request Application:Pharmaceutical Intermedaite Transportation:By Sea/Air/Courier Port:Shanghai
Cas:258273-31-3
Min.Order:0 Metric Ton
Negotiable
Type:Lab/Research institutions
inquiryAfine Chemicals Limited
Company Introduction 1. Established in 2005, with two independent business divisions: Fine chemicals division; Pharmaceutical division. 2. Main product: Optical brightener Textile auxiliary Dye stuff Pigments
Cas:258273-31-3
Min.Order:1 Gram
Negotiable
Type:Lab/Research institutions
inquirySHANGHAI T&W PHARMACEUTICAL CO., LTD.
A substitute for perfluorooctanoic acid, mainly used as a surfactant, dispersant, additive, etc Appearance:White solid or Colorless liquid Purity:99.3 % We will ship the goods in a timely manner as required We can provide relevant documents acc
Cas:258273-31-3
Min.Order:4 Kilogram
Negotiable
Type:Lab/Research institutions
inquiryHangzhou J&H Chemical Co., Ltd.
J&H CHEM R&D center can offer custom synthesis according to the contract research and development services for the fine chemicals, pharmaceutical, biotechnique and some of the other chemicals. J&H CHEM has some Manufacturing base in Jia
Cas:258273-31-3
Min.Order:0
Negotiable
Type:Lab/Research institutions
inquiryZibo Dorne chemical technology co. LTD
Product Details Grade: pharmaceutical grade Purity:99%+ ProductionCapacity: 1000 Kilogram/Month Scope of use: For scientific research only(The product must be used legally) Our Advantage 1. Best quality with competitive price. 2. Quick shipping,
Cas:258273-31-3
Min.Order:1 Gram
Negotiable
Type:Lab/Research institutions
inquiryXiamen Jenny Chemical Technology Co., Ltd.
GMP standard, high purity, competitive price, in stock 1. Quick Response: within 6 hours after receiving your email. 2. Quality Guarantee: All products are strictly tested by our QC, confirmed by QA, and approved by a third-party lab in China, USA,
Cas:258273-31-3
Min.Order:1 Milligram
Negotiable
Type:Trading Company
inquiryShanghai Minstar Chemical Co., Ltd
3-CYANO-4-ISOPROPOXYBENZOIC ACID Basic information Product Name: 3-CYANO-4-ISOPROPOXYBENZOIC ACID Synonyms: 3-CYANO-4-ISOPROPOXYBENZOIC ACID;3-cyano-4-(propan-2-yloxy)benzoic acid;5-(1-hydroxyvinyl)-2-isopropoxybenzonitrile;3-Cyano-4-isopropoxy
Cas:258273-31-3
Min.Order:1 Kilogram
Negotiable
Type:Lab/Research institutions
inquiryHANGZHOU YUNUO CHEMICAL CO.,LTD
Our company advantages: 1 The highest quality with the competitive price. 2、 Professional human services. 3、 The fastest and safest delivery service. 4、Our old customers all around the world. 5、 The high purity products. 6、We have a suf
Cas:258273-31-3
Min.Order:1 Kilogram
Negotiable
Type:Trading Company
inquiryHunan chemfish Pharmaceutical co.,Ltd
Appearance:95%+ Package:R&D,Pilot run Transportation:per client require Port:Express ,Air, Sea
Golden Pharma Co., Limited
GOLDEN PHARMA CO.,LIMITED.is a professional pharmaceutical company,our team have more than 20years expereince in pharmaceutical production and sales. we are a professional technical enterprise specializing in the R & D, production,QA regulation
Bluecrystal chem-union
We are a Union of chemistry in China, consists of chemists,engineers, laboratories,factories in China. We organize surplus capacity of R&D and production as well as custom synthesis for chemical products and chemical business project. We are supp
Cas:258273-31-3
Min.Order:1 Metric Ton
FOB Price: $1.0
Type:Trading Company
inquiryWin-Win chemical Co.Ltd
Stock products, own laboratoryAppearance:White to off-white powder Package:Grams, Kilograms Application:For R&D Transportation:According to customer request Port:Shanghai
Cas:258273-31-3
Min.Order:0
Negotiable
Type:Lab/Research institutions
inquiryGIHI CHEMICALS CO.,LIMITED
high purity,in stock Package:25kg/drum,or as per customers'demand Application:API,Pharmaceutical intermediates Transportation:air,sea,courier
Cas:258273-31-3
Min.Order:0
Negotiable
Type:Lab/Research institutions
inquiryJINHUA HUAYI CHEMICAL CO., LTD.
Jinhua huayi chemical co., ltd. is dedicated to the development, production and marketing of chemicals. On the basis of equality and mutual benefit, and under the principle of customer first, credit first, quality first, we are ready to join hands
Cas:258273-31-3
Min.Order:100 Gram
Negotiable
Type:Lab/Research institutions
inquiryShanghai Acmec Biochemical Technology Co., Ltd.
Acmec is a leading manufacturer and supplier of biochemical reagents and life science products. We have over 40,000 items in stock (real-time inventory) and offer discounted prices to registered members of the online store ( www.acmec.com.cn ) Appea
Cas:258273-31-3
Min.Order:1 bottle
Negotiable
Type:Lab/Research institutions
inquiryEnke Pharma-tech Co.,Ltd. (Cangzhou, China )
high purity Storage:normal temperature Package:DRUM Application:mainly for medical use for R&Dpurpose use only Transportation:AIR,SEA Port:BEIJING,SHANGHAI,TIANJIN,SHENZHEN
Koning Pharmchem Co., Ltd.
KONING PHARMCHEM CO., LTD. has committed itself to a strategy of providing a unique service for companies involved in the manufacture of pharmaceuticals, healthcare products, food, cosmetics and other fine chemicals. Our products including but not li
Cas:258273-31-3
Min.Order:0
Negotiable
Type:Trading Company
inquiryAntimex Chemical Limied
Ansciep Chemical is a professional enterprise manufacturing and distributing fine chemicals and speciality chemicals. We have been dedicated to heterocycle compounds and phenyl rings for tens of years. This is our mature product for export. Our quali
Cas:258273-31-3
Min.Order:0
Negotiable
Type:Lab/Research institutions
inquiryGuangdong Juda Chemical Industrial Co.,Limited
Appearance:solid or liquid Storage:sealed in cool and dry place Package:As customer's requested Application:Pharma Intermediate Transportation:by courier/air/sea Port:Any port in China
Cas:258273-31-3
Min.Order:0
Negotiable
Type:Trading Company
inquirySynthetic route
-
-
213598-11-9
3-cyano-4-isopropoxybenzoic acid methyl ester

-
-
258273-31-3
3-cyano-4-isopropoxybenzoic acid

| Conditions | Yield |
|---|---|
| Stage #1: methyl 3-cyano-4-[(1-methylethyl)oxy]benzoate With ethanol; sodium hydroxide In tetrahydrofuran at 20℃; for 4h; Stage #2: With hydrogenchloride In tetrahydrofuran; water | 92% |
| With ethanol; sodium hydroxide In tetrahydrofuran at 20℃; for 4h; | 92% |
| Stage #1: methyl 3-cyano-4-[(1-methylethyl)oxy]benzoate With potassium hydroxide In tetrahydrofuran; water at 0 - 20℃; for 16h; Stage #2: With hydrogenchloride In water for 2h; | 87% |
-
-
14348-41-5
3-bromo-4-hydroxy-benzoic acid

-
-
258273-31-3
3-cyano-4-isopropoxybenzoic acid

| Conditions | Yield |
|---|---|
| Multi-step reaction with 3 steps 1.1: caesium carbonate / N,N-dimethyl-formamide / 16 h / 80 °C 2.1: 1-methyl-pyrrolidin-2-one / 16 h / 200 °C 3.1: water; lithium hydroxide / 1,4-dioxane / 1.5 h / 30 °C 3.2: 20 °C View Scheme | |
| Multi-step reaction with 4 steps 1: hydrogenchloride / 20 °C 2: potassium carbonate / acetonitrile / Reflux 3: tetrakis(triphenylphosphine) palladium(0) / N,N-dimethyl-formamide / 120 °C / Inert atmosphere 4: sodium hydroxide / methanol / 16 h / 20 °C View Scheme | |
| Multi-step reaction with 4 steps 1.1: hydrogenchloride / water / 20 °C 2.1: potassium carbonate / acetonitrile / Reflux 3.1: tetrakis(triphenylphosphine) palladium(0) / N,N-dimethyl-formamide / 120 °C / Inert atmosphere 4.1: sodium hydroxide; water / isopropyl alcohol / 20 °C 4.2: pH 4 - 5 View Scheme |
-
-
1034689-05-8
1-methylethyl 3-bromo-4-[(1-methylethyl)oxy]benzoate

-
-
258273-31-3
3-cyano-4-isopropoxybenzoic acid

| Conditions | Yield |
|---|---|
| Multi-step reaction with 2 steps 1.1: 1-methyl-pyrrolidin-2-one / 16 h / 200 °C 2.1: water; lithium hydroxide / 1,4-dioxane / 1.5 h / 30 °C 2.2: 20 °C View Scheme |
-
-
1261173-10-7
1-methylethyl 3-cyano-4-[(1-methylethyl)oxy]benzoate

-
-
258273-31-3
3-cyano-4-isopropoxybenzoic acid

| Conditions | Yield |
|---|---|
| Stage #1: 1-methylethyl 3-cyano-4-[(1-methylethyl)oxy]benzoate With water; lithium hydroxide In 1,4-dioxane at 30℃; for 1.5h; Stage #2: With hydrogenchloride In 1,4-dioxane; water at 20℃; | |
| Stage #1: 1-methylethyl 3-cyano-4-[(1-methylethyl)oxy]benzoate With water; sodium hydroxide In isopropyl alcohol at 20℃; Stage #2: With hydrogenchloride In water pH=~ 2; | |
| Stage #1: 1-methylethyl 3-cyano-4-[(1-methylethyl)oxy]benzoate With water; sodium hydroxide In isopropyl alcohol at 20℃; Stage #2: With hydrogenchloride In water pH=2; |
-
-
15126-06-4
methyl 3-iodo-4-hydroxybenzoate

-
-
258273-31-3
3-cyano-4-isopropoxybenzoic acid

| Conditions | Yield |
|---|---|
| Multi-step reaction with 3 steps 1: sodium cyanide / N,N-dimethyl-formamide / 24 h / 120 °C / Inert atmosphere 2: potassium carbonate / N,N-dimethyl-formamide / 12 h / 90 °C / Inert atmosphere 3: sodium hydroxide / methanol / 16 h / 20 °C View Scheme | |
| Multi-step reaction with 2 steps 1.1: copper(l) cyanide / N,N-dimethyl-formamide / 24 h / 120 °C / Inert atmosphere 1.2: 12 h / 90 °C / Cooling with ice 2.1: sodium hydroxide; water / isopropyl alcohol / 20 °C 2.2: pH 4 - 5 View Scheme | |
| Multi-step reaction with 3 steps 1: sodium cyanide / N,N-dimethyl-formamide / 18 h / 105 °C 2: potassium carbonate / N,N-dimethyl-formamide / 14 h / 90 °C 3: sodium hydroxide; ethanol / tetrahydrofuran / 4 h / 20 °C View Scheme |
-
-
99-76-3
methyl 4-hydroxylbenzoate

-
-
258273-31-3
3-cyano-4-isopropoxybenzoic acid

| Conditions | Yield |
|---|---|
| Multi-step reaction with 4 steps 1: Iodine monochloride; acetic acid / 20 - 65 °C 2: sodium cyanide / N,N-dimethyl-formamide / 24 h / 120 °C / Inert atmosphere 3: potassium carbonate / N,N-dimethyl-formamide / 12 h / 90 °C / Inert atmosphere 4: sodium hydroxide / methanol / 16 h / 20 °C View Scheme | |
| Multi-step reaction with 3 steps 1.1: Iodine monochloride / acetic acid / 65 °C 2.1: copper(l) cyanide / N,N-dimethyl-formamide / 24 h / 120 °C / Inert atmosphere 2.2: 12 h / 90 °C / Cooling with ice 3.1: sodium hydroxide; water / isopropyl alcohol / 20 °C 3.2: pH 4 - 5 View Scheme | |
| Multi-step reaction with 4 steps 1: acetic acid; Iodine monochloride / 24.6 h / 20 - 65 °C 2: sodium cyanide / N,N-dimethyl-formamide / 18 h / 105 °C 3: potassium carbonate / N,N-dimethyl-formamide / 14 h / 90 °C 4: sodium hydroxide; ethanol / tetrahydrofuran / 4 h / 20 °C View Scheme | |
| Multi-step reaction with 4 steps 1: magnesium chloride / dichloromethane / 44 °C / Large scale 2: hydroxylamine hydrochloride; acetyl chloride / N,N-dimethyl-formamide; acetonitrile / 2 h / 80 °C / Large scale 3: potassium carbonate / N,N-dimethyl-formamide; acetonitrile / 80 °C / Large scale 4: sodium hydroxide / tetrahydrofuran / 1 h / 60 °C / Large scale View Scheme |
-
-
156001-68-2
methyl 3-cyano-4-hydroxybenzoate

-
-
258273-31-3
3-cyano-4-isopropoxybenzoic acid

| Conditions | Yield |
|---|---|
| Multi-step reaction with 2 steps 1: potassium carbonate / N,N-dimethyl-formamide / 12 h / 90 °C / Inert atmosphere 2: sodium hydroxide / methanol / 16 h / 20 °C View Scheme | |
| Multi-step reaction with 2 steps 1: potassium carbonate / N,N-dimethyl-formamide / 80 °C 2: sodium hydroxide / methanol; water / 2 h / 25 °C View Scheme | |
| Multi-step reaction with 2 steps 1: potassium carbonate / N,N-dimethyl-formamide / 14 h / 90 °C 2: sodium hydroxide; ethanol / tetrahydrofuran / 4 h / 20 °C View Scheme |
-
-
29415-97-2
methyl 3-bromo-4-hydroxybenzoate

-
-
258273-31-3
3-cyano-4-isopropoxybenzoic acid

| Conditions | Yield |
|---|---|
| Multi-step reaction with 3 steps 1: potassium carbonate / acetonitrile / Reflux 2: tetrakis(triphenylphosphine) palladium(0) / N,N-dimethyl-formamide / 120 °C / Inert atmosphere 3: sodium hydroxide / methanol / 16 h / 20 °C View Scheme | |
| Multi-step reaction with 3 steps 1.1: potassium carbonate / acetonitrile / Reflux 2.1: tetrakis(triphenylphosphine) palladium(0) / N,N-dimethyl-formamide / 120 °C / Inert atmosphere 3.1: sodium hydroxide; water / isopropyl alcohol / 20 °C 3.2: pH 4 - 5 View Scheme | |
| Multi-step reaction with 3 steps 1: copper(l) iodide / N,N-dimethyl-formamide / 120 °C 2: potassium carbonate / N,N-dimethyl-formamide / 80 °C 3: sodium hydroxide / methanol; water / 2 h / 25 °C View Scheme |
-
-
676602-31-6
3-cyano-4-fluorobenzoic acid methyl ester

-
-
258273-31-3
3-cyano-4-isopropoxybenzoic acid

| Conditions | Yield |
|---|---|
| Multi-step reaction with 2 steps 1.1: sodium hexamethyldisilazane / tetrahydrofuran / 20 °C / Cooling with ice 1.2: 16 h / 50 °C 2.1: sodium hydroxide; water / isopropyl alcohol / 20 °C 2.2: pH ~ 2 View Scheme | |
| Multi-step reaction with 2 steps 1.1: sodium hexamethyldisilazane / tetrahydrofuran / 17 h / 20 - 50 °C / Cooling with ice 2.1: sodium hydroxide; water / isopropyl alcohol / 20 °C 2.2: pH 2 View Scheme |
-
-
171050-06-9
3-cyano-4-fluorobenzoic acid

-
-
258273-31-3
3-cyano-4-isopropoxybenzoic acid

| Conditions | Yield |
|---|---|
| Multi-step reaction with 3 steps 1.1: toluene-4-sulfonic acid / 80 - 90 °C 2.1: sodium hexamethyldisilazane / tetrahydrofuran / 20 °C / Cooling with ice 2.2: 16 h / 50 °C 3.1: sodium hydroxide; water / isopropyl alcohol / 20 °C 3.2: pH ~ 2 View Scheme | |
| Multi-step reaction with 3 steps 1.1: toluene-4-sulfonic acid / 90 °C 2.1: sodium hexamethyldisilazane / tetrahydrofuran / 17 h / 20 - 50 °C / Cooling with ice 3.1: sodium hydroxide; water / isopropyl alcohol / 20 °C 3.2: pH 2 View Scheme |
-
-
213598-10-8
methyl 3-bromo-4-isopropoxybenzoate

-
-
258273-31-3
3-cyano-4-isopropoxybenzoic acid

| Conditions | Yield |
|---|---|
| Multi-step reaction with 2 steps 1: tetrakis(triphenylphosphine) palladium(0) / N,N-dimethyl-formamide / 120 °C / Inert atmosphere 2: sodium hydroxide / methanol / 16 h / 20 °C View Scheme | |
| Multi-step reaction with 2 steps 1.1: tetrakis(triphenylphosphine) palladium(0) / N,N-dimethyl-formamide / 120 °C / Inert atmosphere 2.1: sodium hydroxide; water / isopropyl alcohol / 20 °C 2.2: pH 4 - 5 View Scheme |
-
-
24589-99-9
methyl 3-formyl-4-hydroxybenzoate

-
-
258273-31-3
3-cyano-4-isopropoxybenzoic acid

| Conditions | Yield |
|---|---|
| Multi-step reaction with 3 steps 1: hydroxylamine hydrochloride; acetyl chloride / N,N-dimethyl-formamide; acetonitrile / 2 h / 80 °C / Large scale 2: potassium carbonate / N,N-dimethyl-formamide; acetonitrile / 80 °C / Large scale 3: sodium hydroxide / tetrahydrofuran / 1 h / 60 °C / Large scale View Scheme |
-
-
258273-31-3
3-cyano-4-isopropoxybenzoic acid

-
-
1072948-37-8
3-cyano-4-[(1-methylethyl)oxy]benzoyl chloride

| Conditions | Yield |
|---|---|
| With oxalyl dichloride; N,N-dimethyl-formamide In dichloromethane at 20℃; for 2h; | 100% |
| With oxalyl dichloride; N,N-dimethyl-formamide at 20℃; for 4h; Product distribution / selectivity; | 100% |
| With oxalyl dichloride; N,N-dimethyl-formamide In dichloromethane at 20℃; for 4h; | 100% |
-
-
258273-31-3
3-cyano-4-isopropoxybenzoic acid

-
-
67-56-1
methanol

-
-
213598-11-9
3-cyano-4-isopropoxybenzoic acid methyl ester

| Conditions | Yield |
|---|---|
| Stage #1: 3-cyano-4-isopropoxybenzoic acid; methanol With diazomethyl-trimethyl-silane In dichloromethane at 0 - 20℃; for 1.8h; Stage #2: With acetic acid In dichloromethane at 20℃; | 100% |
-
-
258273-31-3
3-cyano-4-isopropoxybenzoic acid

-
-
79-19-6
thiosemicarbazide

-
-
1258440-55-9
5-(5-amino-1,3,4-thiadiazol-2-yl)-2-[(1-methylethyl)oxy]benzonitrile

| Conditions | Yield |
|---|---|
| With trichlorophosphate at 90℃; for 3h; | 99% |
| Stage #1: 3-cyano-4-isopropoxybenzoic acid; thiosemicarbazide With trichlorophosphate at 90℃; for 3h; Stage #2: With sodium hydroxide In water at 35℃; pH=10; Cooling with ice; | 99% |
| Stage #1: 3-cyano-4-isopropoxybenzoic acid; thiosemicarbazide With trichlorophosphate at 90℃; for 3h; Stage #2: With sodium hydroxide at 35℃; pH=10; Cooling with ice; | 99% |
-
-
258273-31-3
3-cyano-4-isopropoxybenzoic acid

-
-
1167416-24-1
1,1-dimethylethyl 8-[(hydroxyamino)(imino)methyl]-2,3-dihydro-1,4-benzoxazepine-4(5H)-carboxylate

-
-
1167416-65-0
1,1-dimethylethyl 8-(5-{3-cyano-4-[(1-methylethyl)oxy]phenyl}-1,2,4-oxadiazol-3-yl)-2,3-dihydro-1,4-benzoxazepine-4(5H)-carboxylate

| Conditions | Yield |
|---|---|
| With benzotriazol-1-ol; 1-ethyl-(3-(3-dimethylamino)propyl)-carbodiimide hydrochloride In N,N-dimethyl-formamide at 20℃; Heating; | 92% |
-
-
258273-31-3
3-cyano-4-isopropoxybenzoic acid

| Conditions | Yield |
|---|---|
| Stage #1: 3-cyano-4-isopropoxybenzoic acid With 1,1'-carbonyldiimidazole In N,N-dimethyl-formamide at 20℃; for 1h; Stage #2: 3-ethoxy-N-hydroxy-1H-indene-7-carboximidamide In N,N-dimethyl-formamide for 0.583333h; | 90% |
| Stage #1: 3-cyano-4-isopropoxybenzoic acid With 1,1'-carbonyldiimidazole In water; N,N-dimethyl-formamide at 20℃; for 1h; Stage #2: 3-ethoxy-N-hydroxy-1H-indene-7-carboximidamide In water; N,N-dimethyl-formamide for 0.583333h; | 90% |
| Stage #1: 3-cyano-4-isopropoxybenzoic acid With 1,1'-carbonyldiimidazole In N,N-dimethyl-formamide at 20℃; for 1h; Stage #2: 3-ethoxy-N-hydroxy-1H-indene-7-carboximidamide In N,N-dimethyl-formamide for 0.583333h; | 90% |
-
-
258273-31-3
3-cyano-4-isopropoxybenzoic acid

| Conditions | Yield |
|---|---|
| Stage #1: 3-cyano-4-isopropoxybenzoic acid With 1,1'-carbonyldiimidazole In N,N-dimethyl-formamide at 20℃; for 1h; Stage #2: 3-ethoxy-N-hydroxy-1H-indene-7-carboximidamide In N,N-dimethyl-formamide for 0.583333h; | 90% |
| Stage #1: 3-cyano-4-isopropoxybenzoic acid With 1,1'-carbonyldiimidazole In N,N-dimethyl-formamide at 20℃; for 1h; Stage #2: 3-ethoxy-N-hydroxy-1H-indene-7-carboximidamide In N,N-dimethyl-formamide at 20℃; for 0.583333h; | 90% |
-
-
258273-31-3
3-cyano-4-isopropoxybenzoic acid

-
-
1333996-58-9
tert-butyl 3-(N'-hydroxycarbamimidoyl)-4-methyl-5,6-dihydro-1,7-naphthyridine-7(8H)-carboxylate

-
-
1333996-60-3
tert-butyl 3-(5-(3-cyano-4-isopropoxyphenyl)-1,2,4-oxadiazol-3-yl)-4-methyl-5,6-dihydro-1,7-naphthyridine-7(8H)-carboxylate

| Conditions | Yield |
|---|---|
| Stage #1: 3-cyano-4-isopropoxybenzoic acid With N-ethyl-N,N-diisopropylamine; HATU In N,N-dimethyl-formamide at 20℃; for 0.0833333h; Stage #2: tert-butyl 3-(N'-hydroxycarbamimidoyl)-4-methyl-5,6-dihydro-1,7-naphthyridine-7(8H)-carboxylate In N,N-dimethyl-formamide at 20 - 100℃; for 17.5h; | 88% |
-
-
258273-31-3
3-cyano-4-isopropoxybenzoic acid

| Conditions | Yield |
|---|---|
| Stage #1: 3-cyano-4-isopropoxybenzoic acid; 2-chloro-4-(1,3-dioxolan-2-yl)-N′-hydroxybenzimidamide With potassium carbonate; 1-ethyl-(3-(3-dimethylamino)propyl)-carbodiimide hydrochloride In N,N-dimethyl-formamide at 20℃; for 0.5h; Stage #2: With benzotriazol-1-ol In N,N-dimethyl-formamide at 110℃; for 2h; Inert atmosphere; | 87% |
| Stage #1: 3-cyano-4-isopropoxybenzoic acid With potassium carbonate; benzotriazol-1-ol; 1-ethyl-(3-(3-dimethylamino)propyl)-carbodiimide hydrochloride In N,N-dimethyl-formamide at 20℃; for 0.5h; Stage #2: 2-chloro-4-(1,3-dioxolan-2-yl)-N′-hydroxybenzimidamide In N,N-dimethyl-formamide at 110℃; for 4h; Inert atmosphere; | 87% |
-
-
258273-31-3
3-cyano-4-isopropoxybenzoic acid

| Conditions | Yield |
|---|---|
| Stage #1: 3-cyano-4-isopropoxybenzoic acid; 4-(1,3-dioxolan-2-yl)-2-fluoro-N′-hydroxybenzimidamide With potassium carbonate; 1-ethyl-(3-(3-dimethylamino)propyl)-carbodiimide hydrochloride In N,N-dimethyl-formamide at 20℃; for 0.5h; Stage #2: With benzotriazol-1-ol In N,N-dimethyl-formamide at 110℃; for 2h; Inert atmosphere; | 81.7% |
| Stage #1: 3-cyano-4-isopropoxybenzoic acid With potassium carbonate; benzotriazol-1-ol; 1-ethyl-(3-(3-dimethylamino)propyl)-carbodiimide hydrochloride In N,N-dimethyl-formamide at 20℃; for 0.5h; Stage #2: 4-(1,3-dioxolan-2-yl)-2-fluoro-N′-hydroxybenzimidamide In N,N-dimethyl-formamide at 110℃; for 4h; Inert atmosphere; | 81.7% |
-
-
258273-31-3
3-cyano-4-isopropoxybenzoic acid

| Conditions | Yield |
|---|---|
| Stage #1: (3S)-3-amino-4-(4-bromophenyl)-1-butanol hydrochloride With N-ethyl-N,N-diisopropylamine In DMF (N,N-dimethyl-formamide) for 0.05h; Stage #2: 3-cyano-4-isopropoxybenzoic acid With O-(1H-benzotriazol-1-yl)-N,N,N',N'-tetramethyluronium hexafluorophosphate In DMF (N,N-dimethyl-formamide) at 20℃; for 1.5h; Stage #3: With water In DMF (N,N-dimethyl-formamide) | 78% |
-
-
258273-31-3
3-cyano-4-isopropoxybenzoic acid

-
-
1307231-09-9
N-hydroxybenzofuran-5-carboximidamide

-
-
1307230-52-9
5-(3-(benzofuran-5-yl)-1,2,4-oxadiazol-5-yl)-2-isopropoxybenzonitrile

| Conditions | Yield |
|---|---|
| Stage #1: 3-cyano-4-isopropoxybenzoic acid With benzotriazol-1-ol; 1-ethyl-(3-(3-dimethylamino)propyl)-carbodiimide hydrochloride In N,N-dimethyl-formamide at 20℃; for 0.5h; Inert atmosphere; Stage #2: N-hydroxybenzofuran-5-carboximidamide In N,N-dimethyl-formamide at 20 - 85℃; | 69% |
-
-
258273-31-3
3-cyano-4-isopropoxybenzoic acid

| Conditions | Yield |
|---|---|
| Stage #1: 3-cyano-4-isopropoxybenzoic acid With 1,1'-carbonyldiimidazole In N,N-dimethyl-formamide at 0℃; for 0.5h; Stage #2: (S)-1-azido-N’-hydroxy-2,3-dihydro-1H-indene-4-carboximidamide In N,N-dimethyl-formamide at 115℃; for 24h; Reagent/catalyst; Solvent; | 69% |
-
-
258273-31-3
3-cyano-4-isopropoxybenzoic acid


| Conditions | Yield |
|---|---|
| With benzotriazol-1-ol; 1,2-dichloro-ethane In N,N-dimethyl-formamide at 20 - 85℃; for 1.5h; | 67% |
-
-
258273-31-3
3-cyano-4-isopropoxybenzoic acid


| Conditions | Yield |
|---|---|
| Stage #1: 3-cyano-4-isopropoxybenzoic acid; (1S)-1-({2-[(tert-butyldimethylsilyl)oxy]ethyl}[(S)-2-methylpropane-2-sulfinyl]amino)-N-hydroxy-2,3-dihydro-1H-indene-4-carboximidamide With 2,4,6-tripropyl-1,3,5,2,4,6-trioxatriphosphinane-2,4,6-trioxide; triethylamine In toluene at 20 - 90℃; Inert atmosphere; Stage #2: With hydrogenchloride In water at 0 - 90℃; for 1h; Solvent; | 63.6% |
-
-
213598-11-9
3-cyano-4-isopropoxybenzoic acid methyl ester

-
-
258273-31-3
3-cyano-4-isopropoxybenzoic acid

| Conditions | Yield |
|---|---|
| Stage #1: methyl 3-cyano-4-[(1-methylethyl)oxy]benzoate With ethanol; sodium hydroxide In tetrahydrofuran at 20℃; for 4h; Stage #2: With hydrogenchloride In tetrahydrofuran; water | 92% |
| With ethanol; sodium hydroxide In tetrahydrofuran at 20℃; for 4h; | 92% |
| Stage #1: methyl 3-cyano-4-[(1-methylethyl)oxy]benzoate With potassium hydroxide In tetrahydrofuran; water at 0 - 20℃; for 16h; Stage #2: With hydrogenchloride In water for 2h; | 87% |
-
-
14348-41-5
3-bromo-4-hydroxy-benzoic acid

-
-
258273-31-3
3-cyano-4-isopropoxybenzoic acid

| Conditions | Yield |
|---|---|
| Multi-step reaction with 3 steps 1.1: caesium carbonate / N,N-dimethyl-formamide / 16 h / 80 °C 2.1: 1-methyl-pyrrolidin-2-one / 16 h / 200 °C 3.1: water; lithium hydroxide / 1,4-dioxane / 1.5 h / 30 °C 3.2: 20 °C View Scheme | |
| Multi-step reaction with 4 steps 1: hydrogenchloride / 20 °C 2: potassium carbonate / acetonitrile / Reflux 3: tetrakis(triphenylphosphine) palladium(0) / N,N-dimethyl-formamide / 120 °C / Inert atmosphere 4: sodium hydroxide / methanol / 16 h / 20 °C View Scheme | |
| Multi-step reaction with 4 steps 1.1: hydrogenchloride / water / 20 °C 2.1: potassium carbonate / acetonitrile / Reflux 3.1: tetrakis(triphenylphosphine) palladium(0) / N,N-dimethyl-formamide / 120 °C / Inert atmosphere 4.1: sodium hydroxide; water / isopropyl alcohol / 20 °C 4.2: pH 4 - 5 View Scheme |
-
-
1034689-05-8
1-methylethyl 3-bromo-4-[(1-methylethyl)oxy]benzoate

-
-
258273-31-3
3-cyano-4-isopropoxybenzoic acid

| Conditions | Yield |
|---|---|
| Multi-step reaction with 2 steps 1.1: 1-methyl-pyrrolidin-2-one / 16 h / 200 °C 2.1: water; lithium hydroxide / 1,4-dioxane / 1.5 h / 30 °C 2.2: 20 °C View Scheme |
-
-
1261173-10-7
1-methylethyl 3-cyano-4-[(1-methylethyl)oxy]benzoate

-
-
258273-31-3
3-cyano-4-isopropoxybenzoic acid

| Conditions | Yield |
|---|---|
| Stage #1: 1-methylethyl 3-cyano-4-[(1-methylethyl)oxy]benzoate With water; lithium hydroxide In 1,4-dioxane at 30℃; for 1.5h; Stage #2: With hydrogenchloride In 1,4-dioxane; water at 20℃; | |
| Stage #1: 1-methylethyl 3-cyano-4-[(1-methylethyl)oxy]benzoate With water; sodium hydroxide In isopropyl alcohol at 20℃; Stage #2: With hydrogenchloride In water pH=~ 2; | |
| Stage #1: 1-methylethyl 3-cyano-4-[(1-methylethyl)oxy]benzoate With water; sodium hydroxide In isopropyl alcohol at 20℃; Stage #2: With hydrogenchloride In water pH=2; |
-
-
15126-06-4
methyl 3-iodo-4-hydroxybenzoate

-
-
258273-31-3
3-cyano-4-isopropoxybenzoic acid

| Conditions | Yield |
|---|---|
| Multi-step reaction with 3 steps 1: sodium cyanide / N,N-dimethyl-formamide / 24 h / 120 °C / Inert atmosphere 2: potassium carbonate / N,N-dimethyl-formamide / 12 h / 90 °C / Inert atmosphere 3: sodium hydroxide / methanol / 16 h / 20 °C View Scheme | |
| Multi-step reaction with 2 steps 1.1: copper(l) cyanide / N,N-dimethyl-formamide / 24 h / 120 °C / Inert atmosphere 1.2: 12 h / 90 °C / Cooling with ice 2.1: sodium hydroxide; water / isopropyl alcohol / 20 °C 2.2: pH 4 - 5 View Scheme | |
| Multi-step reaction with 3 steps 1: sodium cyanide / N,N-dimethyl-formamide / 18 h / 105 °C 2: potassium carbonate / N,N-dimethyl-formamide / 14 h / 90 °C 3: sodium hydroxide; ethanol / tetrahydrofuran / 4 h / 20 °C View Scheme |
-
-
99-76-3
methyl 4-hydroxylbenzoate

-
-
258273-31-3
3-cyano-4-isopropoxybenzoic acid

| Conditions | Yield |
|---|---|
| Multi-step reaction with 4 steps 1: Iodine monochloride; acetic acid / 20 - 65 °C 2: sodium cyanide / N,N-dimethyl-formamide / 24 h / 120 °C / Inert atmosphere 3: potassium carbonate / N,N-dimethyl-formamide / 12 h / 90 °C / Inert atmosphere 4: sodium hydroxide / methanol / 16 h / 20 °C View Scheme | |
| Multi-step reaction with 3 steps 1.1: Iodine monochloride / acetic acid / 65 °C 2.1: copper(l) cyanide / N,N-dimethyl-formamide / 24 h / 120 °C / Inert atmosphere 2.2: 12 h / 90 °C / Cooling with ice 3.1: sodium hydroxide; water / isopropyl alcohol / 20 °C 3.2: pH 4 - 5 View Scheme | |
| Multi-step reaction with 4 steps 1: acetic acid; Iodine monochloride / 24.6 h / 20 - 65 °C 2: sodium cyanide / N,N-dimethyl-formamide / 18 h / 105 °C 3: potassium carbonate / N,N-dimethyl-formamide / 14 h / 90 °C 4: sodium hydroxide; ethanol / tetrahydrofuran / 4 h / 20 °C View Scheme | |
| Multi-step reaction with 4 steps 1: magnesium chloride / dichloromethane / 44 °C / Large scale 2: hydroxylamine hydrochloride; acetyl chloride / N,N-dimethyl-formamide; acetonitrile / 2 h / 80 °C / Large scale 3: potassium carbonate / N,N-dimethyl-formamide; acetonitrile / 80 °C / Large scale 4: sodium hydroxide / tetrahydrofuran / 1 h / 60 °C / Large scale View Scheme |
-
-
156001-68-2
methyl 3-cyano-4-hydroxybenzoate

-
-
258273-31-3
3-cyano-4-isopropoxybenzoic acid

| Conditions | Yield |
|---|---|
| Multi-step reaction with 2 steps 1: potassium carbonate / N,N-dimethyl-formamide / 12 h / 90 °C / Inert atmosphere 2: sodium hydroxide / methanol / 16 h / 20 °C View Scheme | |
| Multi-step reaction with 2 steps 1: potassium carbonate / N,N-dimethyl-formamide / 80 °C 2: sodium hydroxide / methanol; water / 2 h / 25 °C View Scheme | |
| Multi-step reaction with 2 steps 1: potassium carbonate / N,N-dimethyl-formamide / 14 h / 90 °C 2: sodium hydroxide; ethanol / tetrahydrofuran / 4 h / 20 °C View Scheme |
-
-
29415-97-2
methyl 3-bromo-4-hydroxybenzoate

-
-
258273-31-3
3-cyano-4-isopropoxybenzoic acid

| Conditions | Yield |
|---|---|
| Multi-step reaction with 3 steps 1: potassium carbonate / acetonitrile / Reflux 2: tetrakis(triphenylphosphine) palladium(0) / N,N-dimethyl-formamide / 120 °C / Inert atmosphere 3: sodium hydroxide / methanol / 16 h / 20 °C View Scheme | |
| Multi-step reaction with 3 steps 1.1: potassium carbonate / acetonitrile / Reflux 2.1: tetrakis(triphenylphosphine) palladium(0) / N,N-dimethyl-formamide / 120 °C / Inert atmosphere 3.1: sodium hydroxide; water / isopropyl alcohol / 20 °C 3.2: pH 4 - 5 View Scheme | |
| Multi-step reaction with 3 steps 1: copper(l) iodide / N,N-dimethyl-formamide / 120 °C 2: potassium carbonate / N,N-dimethyl-formamide / 80 °C 3: sodium hydroxide / methanol; water / 2 h / 25 °C View Scheme |
-
-
676602-31-6
3-cyano-4-fluorobenzoic acid methyl ester

-
-
258273-31-3
3-cyano-4-isopropoxybenzoic acid

| Conditions | Yield |
|---|---|
| Multi-step reaction with 2 steps 1.1: sodium hexamethyldisilazane / tetrahydrofuran / 20 °C / Cooling with ice 1.2: 16 h / 50 °C 2.1: sodium hydroxide; water / isopropyl alcohol / 20 °C 2.2: pH ~ 2 View Scheme | |
| Multi-step reaction with 2 steps 1.1: sodium hexamethyldisilazane / tetrahydrofuran / 17 h / 20 - 50 °C / Cooling with ice 2.1: sodium hydroxide; water / isopropyl alcohol / 20 °C 2.2: pH 2 View Scheme |
-
-
171050-06-9
3-cyano-4-fluorobenzoic acid

-
-
258273-31-3
3-cyano-4-isopropoxybenzoic acid

| Conditions | Yield |
|---|---|
| Multi-step reaction with 3 steps 1.1: toluene-4-sulfonic acid / 80 - 90 °C 2.1: sodium hexamethyldisilazane / tetrahydrofuran / 20 °C / Cooling with ice 2.2: 16 h / 50 °C 3.1: sodium hydroxide; water / isopropyl alcohol / 20 °C 3.2: pH ~ 2 View Scheme | |
| Multi-step reaction with 3 steps 1.1: toluene-4-sulfonic acid / 90 °C 2.1: sodium hexamethyldisilazane / tetrahydrofuran / 17 h / 20 - 50 °C / Cooling with ice 3.1: sodium hydroxide; water / isopropyl alcohol / 20 °C 3.2: pH 2 View Scheme |
-
-
213598-10-8
methyl 3-bromo-4-isopropoxybenzoate

-
-
258273-31-3
3-cyano-4-isopropoxybenzoic acid

| Conditions | Yield |
|---|---|
| Multi-step reaction with 2 steps 1: tetrakis(triphenylphosphine) palladium(0) / N,N-dimethyl-formamide / 120 °C / Inert atmosphere 2: sodium hydroxide / methanol / 16 h / 20 °C View Scheme | |
| Multi-step reaction with 2 steps 1.1: tetrakis(triphenylphosphine) palladium(0) / N,N-dimethyl-formamide / 120 °C / Inert atmosphere 2.1: sodium hydroxide; water / isopropyl alcohol / 20 °C 2.2: pH 4 - 5 View Scheme |
-
-
24589-99-9
methyl 3-formyl-4-hydroxybenzoate

-
-
258273-31-3
3-cyano-4-isopropoxybenzoic acid

| Conditions | Yield |
|---|---|
| Multi-step reaction with 3 steps 1: hydroxylamine hydrochloride; acetyl chloride / N,N-dimethyl-formamide; acetonitrile / 2 h / 80 °C / Large scale 2: potassium carbonate / N,N-dimethyl-formamide; acetonitrile / 80 °C / Large scale 3: sodium hydroxide / tetrahydrofuran / 1 h / 60 °C / Large scale View Scheme |
-
-
258273-31-3
3-cyano-4-isopropoxybenzoic acid

-
-
1072948-37-8
3-cyano-4-[(1-methylethyl)oxy]benzoyl chloride

| Conditions | Yield |
|---|---|
| With oxalyl dichloride; N,N-dimethyl-formamide In dichloromethane at 20℃; for 2h; | 100% |
| With oxalyl dichloride; N,N-dimethyl-formamide at 20℃; for 4h; Product distribution / selectivity; | 100% |
| With oxalyl dichloride; N,N-dimethyl-formamide In dichloromethane at 20℃; for 4h; | 100% |
-
-
258273-31-3
3-cyano-4-isopropoxybenzoic acid

-
-
67-56-1
methanol

-
-
213598-11-9
3-cyano-4-isopropoxybenzoic acid methyl ester

| Conditions | Yield |
|---|---|
| Stage #1: 3-cyano-4-isopropoxybenzoic acid; methanol With diazomethyl-trimethyl-silane In dichloromethane at 0 - 20℃; for 1.8h; Stage #2: With acetic acid In dichloromethane at 20℃; | 100% |
-
-
258273-31-3
3-cyano-4-isopropoxybenzoic acid

-
-
79-19-6
thiosemicarbazide

-
-
1258440-55-9
5-(5-amino-1,3,4-thiadiazol-2-yl)-2-[(1-methylethyl)oxy]benzonitrile

| Conditions | Yield |
|---|---|
| With trichlorophosphate at 90℃; for 3h; | 99% |
| Stage #1: 3-cyano-4-isopropoxybenzoic acid; thiosemicarbazide With trichlorophosphate at 90℃; for 3h; Stage #2: With sodium hydroxide In water at 35℃; pH=10; Cooling with ice; | 99% |
| Stage #1: 3-cyano-4-isopropoxybenzoic acid; thiosemicarbazide With trichlorophosphate at 90℃; for 3h; Stage #2: With sodium hydroxide at 35℃; pH=10; Cooling with ice; | 99% |
-
-
258273-31-3
3-cyano-4-isopropoxybenzoic acid

-
-
1167416-24-1
1,1-dimethylethyl 8-[(hydroxyamino)(imino)methyl]-2,3-dihydro-1,4-benzoxazepine-4(5H)-carboxylate

-
-
1167416-65-0
1,1-dimethylethyl 8-(5-{3-cyano-4-[(1-methylethyl)oxy]phenyl}-1,2,4-oxadiazol-3-yl)-2,3-dihydro-1,4-benzoxazepine-4(5H)-carboxylate

| Conditions | Yield |
|---|---|
| With benzotriazol-1-ol; 1-ethyl-(3-(3-dimethylamino)propyl)-carbodiimide hydrochloride In N,N-dimethyl-formamide at 20℃; Heating; | 92% |
-
-
258273-31-3
3-cyano-4-isopropoxybenzoic acid

| Conditions | Yield |
|---|---|
| Stage #1: 3-cyano-4-isopropoxybenzoic acid With 1,1'-carbonyldiimidazole In N,N-dimethyl-formamide at 20℃; for 1h; Stage #2: 3-ethoxy-N-hydroxy-1H-indene-7-carboximidamide In N,N-dimethyl-formamide for 0.583333h; | 90% |
| Stage #1: 3-cyano-4-isopropoxybenzoic acid With 1,1'-carbonyldiimidazole In water; N,N-dimethyl-formamide at 20℃; for 1h; Stage #2: 3-ethoxy-N-hydroxy-1H-indene-7-carboximidamide In water; N,N-dimethyl-formamide for 0.583333h; | 90% |
| Stage #1: 3-cyano-4-isopropoxybenzoic acid With 1,1'-carbonyldiimidazole In N,N-dimethyl-formamide at 20℃; for 1h; Stage #2: 3-ethoxy-N-hydroxy-1H-indene-7-carboximidamide In N,N-dimethyl-formamide for 0.583333h; | 90% |
-
-
258273-31-3
3-cyano-4-isopropoxybenzoic acid

| Conditions | Yield |
|---|---|
| Stage #1: 3-cyano-4-isopropoxybenzoic acid With 1,1'-carbonyldiimidazole In N,N-dimethyl-formamide at 20℃; for 1h; Stage #2: 3-ethoxy-N-hydroxy-1H-indene-7-carboximidamide In N,N-dimethyl-formamide for 0.583333h; | 90% |
| Stage #1: 3-cyano-4-isopropoxybenzoic acid With 1,1'-carbonyldiimidazole In N,N-dimethyl-formamide at 20℃; for 1h; Stage #2: 3-ethoxy-N-hydroxy-1H-indene-7-carboximidamide In N,N-dimethyl-formamide at 20℃; for 0.583333h; | 90% |
-
-
258273-31-3
3-cyano-4-isopropoxybenzoic acid

-
-
1333996-58-9
tert-butyl 3-(N'-hydroxycarbamimidoyl)-4-methyl-5,6-dihydro-1,7-naphthyridine-7(8H)-carboxylate

-
-
1333996-60-3
tert-butyl 3-(5-(3-cyano-4-isopropoxyphenyl)-1,2,4-oxadiazol-3-yl)-4-methyl-5,6-dihydro-1,7-naphthyridine-7(8H)-carboxylate

| Conditions | Yield |
|---|---|
| Stage #1: 3-cyano-4-isopropoxybenzoic acid With N-ethyl-N,N-diisopropylamine; HATU In N,N-dimethyl-formamide at 20℃; for 0.0833333h; Stage #2: tert-butyl 3-(N'-hydroxycarbamimidoyl)-4-methyl-5,6-dihydro-1,7-naphthyridine-7(8H)-carboxylate In N,N-dimethyl-formamide at 20 - 100℃; for 17.5h; | 88% |
-
-
258273-31-3
3-cyano-4-isopropoxybenzoic acid

| Conditions | Yield |
|---|---|
| Stage #1: 3-cyano-4-isopropoxybenzoic acid; 2-chloro-4-(1,3-dioxolan-2-yl)-N′-hydroxybenzimidamide With potassium carbonate; 1-ethyl-(3-(3-dimethylamino)propyl)-carbodiimide hydrochloride In N,N-dimethyl-formamide at 20℃; for 0.5h; Stage #2: With benzotriazol-1-ol In N,N-dimethyl-formamide at 110℃; for 2h; Inert atmosphere; | 87% |
| Stage #1: 3-cyano-4-isopropoxybenzoic acid With potassium carbonate; benzotriazol-1-ol; 1-ethyl-(3-(3-dimethylamino)propyl)-carbodiimide hydrochloride In N,N-dimethyl-formamide at 20℃; for 0.5h; Stage #2: 2-chloro-4-(1,3-dioxolan-2-yl)-N′-hydroxybenzimidamide In N,N-dimethyl-formamide at 110℃; for 4h; Inert atmosphere; | 87% |
-
-
258273-31-3
3-cyano-4-isopropoxybenzoic acid

| Conditions | Yield |
|---|---|
| Stage #1: 3-cyano-4-isopropoxybenzoic acid; 4-(1,3-dioxolan-2-yl)-2-fluoro-N′-hydroxybenzimidamide With potassium carbonate; 1-ethyl-(3-(3-dimethylamino)propyl)-carbodiimide hydrochloride In N,N-dimethyl-formamide at 20℃; for 0.5h; Stage #2: With benzotriazol-1-ol In N,N-dimethyl-formamide at 110℃; for 2h; Inert atmosphere; | 81.7% |
| Stage #1: 3-cyano-4-isopropoxybenzoic acid With potassium carbonate; benzotriazol-1-ol; 1-ethyl-(3-(3-dimethylamino)propyl)-carbodiimide hydrochloride In N,N-dimethyl-formamide at 20℃; for 0.5h; Stage #2: 4-(1,3-dioxolan-2-yl)-2-fluoro-N′-hydroxybenzimidamide In N,N-dimethyl-formamide at 110℃; for 4h; Inert atmosphere; | 81.7% |
-
-
258273-31-3
3-cyano-4-isopropoxybenzoic acid

| Conditions | Yield |
|---|---|
| Stage #1: (3S)-3-amino-4-(4-bromophenyl)-1-butanol hydrochloride With N-ethyl-N,N-diisopropylamine In DMF (N,N-dimethyl-formamide) for 0.05h; Stage #2: 3-cyano-4-isopropoxybenzoic acid With O-(1H-benzotriazol-1-yl)-N,N,N',N'-tetramethyluronium hexafluorophosphate In DMF (N,N-dimethyl-formamide) at 20℃; for 1.5h; Stage #3: With water In DMF (N,N-dimethyl-formamide) | 78% |
-
-
258273-31-3
3-cyano-4-isopropoxybenzoic acid

-
-
1307231-09-9
N-hydroxybenzofuran-5-carboximidamide

-
-
1307230-52-9
5-(3-(benzofuran-5-yl)-1,2,4-oxadiazol-5-yl)-2-isopropoxybenzonitrile

| Conditions | Yield |
|---|---|
| Stage #1: 3-cyano-4-isopropoxybenzoic acid With benzotriazol-1-ol; 1-ethyl-(3-(3-dimethylamino)propyl)-carbodiimide hydrochloride In N,N-dimethyl-formamide at 20℃; for 0.5h; Inert atmosphere; Stage #2: N-hydroxybenzofuran-5-carboximidamide In N,N-dimethyl-formamide at 20 - 85℃; | 69% |
-
-
258273-31-3
3-cyano-4-isopropoxybenzoic acid

| Conditions | Yield |
|---|---|
| Stage #1: 3-cyano-4-isopropoxybenzoic acid With 1,1'-carbonyldiimidazole In N,N-dimethyl-formamide at 0℃; for 0.5h; Stage #2: (S)-1-azido-N’-hydroxy-2,3-dihydro-1H-indene-4-carboximidamide In N,N-dimethyl-formamide at 115℃; for 24h; Reagent/catalyst; Solvent; | 69% |
-
-
258273-31-3
3-cyano-4-isopropoxybenzoic acid


| Conditions | Yield |
|---|---|
| With benzotriazol-1-ol; 1,2-dichloro-ethane In N,N-dimethyl-formamide at 20 - 85℃; for 1.5h; | 67% |
-
-
258273-31-3
3-cyano-4-isopropoxybenzoic acid


| Conditions | Yield |
|---|---|
| Stage #1: 3-cyano-4-isopropoxybenzoic acid; (1S)-1-({2-[(tert-butyldimethylsilyl)oxy]ethyl}[(S)-2-methylpropane-2-sulfinyl]amino)-N-hydroxy-2,3-dihydro-1H-indene-4-carboximidamide With 2,4,6-tripropyl-1,3,5,2,4,6-trioxatriphosphinane-2,4,6-trioxide; triethylamine In toluene at 20 - 90℃; Inert atmosphere; Stage #2: With hydrogenchloride In water at 0 - 90℃; for 1h; Solvent; | 63.6% |
-
-
258273-31-3
3-cyano-4-isopropoxybenzoic acid

-
-
852691-00-0
4-(1,3-dioxolan-2-yl)-N1-hydroxybenzenecarboximidamide

| Conditions | Yield |
|---|---|
| Stage #1: 3-cyano-4-isopropoxybenzoic acid; 4-(1,3-dioxolan-2-yl)-N1-hydroxybenzenecarboximidamide With potassium carbonate; 1-ethyl-(3-(3-dimethylamino)propyl)-carbodiimide hydrochloride In N,N-dimethyl-formamide at 20℃; for 0.5h; Stage #2: With benzotriazol-1-ol In N,N-dimethyl-formamide at 110℃; for 2h; Inert atmosphere; | 59.7% |
| Stage #1: 3-cyano-4-isopropoxybenzoic acid With potassium carbonate; benzotriazol-1-ol; 1-ethyl-(3-(3-dimethylamino)propyl)-carbodiimide hydrochloride In N,N-dimethyl-formamide at 20℃; for 0.5h; Stage #2: 4-(1,3-dioxolan-2-yl)-N1-hydroxybenzenecarboximidamide In N,N-dimethyl-formamide at 110℃; for 4h; Inert atmosphere; | 59.7% |
| With potassium carbonate; benzotriazol-1-ol; 1-ethyl-(3-(3-dimethylamino)propyl)-carbodiimide hydrochloride In N,N-dimethyl-formamide at 20℃; | 53.1% |
-
-
258273-31-3
3-cyano-4-isopropoxybenzoic acid

-
-
1233500-95-2
tert-butyl 5-(N-hydroxycarbamimidoyl)-1H-indole-1-carboxylate

-
-
1307231-08-8
tert-butyl 5-(5-(3-cyano-4-isopropoxyphenyl)-1,2,4-oxadiazol-3-yl)-1H-indole-1-carboxylate

| Conditions | Yield |
|---|---|
| Stage #1: 3-cyano-4-isopropoxybenzoic acid With benzotriazol-1-ol; 1-ethyl-(3-(3-dimethylamino)propyl)-carbodiimide hydrochloride at 20℃; for 1.5h; Inert atmosphere; Stage #2: tert-butyl 5-(N-hydroxycarbamimidoyl)-1H-indole-1-carboxylate In N,N-dimethyl-formamide at 20℃; for 1h; | 59% |
-
-
258273-31-3
3-cyano-4-isopropoxybenzoic acid

-
-
1167415-97-5
1,1-dimethylethyl 7-[(hydroxyamino)(imino)methyl]-1,3,4,5-tetrahydro-2H-2-benzazepine-2-carboxylate

-
-
1167416-00-3
1,1-dimethylethyl 7-(5-{3-cyano-4-[(1-methylethyl)oxy]phenyl}-1,2,4-oxadiazol-3-yl)-1,3,4,5-tetrahydro-2H-2-benzazepine-2-carboxylate

| Conditions | Yield |
|---|---|
| Stage #1: 3-cyano-4-isopropoxybenzoic acid With benzotriazol-1-ol; 1-ethyl-(3-(3-dimethylamino)propyl)-carbodiimide hydrochloride In N,N-dimethyl-formamide at 20℃; for 0.0833333h; Stage #2: 1,1-dimethylethyl 7-[(hydroxyamino)(imino)methyl]-1,3,4,5-tetrahydro-2H-2-benzazepine-2-carboxylate In N,N-dimethyl-formamide at 20 - 90℃; for 21h; | 46% |
| With benzotriazol-1-ol; 1-ethyl-(3-(3-dimethylamino)propyl)-carbodiimide hydrochloride In N,N-dimethyl-formamide at 20 - 90℃; |
-
-
258273-31-3
3-cyano-4-isopropoxybenzoic acid

-
-
1307231-29-3
N-(3-cyano-4-isopropoxybenzoyloxy)-3-methylisonicotinimidamide

| Conditions | Yield |
|---|---|
| Stage #1: 3-cyano-4-isopropoxybenzoic acid With benzotriazol-1-ol; 1-ethyl-(3-(3-dimethylamino)propyl)-carbodiimide hydrochloride In N,N-dimethyl-formamide at 20℃; for 0.5h; Stage #2: N-hydroxy-3-methylisonicotinimidamide In N,N-dimethyl-formamide | 45% |
-
-
258273-31-3
3-cyano-4-isopropoxybenzoic acid

-
-
1307230-79-0
(R)-N,5-dihydroxy-5,6,7,8-tetrahydronaphthalene-1-carboximidamide

-
-
1307230-03-0
(R)-5-(3-(5-hydroxy-5,6,7,8-tetrahydronaphthalen-1-yl)-1,2,4-oxadiazol-5-yl)-2-isopropoxybenzonitrile

| Conditions | Yield |
|---|---|
| Stage #1: 3-cyano-4-isopropoxybenzoic acid With benzotriazol-1-ol; 1-ethyl-(3-(3-dimethylamino)propyl)-carbodiimide hydrochloride In N,N-dimethyl-formamide for 0.5h; Stage #2: (R)-N,5-dihydroxy-5,6,7,8-tetrahydronaphthalene-1-carboximidamide In N,N-dimethyl-formamide at 20 - 90℃; for 16h; | 42.4% |
-
-
258273-31-3
3-cyano-4-isopropoxybenzoic acid

| Conditions | Yield |
|---|---|
| Stage #1: 3-cyano-4-isopropoxybenzoic acid With benzotriazol-1-ol; 1-ethyl-(3-(3-dimethylamino)propyl)-carbodiimide hydrochloride In N,N-dimethyl-formamide at 20℃; for 16h; Stage #2: (S)-N,1-dihydroxy-2,3-dihydro-1H-indene-4-carboximidamide In N,N-dimethyl-formamide at 20 - 85℃; for 20h; | 40% |
-
-
258273-31-3
3-cyano-4-isopropoxybenzoic acid

-
-
1165923-57-8
1,1-dimethylethyl 5-hydroxyamino(imino)methyl-3,4-dihydro-2(1H)-isoquinolinecarboxylate

-
-
1165923-65-8
1,1-dimethylethyl 5-(5-{3-cyano-4-[(1-methylethyl)oxy]phenyl}-1,2,4-oxadiazol-3-yl)-3,4-dihydro-2(1H)-isoquinolinecarboxylate

| Conditions | Yield |
|---|---|
| Stage #1: 3-cyano-4-isopropoxybenzoic acid With oxalyl dichloride; N,N-dimethyl-formamide In dichloromethane at 20℃; for 1.5h; Stage #2: 1,1-dimethylethyl 5-hydroxyamino(imino)methyl-3,4-dihydro-2(1H)-isoquinolinecarboxylate With dmap; N-ethyl-N,N-diisopropylamine In N,N-dimethyl-formamide at 20 - 95℃; for 19h; | 37% |
| Stage #1: 3-cyano-4-isopropoxybenzoic acid With oxalyl dichloride; N,N-dimethyl-formamide In dichloromethane at 20℃; for 1.5h; Stage #2: 1,1-dimethylethyl 5-hydroxyamino(imino)methyl-3,4-dihydro-2(1H)-isoquinolinecarboxylate With dmap; N-ethyl-N,N-diisopropylamine In N,N-dimethyl-formamide at 20 - 95℃; for 19h; |
-
-
258273-31-3
3-cyano-4-isopropoxybenzoic acid

-
-
1167416-58-1
1,1-dimethylethyl 7-[(hydroxyamino)(imino)methyl]-2,3-dihydro-1,4-benzoxazepine-4(5H)-carboxylate

-
-
1167416-59-2
1,1-dimethylethyl 7-(5-{3-cyano-4-[(1-methylethyl)oxy]phenyl}-1,2,4-oxadiazol-3-yl)-2,3-dihydro-1,4-benzoxazepine-4(5H)-carboxylate

| Conditions | Yield |
|---|---|
| With benzotriazol-1-ol; 1-ethyl-(3-(3-dimethylamino)propyl)-carbodiimide hydrochloride In N,N-dimethyl-formamide at 20 - 120℃; for 36h; | 31% |
| With benzotriazol-1-ol; 1-ethyl-(3-(3-dimethylamino)propyl)-carbodiimide hydrochloride In N,N-dimethyl-formamide at 20 - 120℃; |
Related products
Hot Products
About|Contact|Cas|Product Name|Molecular|Country|Encyclopedia
Message|New Cas|MSDS|Service|Advertisement|CAS DataBase|Article Data|Manufacturers | Chemical Catalog
©2008 LookChem.com,License: ICP
NO.:Zhejiang16009103
complaints:service@lookchem.com Desktop View