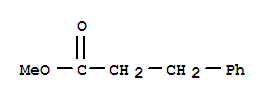Dayang Chem (Hangzhou) Co.,Ltd.
As a leading manufacturer and supplier of chemicals in China, DayangChem not only supply popular chemicals, but also DayangChem's R&D center offer custom synthesis services. DayangChem can provide different quantities of custom synthesis ch
Cas:41639-74-1
Min.Order:1 Kilogram
FOB Price: $3.0
Type:Lab/Research institutions
inquiryHangzhou Think Chemical Co. Ltd
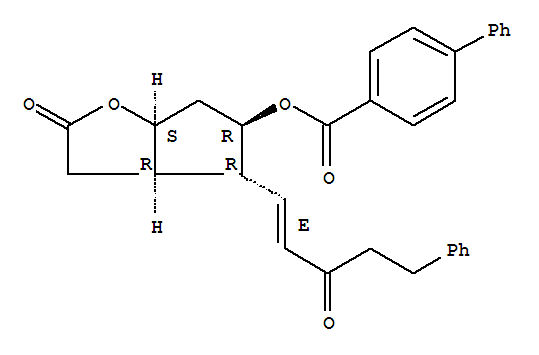
(+)-(3aR,4R,5r,6aS)-Hexahydro-5-hydroxy-4-[(1E,3R)-3-hydroxy-5-phenyl-1-pentenyl]-2H-cyclopenta[b]furan-2-one CAS No.:41639-74-1 Name: (+)-(3aR,4R,5r,6aS)-Hexahydro-5-hydroxy-4-[(1E,3R)-3-hydroxy-5-phenyl-1-pentenyl]-2H-cyclopenta[b]f
Cas:41639-74-1
Min.Order:1 Kilogram
FOB Price: $2.0
Type:Other
inquiryChemwill Asia Co., Ltd.
Cas:41639-74-1
Min.Order:1 Metric Ton
FOB Price: $1.0
Type:Manufacturers
inquiryHenan Tianfu Chemical Co., Ltd.
Product Name: HYDRIDE-L Synonyms: HYDRIDE-L;(+)-(3aR,4R,5r,6aS)-Hexahydro-5-hydroxy-4-[(1E,3R)-3-hydroxy-5-phenyl-1-pentenyl]-2H-cyclopenta[b]furan-2-one;BP-2 (LT-EDI);BP-2 (LT-EDI)(Bimaprost、Latanprost);(3aR,4R,5R,6aS)-5-Hydroxy-4-((S
Cas:41639-74-1
Min.Order:1 Gram
FOB Price: $8900.0
Type:Lab/Research institutions
inquiryZhuozhou Wenxi import and Export Co., Ltd
WITH US,YOUR MONEY IN SAFE,YOUR BUSINESS IN SAFE 1)Quick Response Within 12 hours; 2)Quality Guarantee: All products are strictly tested by our QC, confirmed by QA and approved by third party lab in China, USA, Canada, Germany, UK, Italy, France et
Cas:41639-74-1
Min.Order:1 Kilogram
FOB Price: $139.0 / 210.0
Type:Trading Company
inquiryWuhan Han Sheng New Material Technology Co.,Ltd
Our Advantage: high quality with competitive price High quality standard: BP/USP/EP Enterprise standard All purity customized Fast and safe delivery We have reliable forwarder who can help us deliver our goods more fast and safe. We
Qingdao Beluga Import and Export Co., LTD
HYDRIDE-L CAS:41639-74-1 Qingdao Belugas Import and Export Co., Ltd. is a scientific and technological company integrating research and development, production and trade of chemical intermediates, specializing in high quality organic intermediates,
Cas:41639-74-1
Min.Order:1 Gram
Negotiable
Type:Lab/Research institutions
inquiryHenan Wentao Chemical Product Co., Ltd.
Henan Wentao Chemical Product Co.,Ltd is Located in Zhengzhou High-tech Development Zone with import and export license, We passed ISO 9001:2008 as well, Henan Wentao has developed more than 1000 compounds, which are widely used in the fields of prod
Cas:41639-74-1
Min.Order:0
Negotiable
Type:Lab/Research institutions
inquirySinoway Industrial Co., Ltd.
Why is SINOWAY: 1) Specialized in pharmaceutical and healthcare industrial for 34 years. 2) ISO 9001:2015 & SGS audited supplier . 3) Accept various payment terms : T.T 30-60 days. 4) We have warehouse in USA with quickly shipment . 5) We c
Cas:41639-74-1
Min.Order:1 Kilogram
Negotiable
Type:Trading Company
inquiryTaizhou Crene Biotechnology co.ltd
Our company provides one-stop services of research - development - production for a variety of special prouducts. Not only do we make effective use of our strong technological strength, but also establish of cooperative relations with several well-
Cas:41639-74-1
Min.Order:0 Metric Ton
Negotiable
Type:Lab/Research institutions
inquiryHangzhou Keyingchem Co.,Ltd
Hangzhou KeyingChem Co., Ltd. exported this product to many countries and regions at best price. If you are looking for the material’s manufacturer or supplier in China, KeyingChem is your best choice. Pls contact with us freely for getting det
Afine Chemicals Limited
Company Introduction 1. Established in 2005, with two independent business divisions: Fine chemicals division; Pharmaceutical division. 2. Main product: Optical brightener Textile auxiliary Dye stuff Pigments
Zibo Hangyu Biotechnology Development Co., Ltd
Zibo Hangyu Biotechnology Development Co., Ltd is a leading manufacturer and supplier of chemicals in China. We develop produce and distribute high quality pharmaceuticals, intermediates, special chemicals and OLED intermediates and other fine chemi
SHANGHAI SYSTEAM BIOCHEM CO., LTD
We are one of a few suppliers that can offer custom synthesis service of this product We are specialized in custom synthesis, chemical/pharmaceutical/ pesticides outsourcing and contract research. We are committed to prov
Ality Chemical Corporation
The above product is Ality Chemical's strong item with best price, good quality and fast supply. Ality Chemical has been focusing on the research and production of this field for over 14 years. At the same time, we are always committed to providing h
Siwei Development Group Ltd.
Product name: 2H-Cyclopenta[b]furan-2-One, Hexahydro-5-Hydroxy-4-[(1E,3S)-3-Hydroxy-5-Phenyl-1-Penten-1-yl]-,(3aR,4R,5R,6aS)- CAS No.:41639-74-1 Molecule Formula:C18H22O4 Molecule Weight:302.36 Purity: 98.0% Package: 25kg/drum Description:White
Cas:41639-74-1
Min.Order:1 Kilogram
Negotiable
Type:Lab/Research institutions
inquiryZibo Dorne chemical technology co. LTD
Product Details Grade: pharmaceutical grade Purity:99%+ ProductionCapacity: 1000 Kilogram/Month Scope of use: For scientific research only(The product must be used legally) Our Advantage 1. Best quality with competitive price. 2. Quick shipping,
EAST CHEMSOURCES LIMITED
factory?direct?saleAppearance:White Powder Storage:Store In Dry, Cool And Ventilated Place Package:25kg/drum, also according to the clients requirement Application:It is widely used as a thickener, emulsifier and stabilizer Transportation:By Sea Or B
Hangzhou JINLAN Pharm-Drugs Technology Co., Ltd
Product name: BP-2;(3aR,4R,5R,6aS)-Hexahydro-5-hydroxy-4-[(1E,3R)-3-hydroxy-5-phenyl-1-pentenyl]-2H-cyclopenta[b]furan-2-one CAS No.: 41639-74-1 Chemical name: HYDRIDE-L;(+)-(3aR,4R,5r,6aS)-Hexahydro-5-hydroxy-4-[(1E,3R)-3-hydroxy-5-phenyl
Cas:41639-74-1
Min.Order:1 Kilogram
Negotiable
Type:Trading Company
inquiryShandong Mopai Biotechnology Co., LTD
Shandong Mopai Biotechnology Co., LTD is a leading manufacturer and supplier of chemicals in China. We develop produce and distribute high quality pharmaceuticals, intermediates, special chemicals and OLED intermediates and other fine chemicals. W
KAISA GROUP INC
1.Applied in food field.it can improve the immune system and prolong life. 2.Appliedin cosmetic field.it can improve the skin care. 3.Applied in pharmaceutical field.it can treat various dieases. 4.Our product quality assurance will make our customer
Senova Technology Company Limited
1.High quality 2.High purity 3.Best price 4.Best service 5.Professional manufacturer 6.Loyal and honest supplier 7.Fast response to customer within 6 hours Application:Pharmaceutical Intermediate
Cas:41639-74-1
Min.Order:100 Gram
Negotiable
Type:Trading Company
inquirySAGECHEM LIMITED
SAGECHEM is a chemical R&D, manufacturing and distribution company in China since 2009, including pharmaceutical intermediates, agrochemical, dyestuff intermediates, organosilicone, API and etc. We also offer a full range of services in custom synthe
Cas:41639-74-1
Min.Order:0
Negotiable
Type:Lab/Research institutions
inquirySuzhou Health Chemicals Co., Ltd.
High quality,stable supply chain.Appearance:white/off-white or light yellow Storage:Store in cool and dry place, keep away from strong light and heat. Package:aluminum bottle,glass bottle,PTFE bottle,cardboard drum Application:This product can be use
Cas:41639-74-1
Min.Order:0
Negotiable
Type:Lab/Research institutions
inquiryCHANGZHOU HANGYU PHARMACEUTICAL TECHNOLOGY CO., LTD
CHANGZHOU HANGYU PHARMACEUTICAL TECHNOLOGY CO., LTD. founded in 1984, engages in pharmaceutical research, development, production, process design and technical consultation of synthetic and fermentation pharmaceutical products. The organizational s
Cas:41639-74-1
Min.Order:1 Kilogram
Negotiable
Type:Lab/Research institutions
inquiryBluecrystal chem-union
We are a Union of chemistry in China, consists of chemists,engineers, laboratories,factories in China. We organize surplus capacity of R&D and production as well as custom synthesis for chemical products and chemical business project. We are supp
GIHI CHEMICALS CO.,LIMITED
Lower price, sample is available,SDS test documents are available,large stock in warehouseAppearance:White powder Storage:Sealed and preserved Package:200/Kilograms Application:Fine chemical intermediates, used as the main raw material for the synthe
Cas:41639-74-1
Min.Order:0
Negotiable
Type:Lab/Research institutions
inquiryHangzhou J&H Chemical Co., Ltd.
J&H CHEM is one of China's leading providers of integrated fine chemical services including offering, research and development, Custom manufacturing business, as well as other Value-added customer services, for diversified range products of chemicals
Antimex Chemical Limied
hight degree of purity Application:Fine chemical intermediates, used as the main raw material for he synthesis of various pesticides, medicines, surfactants, polymer monomers, Ond Ontifungal agents
Cas:41639-74-1
Min.Order:0
Negotiable
Type:Lab/Research institutions
inquiryWuhan Circle Star Chem-medical Technology co.,Ltd.
good quality, competitive price, thoughtful after sale serviceAppearance:White to Off-White Solid Storage:Keep it in dry,shady and cool place Package:as your requirement Application:Pharma;Industry;Agricultural;chemical reaserch Transportation:by Sea
Synthetic route

-
-
41639-74-1
(3aR,4R,5R,6aS)-hexahydro-5-hydroxy-4-[(1E,3S)-5-phenyl-3-hydroxy-(1E)-pentenyl]-2H-cyclopentane [β] furan-2-one

| Conditions | Yield |
|---|---|
| Stage #1: (3aR,4R,5R,6aS)-5-hydroxy-4-((E)-3-oxo-5-phenylpent-1-en-1-yl)hexahydro-2H-cyclopenta[b]furan-2-one With chlorobis(2,6,6-trimethylbicyclo[3.1.1]heptan-3-yl)borane In tetrahydrofuran at -40℃; for 12h; Stage #2: With hydrogenchloride In water at 20℃; for 0.5h; Reagent/catalyst; Temperature; | 88% |
| With C20H35B In tetrahydrofuran; hexane at -40℃; for 12h; | 76% |
-
-
55444-68-3
(3aR,4R,5R,6aS)-4-[(1E,3S)-3-hydroxy-5-phenylpent-1-en-1-yl]-2-oxo-hexahydro-2H-cyclopenta[b]furan-5-yl benzoate

-
-
41639-74-1
(3aR,4R,5R,6aS)-hexahydro-5-hydroxy-4-[(1E,3S)-5-phenyl-3-hydroxy-(1E)-pentenyl]-2H-cyclopentane [β] furan-2-one

| Conditions | Yield |
|---|---|
| With potassium carbonate In methanol at 25℃; for 2h; | 83% |
| With methanol; potassium carbonate deprotection; |
-
-
41639-73-0
(1S,5R,6R,7R)-6-<(3R)-3-hydroxy-5-phenyl-1-pentenyl>-7-<(4-phenylbenzoyl)oxy>-2-oxabicyclo<3.3.0>octan-3-one

-
-
41639-74-1
(3aR,4R,5R,6aS)-hexahydro-5-hydroxy-4-[(1E,3S)-5-phenyl-3-hydroxy-(1E)-pentenyl]-2H-cyclopentane [β] furan-2-one

| Conditions | Yield |
|---|---|
| With methanol; potassium carbonate | 80% |
| With potassium carbonate In tetrahydrofuran; methanol for 2.5h; | |
| Stage #1: (1S,5R,6R,7R)-6-<(3R)-3-hydroxy-5-phenyl-1-pentenyl>-7-<(4-phenylbenzoyl)oxy>-2-oxabicyclo<3.3.0>octan-3-one With methanol; potassium carbonate at 20 - 30℃; Stage #2: With hydrogenchloride In water; ethyl acetate at 20 - 30℃; for 0.5h; |
-
-
944269-28-7
benzyl(1R,2R,3R)-3-hydroxy-2-[5-phenyl-(3S)-hydroxy-(1E)-pentenyl]-5-oxo-cyclopentane-acetate

-
-
41639-74-1
(3aR,4R,5R,6aS)-hexahydro-5-hydroxy-4-[(1E,3S)-5-phenyl-3-hydroxy-(1E)-pentenyl]-2H-cyclopentane [β] furan-2-one

| Conditions | Yield |
|---|---|
| With L-Selectride In tetrahydrofuran at -70℃; for 2h; Product distribution / selectivity; | 78% |

-
-
41639-74-1
(3aR,4R,5R,6aS)-hexahydro-5-hydroxy-4-[(1E,3S)-5-phenyl-3-hydroxy-(1E)-pentenyl]-2H-cyclopentane [β] furan-2-one

| Conditions | Yield |
|---|---|
| Stage #1: benzyl (1R,2R,3R)-3-hydroxy-2-[5-phenyl-(3S)-3-hydroxy-1-pentenyl]-5-oxo-cyclopentaneacetate With L-Selectride In tetrahydrofuran at -70℃; for 2h; Cooling with methanol-dry ice; Stage #2: With water; ammonium chloride In tetrahydrofuran Product distribution / selectivity; | 78% |

-
-
41639-74-1
(3aR,4R,5R,6aS)-hexahydro-5-hydroxy-4-[(1E,3S)-5-phenyl-3-hydroxy-(1E)-pentenyl]-2H-cyclopentane [β] furan-2-one

| Conditions | Yield |
|---|---|
| With L-Selectride In tetrahydrofuran at -70℃; for 2h; Product distribution / selectivity; | 77% |

-
-
41639-74-1
(3aR,4R,5R,6aS)-hexahydro-5-hydroxy-4-[(1E,3S)-5-phenyl-3-hydroxy-(1E)-pentenyl]-2H-cyclopentane [β] furan-2-one

| Conditions | Yield |
|---|---|
| Stage #1: (1'R,2'S,5'R)-menthyl (1R,2R,3R)-3-hydroxy-2-[5-phenyl-(3S)-3-hydroxy-1-pentenyl]-5-oxo-cyclopentaneacetate With L-Selectride In tetrahydrofuran at -70℃; for 2h; Cooling with methanol-dry ice; Stage #2: With water; ammonium chloride In tetrahydrofuran Product distribution / selectivity; | 77% |

-
-
41639-74-1
(3aR,4R,5R,6aS)-hexahydro-5-hydroxy-4-[(1E,3S)-5-phenyl-3-hydroxy-(1E)-pentenyl]-2H-cyclopentane [β] furan-2-one

| Conditions | Yield |
|---|---|
| With potassium carbonate In methanol; dichloromethane at 25 - 30℃; Inert atmosphere; | 77% |

-
-
41639-74-1
(3aR,4R,5R,6aS)-hexahydro-5-hydroxy-4-[(1E,3S)-5-phenyl-3-hydroxy-(1E)-pentenyl]-2H-cyclopentane [β] furan-2-one

| Conditions | Yield |
|---|---|
| With L-Selectride In tetrahydrofuran at -70℃; for 2h; Product distribution / selectivity; | 75% |

-
-
41639-74-1
(3aR,4R,5R,6aS)-hexahydro-5-hydroxy-4-[(1E,3S)-5-phenyl-3-hydroxy-(1E)-pentenyl]-2H-cyclopentane [β] furan-2-one

| Conditions | Yield |
|---|---|
| Stage #1: ethyl (1R,2R,3R)-3-hydroxy-2-[5-phenyl-(3S)-3-hydroxy-1-pentenyl]-5-oxo-cyclopentaneacetate With L-Selectride In tetrahydrofuran at -70℃; for 2h; Cooling with methanol-dry ice; Stage #2: With water; ammonium chloride In tetrahydrofuran Product distribution / selectivity; | 75% |

-
-
41639-74-1
(3aR,4R,5R,6aS)-hexahydro-5-hydroxy-4-[(1E,3S)-5-phenyl-3-hydroxy-(1E)-pentenyl]-2H-cyclopentane [β] furan-2-one

| Conditions | Yield |
|---|---|
| With L-Selectride In tetrahydrofuran at -70℃; for 2h; Product distribution / selectivity; | 68% |

-
-
41639-74-1
(3aR,4R,5R,6aS)-hexahydro-5-hydroxy-4-[(1E,3S)-5-phenyl-3-hydroxy-(1E)-pentenyl]-2H-cyclopentane [β] furan-2-one

| Conditions | Yield |
|---|---|
| Stage #1: 2-naphthyl (1R,2R,3R)-3-hydroxy-2-[5-phenyl-(3S)-3-hydroxy-1-pentenyl]-5-oxo-cyclopentaneacetate With L-Selectride In tetrahydrofuran at -70℃; for 2h; Cooling with methanol-dry ice; Stage #2: With water; ammonium chloride In tetrahydrofuran Product distribution / selectivity; | 68% |
-
-
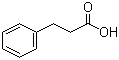
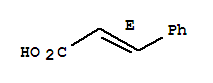
140-10-3
(E)-3-phenylacrylic acid

-
-
41639-74-1
(3aR,4R,5R,6aS)-hexahydro-5-hydroxy-4-[(1E,3S)-5-phenyl-3-hydroxy-(1E)-pentenyl]-2H-cyclopentane [β] furan-2-one

| Conditions | Yield |
|---|---|
| Multi-step reaction with 6 steps 1.1: H2 / 5 percent Pd/C / ethyl acetate 2.1: MeOH 3.1: N-BuLi / tetrahydrofuran / 0 °C 3.2: tetrahydrofuran 4.1: NaHMDS / 1,2-dimethoxy-ethane 4.2: 1,2-dimethoxy-ethane 5.1: (S)-BINAL-H; LiAlH4 / tetrahydrofuran 6.1: K2CO3; MeOH View Scheme |
-
-
103-25-3
3-phenylpropanoic acid methyl ester

-
-
41639-74-1
(3aR,4R,5R,6aS)-hexahydro-5-hydroxy-4-[(1E,3S)-5-phenyl-3-hydroxy-(1E)-pentenyl]-2H-cyclopentane [β] furan-2-one

| Conditions | Yield |
|---|---|
| Multi-step reaction with 4 steps 1.1: N-BuLi / tetrahydrofuran / 0 °C 1.2: tetrahydrofuran 2.1: NaHMDS / 1,2-dimethoxy-ethane 2.2: 1,2-dimethoxy-ethane 3.1: (S)-BINAL-H; LiAlH4 / tetrahydrofuran 4.1: K2CO3; MeOH View Scheme |
-
-
41162-19-0
dimethyl(2-oxo-4-phenylbutyl)phosphonate

-
-
41639-74-1
(3aR,4R,5R,6aS)-hexahydro-5-hydroxy-4-[(1E,3S)-5-phenyl-3-hydroxy-(1E)-pentenyl]-2H-cyclopentane [β] furan-2-one

| Conditions | Yield |
|---|---|
| Multi-step reaction with 3 steps 1.1: NaHMDS / 1,2-dimethoxy-ethane 1.2: 1,2-dimethoxy-ethane 2.1: (S)-BINAL-H; LiAlH4 / tetrahydrofuran 3.1: K2CO3; MeOH View Scheme | |
| Multi-step reaction with 3 steps 1: 1.) NaH / 1.) THF, 90 min, 2.) THF, 30 min 2: lithium triethylborohydride / tetrahydrofuran / 0.5 h 3: potassium carbonate / methanol; tetrahydrofuran / 2.5 h View Scheme |
-
-
55076-60-3
(3aR,4R,5R,6aS)-2-oxo-4-((1E)-3-oxo-5-phenylpent-1-en-1-yl)-hexahydro-2H-cyclopenta[b]furan-5-yl benzoate

-
-
41639-74-1
(3aR,4R,5R,6aS)-hexahydro-5-hydroxy-4-[(1E,3S)-5-phenyl-3-hydroxy-(1E)-pentenyl]-2H-cyclopentane [β] furan-2-one

| Conditions | Yield |
|---|---|
| Multi-step reaction with 2 steps 1: (S)-BINAL-H; LiAlH4 / tetrahydrofuran 2: K2CO3; MeOH View Scheme | |
| Multi-step reaction with 2 steps 1: (-)-diisopinocamphenylborane chloride / dichloromethane / 26 h / -25 - 30 °C 2: potassium carbonate / methanol / 2 h / 25 °C View Scheme |
-
-
501-52-0
3-Phenylpropionic acid

-
-
41639-74-1
(3aR,4R,5R,6aS)-hexahydro-5-hydroxy-4-[(1E,3S)-5-phenyl-3-hydroxy-(1E)-pentenyl]-2H-cyclopentane [β] furan-2-one

| Conditions | Yield |
|---|---|
| Multi-step reaction with 5 steps 1.1: MeOH 2.1: N-BuLi / tetrahydrofuran / 0 °C 2.2: tetrahydrofuran 3.1: NaHMDS / 1,2-dimethoxy-ethane 3.2: 1,2-dimethoxy-ethane 4.1: (S)-BINAL-H; LiAlH4 / tetrahydrofuran 5.1: K2CO3; MeOH View Scheme |
-
-
41639-72-9
(1S,5R,6R,7R)-6-(3-oxo-5-phenyl-1E-pentenyl)-7-<(4-phenylbenzoyl)oxy>-2-oxabicyclo<3.3.0>octan-3-one

-
-
41639-74-1
(3aR,4R,5R,6aS)-hexahydro-5-hydroxy-4-[(1E,3S)-5-phenyl-3-hydroxy-(1E)-pentenyl]-2H-cyclopentane [β] furan-2-one

| Conditions | Yield |
|---|---|
| Multi-step reaction with 2 steps 1: lithium triethylborohydride / tetrahydrofuran / 0.5 h 2: potassium carbonate / methanol; tetrahydrofuran / 2.5 h View Scheme |
-
-
38754-71-1
(3aR,4R,5R,6aS)-4-formyl-2-oxohexahydro-2H-cyclopenta[b]furan-5-yl biphenyl-4-carboxylate

-
-
41639-74-1
(3aR,4R,5R,6aS)-hexahydro-5-hydroxy-4-[(1E,3S)-5-phenyl-3-hydroxy-(1E)-pentenyl]-2H-cyclopentane [β] furan-2-one

| Conditions | Yield |
|---|---|
| Multi-step reaction with 3 steps 1: 1.) NaH / 1.) THF, 90 min, 2.) THF, 30 min 2: lithium triethylborohydride / tetrahydrofuran / 0.5 h 3: potassium carbonate / methanol; tetrahydrofuran / 2.5 h View Scheme |

-
-
41639-74-1
(3aR,4R,5R,6aS)-hexahydro-5-hydroxy-4-[(1E,3S)-5-phenyl-3-hydroxy-(1E)-pentenyl]-2H-cyclopentane [β] furan-2-one

| Conditions | Yield |
|---|---|
| Stage #1: benzoic acid (3aR,4R,5R,6aS)-4-((E)-3-hydroxy-5-phenyl-pent-1-enyl)-2-oxo-hexahydro-cyclopenta[b]furan-5-yl ester With methanol; sodium methylate for 2h; Stage #2: With hydrogenchloride In methanol; water at 0 - 5℃; pH=6.5; Product distribution / selectivity; |

-
-
41639-74-1
(3aR,4R,5R,6aS)-hexahydro-5-hydroxy-4-[(1E,3S)-5-phenyl-3-hydroxy-(1E)-pentenyl]-2H-cyclopentane [β] furan-2-one

| Conditions | Yield |
|---|---|
| Multi-step reaction with 2 steps 1.1: (3aR)-1-methyl-3,3-diphenyl-tetrahydro-pyrrolo[1,2-c][1,3,2]oxazaborole / toluene; tetrahydrofuran / 0.5 h / 25 °C / Inert atmosphere 1.2: -20 - 0 °C / Inert atmosphere 1.3: pH 3 2.1: potassium carbonate; methanol / 2 h / 25 °C View Scheme |

-
-
41639-74-1
(3aR,4R,5R,6aS)-hexahydro-5-hydroxy-4-[(1E,3S)-5-phenyl-3-hydroxy-(1E)-pentenyl]-2H-cyclopentane [β] furan-2-one

| Conditions | Yield |
|---|---|
| With methanol; potassium carbonate at 25℃; for 2h; |

-
-
41639-74-1
(3aR,4R,5R,6aS)-hexahydro-5-hydroxy-4-[(1E,3S)-5-phenyl-3-hydroxy-(1E)-pentenyl]-2H-cyclopentane [β] furan-2-one

| Conditions | Yield |
|---|---|
| Multi-step reaction with 3 steps 1.1: sodium hydride / dichloromethane / 1 h / 0 °C 1.2: 0 - 5 °C 2.1: (3aR)-1-methyl-3,3-diphenyl-tetrahydro-pyrrolo[1,2-c][1,3,2]oxazaborole / toluene; tetrahydrofuran / 0.5 h / 25 °C / Inert atmosphere 2.2: -20 - 0 °C / Inert atmosphere 2.3: pH 3 3.1: potassium carbonate; methanol / 2 h / 25 °C View Scheme |

-
-
41639-74-1
(3aR,4R,5R,6aS)-hexahydro-5-hydroxy-4-[(1E,3S)-5-phenyl-3-hydroxy-(1E)-pentenyl]-2H-cyclopentane [β] furan-2-one

| Conditions | Yield |
|---|---|
| Multi-step reaction with 4 steps 1.1: oxalyl dichloride; dimethyl sulfoxide / dichloromethane / 1 h / -70 °C / Inert atmosphere 1.2: 1 h / Inert atmosphere 2.1: sodium hydride / dichloromethane / 1 h / 0 °C 2.2: 0 - 5 °C 3.1: (3aR)-1-methyl-3,3-diphenyl-tetrahydro-pyrrolo[1,2-c][1,3,2]oxazaborole / toluene; tetrahydrofuran / 0.5 h / 25 °C / Inert atmosphere 3.2: -20 - 0 °C / Inert atmosphere 3.3: pH 3 4.1: potassium carbonate; methanol / 2 h / 25 °C View Scheme |
-
-
2550-26-7
4-Phenyl-2-butanone

-
-
41639-74-1
(3aR,4R,5R,6aS)-hexahydro-5-hydroxy-4-[(1E,3S)-5-phenyl-3-hydroxy-(1E)-pentenyl]-2H-cyclopentane [β] furan-2-one

| Conditions | Yield |
|---|---|
| Multi-step reaction with 5 steps 1: acetic acid; bromine / methanol / 2 h / -5 - 20 °C 2: acetonitrile / 1 h / 80 °C 3: lithium chloride; triethylamine / tetrahydrofuran / 2 h / -4 - 20 °C 4: (-)-diisopinocamphenylborane chloride / dichloromethane / 26 h / -25 - 30 °C 5: potassium carbonate / methanol / 2 h / 25 °C View Scheme |
-
-
40601-45-4
diethyl (2-oxo-4-phenylbutyl)phosphonate

-
-
41639-74-1
(3aR,4R,5R,6aS)-hexahydro-5-hydroxy-4-[(1E,3S)-5-phenyl-3-hydroxy-(1E)-pentenyl]-2H-cyclopentane [β] furan-2-one

| Conditions | Yield |
|---|---|
| Multi-step reaction with 3 steps 1: lithium chloride; triethylamine / tetrahydrofuran / 2 h / -4 - 20 °C 2: (-)-diisopinocamphenylborane chloride / dichloromethane / 26 h / -25 - 30 °C 3: potassium carbonate / methanol / 2 h / 25 °C View Scheme |
-
-
31984-10-8
1-bromo-4-phenylbutan-2-one

-
-
41639-74-1
(3aR,4R,5R,6aS)-hexahydro-5-hydroxy-4-[(1E,3S)-5-phenyl-3-hydroxy-(1E)-pentenyl]-2H-cyclopentane [β] furan-2-one

| Conditions | Yield |
|---|---|
| Multi-step reaction with 4 steps 1: acetonitrile / 1 h / 80 °C 2: lithium chloride; triethylamine / tetrahydrofuran / 2 h / -4 - 20 °C 3: (-)-diisopinocamphenylborane chloride / dichloromethane / 26 h / -25 - 30 °C 4: potassium carbonate / methanol / 2 h / 25 °C View Scheme |
-
-
5307-99-3
(+/-)-7,7-dichlorobicyclo[3.2.0]hept-2-en-6-one

-
-
41639-74-1
(3aR,4R,5R,6aS)-hexahydro-5-hydroxy-4-[(1E,3S)-5-phenyl-3-hydroxy-(1E)-pentenyl]-2H-cyclopentane [β] furan-2-one

| Conditions | Yield |
|---|---|
| Multi-step reaction with 6 steps 1.1: (S)-6,6'-bis(2,4,6-triisopropylphenyl)-1,1'-spirobiindane-7,7'-diyl hydrogenphosphate; dihydrogen peroxide / chloroform; water / -15 °C / Schlenk technique 2.1: ammonium chloride; zinc / methanol / 3 h / 70 °C 3.1: formic acid; sulfuric acid / 24 h / 80 °C 3.2: 2 h / 0 - 20 °C 4.1: [bis(acetoxy)iodo]benzene; 2,2,6,6-Tetramethyl-1-piperidinyloxy free radical / dichloromethane; N,N-dimethyl acetamide / 6 h / 20 °C 5.1: sodium hydride / tetrahydrofuran / 1 h / 0 °C / Inert atmosphere 5.2: 6 h / 0 - 20 °C / Inert atmosphere 6.1: C20H35B / tetrahydrofuran; hexane / 12 h / -40 °C View Scheme | |
| Multi-step reaction with 6 steps 1.1: (S)-6,6'-bis(2,4,6-triisopropylphenyl)-1,1'-spirobiindane-7,7'-diyl hydrogenphosphate; dihydrogen peroxide / chloroform; water / 48 h / 0 °C / Schlenk technique 2.1: ammonium chloride; zinc / methanol / 3 h / 70 °C 3.1: formic acid; sulfuric acid / 24 h / 80 °C 3.2: 2 h / 0 - 20 °C 4.1: [bis(acetoxy)iodo]benzene; 2,2,6,6-Tetramethyl-1-piperidinyloxy free radical / dichloromethane; N,N-dimethyl acetamide / 6 h / 20 °C 5.1: sodium hydride / tetrahydrofuran / 1 h / 0 °C / Inert atmosphere 5.2: 6 h / 0 - 20 °C / Inert atmosphere 6.1: C20H35B / tetrahydrofuran; hexane / 12 h / -40 °C View Scheme |
-
-
26054-46-6
(-)-(1S,5R)-2-oxabicyclo[3.3.0]oct-6-en-3-one

-
-
41639-74-1
(3aR,4R,5R,6aS)-hexahydro-5-hydroxy-4-[(1E,3S)-5-phenyl-3-hydroxy-(1E)-pentenyl]-2H-cyclopentane [β] furan-2-one

| Conditions | Yield |
|---|---|
| Multi-step reaction with 4 steps 1.1: formic acid; sulfuric acid / 24 h / 80 °C 1.2: 2 h / 0 - 20 °C 2.1: [bis(acetoxy)iodo]benzene; 2,2,6,6-Tetramethyl-1-piperidinyloxy free radical / dichloromethane; N,N-dimethyl acetamide / 6 h / 20 °C 3.1: sodium hydride / tetrahydrofuran / 1 h / 0 °C / Inert atmosphere 3.2: 6 h / 0 - 20 °C / Inert atmosphere 4.1: C20H35B / tetrahydrofuran; hexane / 12 h / -40 °C View Scheme |
-
-
41639-74-1
(3aR,4R,5R,6aS)-hexahydro-5-hydroxy-4-[(1E,3S)-5-phenyl-3-hydroxy-(1E)-pentenyl]-2H-cyclopentane [β] furan-2-one

-
-
145667-75-0
(1S,5R,6R,7R)-6-<(3S)-3-hydroxy-5-phenyl-1-pentyl>-7(R)-hydroxy-2-oxabicyclo<3.3.0>octan-3-one

| Conditions | Yield |
|---|---|
| With 5%-palladium/activated carbon; hydrogen; sodium hydroxide In ethanol at 20℃; under 760.051 Torr; for 5h; | 100% |
| With palladium 10% on activated carbon; hydrogen; sodium hydroxide In ethanol at 20℃; under 3000.3 Torr; | 85% |
| With sodium hydroxide; hydrogen; 5%-palladium/activated carbon In ethyl acetate Product distribution / selectivity; | 78% |
-
-
110-87-2
3,4-dihydro-2H-pyran

-
-
41639-74-1
(3aR,4R,5R,6aS)-hexahydro-5-hydroxy-4-[(1E,3S)-5-phenyl-3-hydroxy-(1E)-pentenyl]-2H-cyclopentane [β] furan-2-one

-
-
69610-63-5
(3aR,4R,5R,6aS)-hexahydro-4-((3S,E)-5-phenyl-3-(tetrahydro-2H-pyran-2-yloxy)pent-1-enyl)-5-(tetrahydro-2H-pyran-2-yloxy)cyclopenta[b]furan-2-one

| Conditions | Yield |
|---|---|
| With toluene-4-sulfonic acid In dichloromethane at 20℃; for 2.5h; | 89.9% |
| With toluene-4-sulfonic acid In dichloromethane at 20℃; for 2.5h; | 89.9% |
| With toluene-4-sulfonic acid In dichloromethane for 0.5h; | |
| With toluene-4-sulfonic acid In dichloromethane Etherification; |
-
-
41639-74-1
(3aR,4R,5R,6aS)-hexahydro-5-hydroxy-4-[(1E,3S)-5-phenyl-3-hydroxy-(1E)-pentenyl]-2H-cyclopentane [β] furan-2-one

-
-
856240-62-5
(3aR,4R,5R,6aS)-4-[(1E,3S)-3-hydroxy-5-phenylpent-1-en-1-yl]-hexahydro-2H-cyclopenta[b]furan-2,5-diol

| Conditions | Yield |
|---|---|
| With diisobutylaluminium hydride In tetrahydrofuran at -70℃; for 2h; | 81% |
| With diisobutylaluminium hydride | |
| With diisobutylaluminium hydride In dichloromethane at -78℃; for 2h; | |
| Stage #1: (3aR,4R,5R,6aS)-hexahydro-5-hydroxy-4-[(1E,3S)-5-phenyl-3-hydroxy-(1E)-pentenyl]-2H-cyclopentane [β] furan-2-one With diisobutylaluminium hydride In dichloromethane at -78℃; for 2h; Stage #2: With ammonium chloride at 20℃; for 0.25h; Reagent/catalyst; Temperature; |
-
-
41639-74-1
(3aR,4R,5R,6aS)-hexahydro-5-hydroxy-4-[(1E,3S)-5-phenyl-3-hydroxy-(1E)-pentenyl]-2H-cyclopentane [β] furan-2-one

| Conditions | Yield |
|---|---|
| Multi-step reaction with 4 steps 1: p-TSA / CH2Cl2 2: DIBAL-H / -78 °C 3: NaHMDS 4: aq. AcOH / tetrahydrofuran View Scheme |
-
-
41639-74-1
(3aR,4R,5R,6aS)-hexahydro-5-hydroxy-4-[(1E,3S)-5-phenyl-3-hydroxy-(1E)-pentenyl]-2H-cyclopentane [β] furan-2-one

| Conditions | Yield |
|---|---|
| Multi-step reaction with 5 steps 1: p-TSA / CH2Cl2 2: DIBAL-H / -78 °C 3: NaHMDS 4: aq. AcOH / tetrahydrofuran 5: H2 / 5 percent Pd/C / ethyl acetate View Scheme |
-
-
41639-74-1
(3aR,4R,5R,6aS)-hexahydro-5-hydroxy-4-[(1E,3S)-5-phenyl-3-hydroxy-(1E)-pentenyl]-2H-cyclopentane [β] furan-2-one

| Conditions | Yield |
|---|---|
| Multi-step reaction with 2 steps 1: p-TSA / CH2Cl2 2: DIBAL-H / -78 °C View Scheme |
-
-
41639-74-1
(3aR,4R,5R,6aS)-hexahydro-5-hydroxy-4-[(1E,3S)-5-phenyl-3-hydroxy-(1E)-pentenyl]-2H-cyclopentane [β] furan-2-one

| Conditions | Yield |
|---|---|
| Multi-step reaction with 3 steps 1: p-TSA / CH2Cl2 2: DIBAL-H / -78 °C 3: NaHMDS View Scheme | |
| Multi-step reaction with 3 steps 1.1: toluene-4-sulfonic acid / dichloromethane / 2.5 h / 20 °C 2.1: diisobutylaluminium hydride / toluene; hexane / -70 °C 3.1: potassium tert-butylate / tetrahydrofuran / 0.5 h / -20 °C 3.2: 16 h / -20 °C View Scheme | |
| Multi-step reaction with 3 steps 1.1: toluene-4-sulfonic acid / dichloromethane / 2.5 h / 20 °C 2.1: diisobutylaluminium hydride / hexane; toluene / -70 °C 3.1: potassium tert-butylate / tetrahydrofuran / 0.5 h / -20 °C 3.2: 16 h / -20 °C View Scheme |
-
-
41639-74-1
(3aR,4R,5R,6aS)-hexahydro-5-hydroxy-4-[(1E,3S)-5-phenyl-3-hydroxy-(1E)-pentenyl]-2H-cyclopentane [β] furan-2-one

-
-
69590-43-8
N-(methanesulfonyl)-17-phenyl-ω-trinor-PGE2-carboxamide

| Conditions | Yield |
|---|---|
| Multi-step reaction with 5 steps 1: p-toluenesulfonic acid / CH2Cl2 / 0.5 h 2: diisobutylaluminum hydride / toluene; hexane / 0.33 h 3: 1.) sodium methylsulfinylcarbanide / 1.) Me2SO, 2.) Me2SO, 2 h 4: Jones reagent / acetone / 0.08 h / -20 °C 5: 65percent aq. acetic acid / 14 h / Ambient temperature View Scheme |
-
-
41639-74-1
(3aR,4R,5R,6aS)-hexahydro-5-hydroxy-4-[(1E,3S)-5-phenyl-3-hydroxy-(1E)-pentenyl]-2H-cyclopentane [β] furan-2-one

-
-
856453-32-2
(3aR,4R,5R,6aS)-hexahydro-4-((3S,E)-5-phenyl-3-(tetrahydro-2H-pyran-2-yloxy)pent-1-enyl)-5-(tetrahydro-2H-pyran-2-yloxy)-2H-cyclopenta[b]furan-2-ol

| Conditions | Yield |
|---|---|
| Multi-step reaction with 2 steps 1: p-toluenesulfonic acid / CH2Cl2 / 0.5 h 2: diisobutylaluminum hydride / toluene; hexane / 0.33 h View Scheme | |
| Multi-step reaction with 2 steps 1: toluene-4-sulfonic acid / dichloromethane / 2.5 h / 20 °C 2: diisobutylaluminium hydride / toluene; hexane / -70 °C View Scheme | |
| Multi-step reaction with 2 steps 1: toluene-4-sulfonic acid / dichloromethane / 2.5 h / 20 °C 2: diisobutylaluminium hydride / hexane; toluene / -70 °C View Scheme |
-
-
41639-74-1
(3aR,4R,5R,6aS)-hexahydro-5-hydroxy-4-[(1E,3S)-5-phenyl-3-hydroxy-(1E)-pentenyl]-2H-cyclopentane [β] furan-2-one

-
-
69590-40-5
N-{(Z)-7-[(1R,2R,3R,5S)-5-Hydroxy-2-[(E)-(S)-5-phenyl-3-(tetrahydro-pyran-2-yloxy)-pent-1-enyl]-3-(tetrahydro-pyran-2-yloxy)-cyclopentyl]-hept-5-enoyl}-methanesulfonamide

| Conditions | Yield |
|---|---|
| Multi-step reaction with 3 steps 1: p-toluenesulfonic acid / CH2Cl2 / 0.5 h 2: diisobutylaluminum hydride / toluene; hexane / 0.33 h 3: 1.) sodium methylsulfinylcarbanide / 1.) Me2SO, 2.) Me2SO, 2 h View Scheme |
-
-
41639-74-1
(3aR,4R,5R,6aS)-hexahydro-5-hydroxy-4-[(1E,3S)-5-phenyl-3-hydroxy-(1E)-pentenyl]-2H-cyclopentane [β] furan-2-one

-
-
69590-42-7
N-{(Z)-7-[(1R,2R,3R)-5-Oxo-2-[(E)-(S)-5-phenyl-3-(tetrahydro-pyran-2-yloxy)-pent-1-enyl]-3-(tetrahydro-pyran-2-yloxy)-cyclopentyl]-hept-5-enoyl}-methanesulfonamide

| Conditions | Yield |
|---|---|
| Multi-step reaction with 4 steps 1: p-toluenesulfonic acid / CH2Cl2 / 0.5 h 2: diisobutylaluminum hydride / toluene; hexane / 0.33 h 3: 1.) sodium methylsulfinylcarbanide / 1.) Me2SO, 2.) Me2SO, 2 h 4: Jones reagent / acetone / 0.08 h / -20 °C View Scheme |
Related products
Raw Materials
Downstream Products
Hot Products
About|Contact|Cas|Product Name|Molecular|Country|Encyclopedia
Message|New Cas|MSDS|Service|Advertisement|CAS DataBase|Article Data|Manufacturers | Chemical Catalog
©2008 LookChem.com,License: ICP
NO.:Zhejiang16009103
complaints:service@lookchem.com Desktop View