Dayang Chem (Hangzhou) Co.,Ltd.
As a leading manufacturer and supplier of chemicals in China, DayangChem not only supply popular chemicals, but also DayangChem's R&D center offer custom synthesis services. DayangChem can provide different quantities of custom synthesis ch
Cas:519-09-5
Min.Order:1 Kilogram
FOB Price: $3.0
Type:Lab/Research institutions
inquiryXi'an Xszo Chem Co., Ltd.
1. Factory price and high quality must be guaranteed, base on 8 years of production and R&D experience2. Free samples will be provided,ensure specifications and quality are right for customer3. Customers will receive the most professional technical s
Cas:519-09-5
Min.Order:1 Gram
FOB Price: $0.1
Type:Manufacturers
inquiryChemwill Asia Co., Ltd.
Our main production base is located in Xuzhou industry park. We are certified both to the ISO 9001 and ISO 14001 Standards, have a safety management system in place.Our R&D team masters core technology for process-design of target building block
Henan Tianfu Chemical Co., Ltd.
BENZOYLECGONINE Basic information Product Name: BENZOYLECGONINE Synonyms: BENZOYLECGONINE;1alphaH,5alphaH-Tropane-2beta-carboxylic acid, 3beta-hydroxy-, benzoate (e
Cas:519-09-5
Min.Order:1 Kilogram
Negotiable
Type:Lab/Research institutions
inquiryHebei Nengqian Chemical Import and Export Co., LTD
With our good experience, we offer detailed technical support and advice to assist customers. We communicate closely with customers to establish their quality requirements. Consistent Quality Our plant has strict quality control in each manufacturin
Cas:519-09-5
Min.Order:1 Kilogram
FOB Price: $70.0 / 90.0
Type:Trading Company
inquiryShanghai Upbio Tech Co.,Ltd
1.No Less 8 years exporting experience. Clients can 100% received goods 2.Lower Price with higher quality 3,Free sample 4,We are sincerely responsible for the "product quality" and "After Service" Upbio is Specialized
Cas:519-09-5
Min.Order:1 Kilogram
Negotiable
Type:Lab/Research institutions
inquiryQingdao Beluga Import and Export Co., LTD
BENZOYLECGONINE CAS:519-09-5 Qingdao Belugas Import and Export Co., Ltd. is a scientific and technological company integrating research and development, production and trade of chemical intermediates, specializing in high quality organic intermediat
Cas:519-09-5
Min.Order:1 Gram
Negotiable
Type:Lab/Research institutions
inquiryShandong Hanjiang Chemical Co., Ltd.
Hello, dear friend! I'm Hansen and Allen from China. Welcome to my lookchem mall! The following is a brief introduction of our company's products and services. If you are interested in our products, please contact us by emai
Cas:519-09-5
Min.Order:1 Kilogram
Negotiable
Type:Lab/Research institutions
inquiryHebei Quanhe Biotechnology Co. LTD
1. Timely and efficient service to ensure communication with customers 2. Produce products of different specifications and sizes according to your requirements. 3. Quality procedures and standards recognized by SGS. Advanced plant equipment ensures s
Cas:519-09-5
Min.Order:1 Kilogram
FOB Price: $50.0 / 100.0
Type:Lab/Research institutions
inquiryHenan Wentao Chemical Product Co., Ltd.
Henan Wentao Chemical Product Co.,Ltd is Located in Zhengzhou High-tech Development Zone with import and export license, We passed ISO 9001:2008 as well, Henan Wentao has developed more than 1000 compounds, which are widely used in the fields of prod
Cas:519-09-5
Min.Order:0
Negotiable
Type:Lab/Research institutions
inquiryWuhan Han Sheng New Material Technology Co.,Ltd
Our Advantage: high quality with competitive price High quality standard: BP/USP/EP Enterprise standard All purity customized Fast and safe delivery We have reliable forwarder who can help us deliver our goods more fast and safe. We
Cas:519-09-5
Min.Order:10 Kilogram
Negotiable
Type:Trading Company
inquiryHangzhou Keyingchem Co.,Ltd
Hangzhou KeyingChem Co., Ltd. exported this product to many countries and regions at best price. If you are looking for the material’s manufacturer or supplier in China, KeyingChem is your best choice. Pls contact with us freely for getting det
Cas:519-09-5
Min.Order:0 Metric Ton
Negotiable
Type:Lab/Research institutions
inquiryZibo Hangyu Biotechnology Development Co., Ltd
Zibo Hangyu Biotechnology Development Co., Ltd is a leading manufacturer and supplier of chemicals in China. We develop produce and distribute high quality pharmaceuticals, intermediates, special chemicals and OLED intermediates and other fine chemi
Cas:519-09-5
Min.Order:10 Gram
FOB Price: $100.0
Type:Lab/Research institutions
inquiryZibo Dorne chemical technology co. LTD
Product Details Grade: pharmaceutical grade Purity:99%+ ProductionCapacity: 1000 Kilogram/Month Scope of use: For scientific research only(The product must be used legally) Our Advantage 1. Best quality with competitive price. 2. Quick shipping,
Cas:519-09-5
Min.Order:1 Metric Ton
Negotiable
Type:Lab/Research institutions
inquiryJiangsu Qianyu Molecular Technology Co., LTD.
Our Advantages A. International Top level TechnologyOur company owned biomedicine experts are famous at home and abroad with rich experience in research and development in the field of efficient chiral functional molecules research and development an
EAST CHEMSOURCES LIMITED
factory?direct?saleAppearance:White Powder Storage:Store In Dry, Cool And Ventilated Place Package:25kg/drum, also according to the clients requirement Application:It is widely used as a thickener, emulsifier and stabilizer Transportation:By Sea Or B
Cas:519-09-5
Min.Order:1 Kilogram
FOB Price: $18.0 / 20.0
Type:Trading Company
inquiryTAIZHOU ZHENYU BIOTECHNOLOGY CO., LTD
Zhenyu biotech exported this product to many countries and regions at best price. if you are looking for the material's manufacturer or supplier in china, zhenyu biotech is your best choice. pls contact with us freely for getting detailed
Cas:519-09-5
Min.Order:1 Kilogram
FOB Price: $2.0
Type:Lab/Research institutions
inquiryShandong Mopai Biotechnology Co., LTD
Shandong Mopai Biotechnology Co., LTD is a leading manufacturer and supplier of chemicals in China. We develop produce and distribute high quality pharmaceuticals, intermediates, special chemicals and OLED intermediates and other fine chemicals. W
Cas:519-09-5
Min.Order:1 Gram
Negotiable
Type:Lab/Research institutions
inquiryKAISA GROUP INC
1.Applied in food field.it can improve the immune system and prolong life. 2.Appliedin cosmetic field.it can improve the skin care. 3.Applied in pharmaceutical field.it can treat various dieases. 4.Our product quality assurance will make our customer
Cas:519-09-5
Min.Order:1 Metric Ton
FOB Price: $1.5
Type:Trading Company
inquiryGolden Pharma Co., Limited
GOLDEN PHARMA CO.,LIMITED.is a professional pharmaceutical company,our team have more than 20years expereince in pharmaceutical production and sales. we are a professional technical enterprise specializing in the R & D, production,QA regulation
Bluecrystal chem-union
We are a Union of chemistry in China, consists of chemists,engineers, laboratories,factories in China. We organize surplus capacity of R&D and production as well as custom synthesis for chemical products and chemical business project. We are supp
Cas:519-09-5
Min.Order:1 Metric Ton
FOB Price: $1.0
Type:Trading Company
inquiryHebei Mojin Biotechnology Co.,Ltd
1, High quality with competitive price:2, Fast and safe delivery3.Excellent pre-sales and after-sales service4. Well-trained and professional technologist and sales with rich experience in the field for 5-10 yearsAppearance:see detailed specification
Cas:519-09-5
Min.Order:0
Negotiable
Type:Trading Company
inquiryHangzhou Dingyan Chem Co., Ltd
R & D enterprises have their own stock in stock Package:1kg Application:pharmaceutical intermediates
Cas:519-09-5
Min.Order:0
Negotiable
Type:Trading Company
inquiryHenan Allgreen Chemical Co.,Ltd
high qualityAppearance:white crystalline powder Storage:Sealed, dry, microtherm , avoid light and smell. Package:According to the demand of customer Application:Organic synthesis Transportation:by air or by sea
Hangzhou J&H Chemical Co., Ltd.
J&H CHEM is one of China's leading providers of integrated fine chemical services including offering, research and development, Custom manufacturing business, as well as other Value-added customer services, for diversified range products of chemicals
Wuhan Circle Star Chem-medical Technology co.,Ltd.
1,we produce and sell good chemicals around the world.2,our success rate is about 95%. this means, if customer order is accepted, the probability that the customer will obtain the ordered substances, is 95%.3,our staff consists of highly qualified in
Antimex Chemical Limied
Ansciep Chemical is a professional enterprise manufacturing and distributing fine chemicals and speciality chemicals. We have been dedicated to heterocycle compounds and phenyl rings for tens of years. This is our mature product for export. Our quali
Cas:519-09-5
Min.Order:0
Negotiable
Type:Lab/Research institutions
inquiryHANGZHOU TIANYE CHEMICALS CO., LTD.
We product this chemical more than 10 years . We are very experience to export it to many countries, Our superior & stable quality , competitive price gain warm reception from our customers. Application:intermediate
Hubei Langyou International Trading Co., Ltd
TIANFUCHEM--High purity Rutin factory priceBENZOYLECGONINE Application:TIANFUCHEM--High purity Rutin factory priceBENZOYLECGONINE
Cas:519-09-5
Min.Order:0
Negotiable
Type:Other
inquirySynthetic route
-
-
155748-84-8
RTI-128

-
-
519-09-5
benzylecgonine

| Conditions | Yield |
|---|---|
| With sodium nitrite In acetic anhydride; acetic acid 1.) 0 deg C, 16 h, 2.) room temperature, 8 h; | 93% |

| Conditions | Yield |
|---|---|
| With ammonia; water | 86% |
| With dipotassium hydrogenphosphate; potassium dihydrogenphosphate In tetrahydrofuran; water at 80℃; for 3h; | 83% |
| With sodium hydroxide for 12h; Heating; Yield given; |

| Conditions | Yield |
|---|---|
| With water for 24h; Reflux; | 84% |
| With human carboxylesterase 1b, recombinant In aq. phosphate buffer at 37℃; for 1h; pH=7.4; Enzymatic reaction; | |
| With water In 1,4-dioxane at 130℃; for 2h; Microwave irradiation; |

| Conditions | Yield |
|---|---|
| With acetone | |
| With water at 100℃; |
-
-
50-36-2
Cocaine

-
A
-
67-56-1
methanol

-
B
-
65-85-0
benzoic acid

-
C
-
481-37-8
ecgonine

-
D
-
7143-09-1
methyl ecgonine

-
E
-
519-09-5
benzylecgonine

| Conditions | Yield |
|---|---|
| With hydrogenchloride In water at 70 - 90℃; Product distribution; Rate constant; Thermodynamic data; also in plasma and with other reagents ; ΔHa, ΔS(excit.), Arrhenius parameters; |


| Conditions | Yield |
|---|---|
| at 37℃; Rate constant; various pH from 6.9 to 10.4; I=0.1 M; |

| Conditions | Yield |
|---|---|
| at 100℃; | |
| at 100℃; |


-
-
519-09-5
benzylecgonine

| Conditions | Yield |
|---|---|
| With water |

| Conditions | Yield |
|---|---|
| cocE-pIX Enzyme kinetics; Enzymatic reaction; | |
| cocE-pIII Enzyme kinetics; Enzymatic reaction; | |
| cocE Enzyme kinetics; Enzymatic reaction; |
-
-
74242-55-0
anhydroecgonine hydrochloride

-
-
519-09-5
benzylecgonine

| Conditions | Yield |
|---|---|
| Multi-step reaction with 13 steps 1: 93 percent / H2 / Pd/C / methanol / 6.5 h / 3102.9 Torr / Ambient temperature 2: 1.) (COCl)2, DMF, 2.) N-hydroxy-2-thiopyridone / 1.) CH2ClCH2Cl, room temperature, 12 h, 2.) CH2ClCH2Cl, room temperature, 1.5 h, 3.) CH2Cl2, irradiation, 0 deg C, 2 h 3: toluene / 24 h / Heating 4: 2.) Et3N / 1.) MeOH, reflux, 2 h, 2.) MeOH, room temperature, 15 h 5: 91 percent / NaIO4 / tetrahydrofuran; H2O / 2 h / Ambient temperature 6: 78 percent / Na2CO3*H2O / tetrahydrofuran; H2O / 16 h 7: 96 percent / aq. NaOH / ethanol / 1.) 0 deg C, 1 h, 2.) room temperature, 1.5 h 8: 80 percent / 0.67 h / 105 - 110 °C 9: 94 percent / Na2CO3, aq. H2O2 / H2O / 24 h / Ambient temperature 10: 96 percent / DMAP / CH2Cl2 / 12 h / Ambient temperature 11: 90 percent / TFA / CH2Cl2 / 1 h / Ambient temperature 12: 75 percent / NaBH3CN / acetonitrile / 1 h / Ambient temperature 13: 93 percent / NaNO2 / acetic acid; acetic anhydride / 1.) 0 deg C, 16 h, 2.) room temperature, 8 h View Scheme | |
| Multi-step reaction with 13 steps 1: 93 percent / H2 / Pd/C / methanol / 6.5 h / 3102.9 Torr / Ambient temperature 2: 1.) (COCl)2, DMF, 2.) N-hydroxy-2-thiopyridone / 1.) CH2ClCH2Cl, room temperature, 12 h, 2.) CH2ClCH2Cl, room temperature, 1.5 h, 3.) CH2Cl2, irradiation, 0 deg C, 2 h 3: toluene / 24 h / Heating 4: 2.) Et3N / 1.) MeOH, reflux, 2 h, 2.) MeOH, room temperature, 15 h 5: 91 percent / NaIO4 / tetrahydrofuran; H2O / 2 h / Ambient temperature 6: 78 percent / Na2CO3*H2O / tetrahydrofuran; H2O / 16 h 7: 96 percent / aq. NaOH / ethanol / 1.) 0 deg C, 1 h, 2.) room temperature, 1.5 h 8: 80 percent / 0.67 h / 105 - 110 °C 9: 94 percent / Na2CO3, aq. H2O2 / H2O / 24 h / Ambient temperature 10: 96 percent / DMAP / CH2Cl2 / 12 h / Ambient temperature 11: 90 percent / TFA / CH2Cl2 / 1 h / Ambient temperature 12: 75 percent / NaBH3CN / acetonitrile / 1 h / Ambient temperature 13: 93 percent / NaNO2 / acetic acid; acetic anhydride / 1.) 0 deg C, 16 h, 2.) room temperature, 8 h View Scheme | |
| Multi-step reaction with 13 steps 1: 93 percent / H2 / Pd/C / methanol / 6.5 h / 3102.9 Torr / Ambient temperature 2: 1.) (COCl)2, DMF, 2.) N-hydroxy-2-thiopyridone / 1.) CH2ClCH2Cl, room temperature, 12 h, 2.) CH2ClCH2Cl, room temperature, 1.5 h, 3.) CH2Cl2, irradiation, 0 deg C, 2 h 3: NaIO4 / H2O / 2 h / Ambient temperature 4: 57 percent / 75 °C / 0.08 - 0.1 Torr 5: 1.) 1-chloroethyl chloroformate, 3.) Et3N / 1.) toluene, reflux, 16 h, 2.) MeOH, reflux, 16 h, 3.) room temperature, 14 h 6: 78 percent / Na2CO3*H2O / tetrahydrofuran; H2O / 16 h 7: 96 percent / aq. NaOH / ethanol / 1.) 0 deg C, 1 h, 2.) room temperature, 1.5 h 8: 80 percent / 0.67 h / 105 - 110 °C 9: 94 percent / Na2CO3, aq. H2O2 / H2O / 24 h / Ambient temperature 10: 96 percent / DMAP / CH2Cl2 / 12 h / Ambient temperature 11: 90 percent / TFA / CH2Cl2 / 1 h / Ambient temperature 12: 75 percent / NaBH3CN / acetonitrile / 1 h / Ambient temperature 13: 93 percent / NaNO2 / acetic acid; acetic anhydride / 1.) 0 deg C, 16 h, 2.) room temperature, 8 h View Scheme |

-
-
519-09-5
benzylecgonine

| Conditions | Yield |
|---|---|
| Multi-step reaction with 11 steps 1: toluene / 24 h / Heating 2: 2.) Et3N / 1.) MeOH, reflux, 2 h, 2.) MeOH, room temperature, 15 h 3: 91 percent / NaIO4 / tetrahydrofuran; H2O / 2 h / Ambient temperature 4: 78 percent / Na2CO3*H2O / tetrahydrofuran; H2O / 16 h 5: 96 percent / aq. NaOH / ethanol / 1.) 0 deg C, 1 h, 2.) room temperature, 1.5 h 6: 80 percent / 0.67 h / 105 - 110 °C 7: 94 percent / Na2CO3, aq. H2O2 / H2O / 24 h / Ambient temperature 8: 96 percent / DMAP / CH2Cl2 / 12 h / Ambient temperature 9: 90 percent / TFA / CH2Cl2 / 1 h / Ambient temperature 10: 75 percent / NaBH3CN / acetonitrile / 1 h / Ambient temperature 11: 93 percent / NaNO2 / acetic acid; acetic anhydride / 1.) 0 deg C, 16 h, 2.) room temperature, 8 h View Scheme | |
| Multi-step reaction with 11 steps 1: toluene / 24 h / Heating 2: 2.) Et3N / 1.) MeOH, reflux, 2 h, 2.) MeOH, room temperature, 15 h 3: 91 percent / NaIO4 / tetrahydrofuran; H2O / 2 h / Ambient temperature 4: 78 percent / Na2CO3*H2O / tetrahydrofuran; H2O / 16 h 5: 96 percent / aq. NaOH / ethanol / 1.) 0 deg C, 1 h, 2.) room temperature, 1.5 h 6: 80 percent / 0.67 h / 105 - 110 °C 7: 94 percent / Na2CO3, aq. H2O2 / H2O / 24 h / Ambient temperature 8: 96 percent / DMAP / CH2Cl2 / 12 h / Ambient temperature 9: 90 percent / TFA / CH2Cl2 / 1 h / Ambient temperature 10: 75 percent / NaBH3CN / acetonitrile / 1 h / Ambient temperature 11: 93 percent / NaNO2 / acetic acid; acetic anhydride / 1.) 0 deg C, 16 h, 2.) room temperature, 8 h View Scheme | |
| Multi-step reaction with 11 steps 1: NaIO4 / H2O / 2 h / Ambient temperature 2: 57 percent / 75 °C / 0.08 - 0.1 Torr 3: 1.) 1-chloroethyl chloroformate, 3.) Et3N / 1.) toluene, reflux, 16 h, 2.) MeOH, reflux, 16 h, 3.) room temperature, 14 h 4: 78 percent / Na2CO3*H2O / tetrahydrofuran; H2O / 16 h 5: 96 percent / aq. NaOH / ethanol / 1.) 0 deg C, 1 h, 2.) room temperature, 1.5 h 6: 80 percent / 0.67 h / 105 - 110 °C 7: 94 percent / Na2CO3, aq. H2O2 / H2O / 24 h / Ambient temperature 8: 96 percent / DMAP / CH2Cl2 / 12 h / Ambient temperature 9: 90 percent / TFA / CH2Cl2 / 1 h / Ambient temperature 10: 75 percent / NaBH3CN / acetonitrile / 1 h / Ambient temperature 11: 93 percent / NaNO2 / acetic acid; acetic anhydride / 1.) 0 deg C, 16 h, 2.) room temperature, 8 h View Scheme |
-
-
208038-05-5
C14H19NOSe

-
-
519-09-5
benzylecgonine

| Conditions | Yield |
|---|---|
| Multi-step reaction with 10 steps 1: 57 percent / 75 °C / 0.08 - 0.1 Torr 2: 1.) 1-chloroethyl chloroformate, 3.) Et3N / 1.) toluene, reflux, 16 h, 2.) MeOH, reflux, 16 h, 3.) room temperature, 14 h 3: 78 percent / Na2CO3*H2O / tetrahydrofuran; H2O / 16 h 4: 96 percent / aq. NaOH / ethanol / 1.) 0 deg C, 1 h, 2.) room temperature, 1.5 h 5: 80 percent / 0.67 h / 105 - 110 °C 6: 94 percent / Na2CO3, aq. H2O2 / H2O / 24 h / Ambient temperature 7: 96 percent / DMAP / CH2Cl2 / 12 h / Ambient temperature 8: 90 percent / TFA / CH2Cl2 / 1 h / Ambient temperature 9: 75 percent / NaBH3CN / acetonitrile / 1 h / Ambient temperature 10: 93 percent / NaNO2 / acetic acid; acetic anhydride / 1.) 0 deg C, 16 h, 2.) room temperature, 8 h View Scheme |

-
-
519-09-5
benzylecgonine

| Conditions | Yield |
|---|---|
| Multi-step reaction with 12 steps 1: 1.) (COCl)2, DMF, 2.) N-hydroxy-2-thiopyridone / 1.) CH2ClCH2Cl, room temperature, 12 h, 2.) CH2ClCH2Cl, room temperature, 1.5 h, 3.) CH2Cl2, irradiation, 0 deg C, 2 h 2: toluene / 24 h / Heating 3: 2.) Et3N / 1.) MeOH, reflux, 2 h, 2.) MeOH, room temperature, 15 h 4: 91 percent / NaIO4 / tetrahydrofuran; H2O / 2 h / Ambient temperature 5: 78 percent / Na2CO3*H2O / tetrahydrofuran; H2O / 16 h 6: 96 percent / aq. NaOH / ethanol / 1.) 0 deg C, 1 h, 2.) room temperature, 1.5 h 7: 80 percent / 0.67 h / 105 - 110 °C 8: 94 percent / Na2CO3, aq. H2O2 / H2O / 24 h / Ambient temperature 9: 96 percent / DMAP / CH2Cl2 / 12 h / Ambient temperature 10: 90 percent / TFA / CH2Cl2 / 1 h / Ambient temperature 11: 75 percent / NaBH3CN / acetonitrile / 1 h / Ambient temperature 12: 93 percent / NaNO2 / acetic acid; acetic anhydride / 1.) 0 deg C, 16 h, 2.) room temperature, 8 h View Scheme | |
| Multi-step reaction with 12 steps 1: 1.) (COCl)2, DMF, 2.) N-hydroxy-2-thiopyridone / 1.) CH2ClCH2Cl, room temperature, 12 h, 2.) CH2ClCH2Cl, room temperature, 1.5 h, 3.) CH2Cl2, irradiation, 0 deg C, 2 h 2: toluene / 24 h / Heating 3: 2.) Et3N / 1.) MeOH, reflux, 2 h, 2.) MeOH, room temperature, 15 h 4: 91 percent / NaIO4 / tetrahydrofuran; H2O / 2 h / Ambient temperature 5: 78 percent / Na2CO3*H2O / tetrahydrofuran; H2O / 16 h 6: 96 percent / aq. NaOH / ethanol / 1.) 0 deg C, 1 h, 2.) room temperature, 1.5 h 7: 80 percent / 0.67 h / 105 - 110 °C 8: 94 percent / Na2CO3, aq. H2O2 / H2O / 24 h / Ambient temperature 9: 96 percent / DMAP / CH2Cl2 / 12 h / Ambient temperature 10: 90 percent / TFA / CH2Cl2 / 1 h / Ambient temperature 11: 75 percent / NaBH3CN / acetonitrile / 1 h / Ambient temperature 12: 93 percent / NaNO2 / acetic acid; acetic anhydride / 1.) 0 deg C, 16 h, 2.) room temperature, 8 h View Scheme | |
| Multi-step reaction with 12 steps 1: 1.) (COCl)2, DMF, 2.) N-hydroxy-2-thiopyridone / 1.) CH2ClCH2Cl, room temperature, 12 h, 2.) CH2ClCH2Cl, room temperature, 1.5 h, 3.) CH2Cl2, irradiation, 0 deg C, 2 h 2: NaIO4 / H2O / 2 h / Ambient temperature 3: 57 percent / 75 °C / 0.08 - 0.1 Torr 4: 1.) 1-chloroethyl chloroformate, 3.) Et3N / 1.) toluene, reflux, 16 h, 2.) MeOH, reflux, 16 h, 3.) room temperature, 14 h 5: 78 percent / Na2CO3*H2O / tetrahydrofuran; H2O / 16 h 6: 96 percent / aq. NaOH / ethanol / 1.) 0 deg C, 1 h, 2.) room temperature, 1.5 h 7: 80 percent / 0.67 h / 105 - 110 °C 8: 94 percent / Na2CO3, aq. H2O2 / H2O / 24 h / Ambient temperature 9: 96 percent / DMAP / CH2Cl2 / 12 h / Ambient temperature 10: 90 percent / TFA / CH2Cl2 / 1 h / Ambient temperature 11: 75 percent / NaBH3CN / acetonitrile / 1 h / Ambient temperature 12: 93 percent / NaNO2 / acetic acid; acetic anhydride / 1.) 0 deg C, 16 h, 2.) room temperature, 8 h View Scheme |
-
-
208037-81-4
tropidine

-
-
519-09-5
benzylecgonine

| Conditions | Yield |
|---|---|
| Multi-step reaction with 9 steps 1: 1.) 1-chloroethyl chloroformate, 3.) Et3N / 1.) toluene, reflux, 16 h, 2.) MeOH, reflux, 16 h, 3.) room temperature, 14 h 2: 78 percent / Na2CO3*H2O / tetrahydrofuran; H2O / 16 h 3: 96 percent / aq. NaOH / ethanol / 1.) 0 deg C, 1 h, 2.) room temperature, 1.5 h 4: 80 percent / 0.67 h / 105 - 110 °C 5: 94 percent / Na2CO3, aq. H2O2 / H2O / 24 h / Ambient temperature 6: 96 percent / DMAP / CH2Cl2 / 12 h / Ambient temperature 7: 90 percent / TFA / CH2Cl2 / 1 h / Ambient temperature 8: 75 percent / NaBH3CN / acetonitrile / 1 h / Ambient temperature 9: 93 percent / NaNO2 / acetic acid; acetic anhydride / 1.) 0 deg C, 16 h, 2.) room temperature, 8 h View Scheme |
-
-
208037-76-7
(1R,5S)-8-benzyl-8-azabicyclo[3.2.1]octan-2-one

-
-
519-09-5
benzylecgonine

| Conditions | Yield |
|---|---|
| Multi-step reaction with 11 steps 1: 96 percent / H2 / Pd/C / methanol / 5 h 2: 88 percent / methanol / 2 h / Ambient temperature 3: 70 percent / NaH / toluene / 1.) room temperature, 30 min, 2.) reflux, 5 h 4: 78 percent / Na2CO3*H2O / tetrahydrofuran; H2O / 16 h 5: 96 percent / aq. NaOH / ethanol / 1.) 0 deg C, 1 h, 2.) room temperature, 1.5 h 6: 80 percent / 0.67 h / 105 - 110 °C 7: 94 percent / Na2CO3, aq. H2O2 / H2O / 24 h / Ambient temperature 8: 96 percent / DMAP / CH2Cl2 / 12 h / Ambient temperature 9: 90 percent / TFA / CH2Cl2 / 1 h / Ambient temperature 10: 75 percent / NaBH3CN / acetonitrile / 1 h / Ambient temperature 11: 93 percent / NaNO2 / acetic acid; acetic anhydride / 1.) 0 deg C, 16 h, 2.) room temperature, 8 h View Scheme |
-
-
208037-78-9
(1R,5S)-8-azabicyclo[3.2.1]oct-2-ene-8-carboxylic acid tert-butyl ester

-
-
519-09-5
benzylecgonine

| Conditions | Yield |
|---|---|
| Multi-step reaction with 8 steps 1: 78 percent / Na2CO3*H2O / tetrahydrofuran; H2O / 16 h 2: 96 percent / aq. NaOH / ethanol / 1.) 0 deg C, 1 h, 2.) room temperature, 1.5 h 3: 80 percent / 0.67 h / 105 - 110 °C 4: 94 percent / Na2CO3, aq. H2O2 / H2O / 24 h / Ambient temperature 5: 96 percent / DMAP / CH2Cl2 / 12 h / Ambient temperature 6: 90 percent / TFA / CH2Cl2 / 1 h / Ambient temperature 7: 75 percent / NaBH3CN / acetonitrile / 1 h / Ambient temperature 8: 93 percent / NaNO2 / acetic acid; acetic anhydride / 1.) 0 deg C, 16 h, 2.) room temperature, 8 h View Scheme |
-
-
208037-77-8
(1R,5S)-8-(tert-butyloxycarbonyl)-8-azabicyclo[3.2.1]-2-octanone

-
-
519-09-5
benzylecgonine

| Conditions | Yield |
|---|---|
| Multi-step reaction with 10 steps 1: 88 percent / methanol / 2 h / Ambient temperature 2: 70 percent / NaH / toluene / 1.) room temperature, 30 min, 2.) reflux, 5 h 3: 78 percent / Na2CO3*H2O / tetrahydrofuran; H2O / 16 h 4: 96 percent / aq. NaOH / ethanol / 1.) 0 deg C, 1 h, 2.) room temperature, 1.5 h 5: 80 percent / 0.67 h / 105 - 110 °C 6: 94 percent / Na2CO3, aq. H2O2 / H2O / 24 h / Ambient temperature 7: 96 percent / DMAP / CH2Cl2 / 12 h / Ambient temperature 8: 90 percent / TFA / CH2Cl2 / 1 h / Ambient temperature 9: 75 percent / NaBH3CN / acetonitrile / 1 h / Ambient temperature 10: 93 percent / NaNO2 / acetic acid; acetic anhydride / 1.) 0 deg C, 16 h, 2.) room temperature, 8 h View Scheme |
-
-
208037-73-4
(2R,5S)-5-[1'-(2'-methoxycarbonyl)ethyl]proline methyl ester

-
-
519-09-5
benzylecgonine

| Conditions | Yield |
|---|---|
| Multi-step reaction with 13 steps 1: 93 percent / Et3N / methanol / 14 h / Ambient temperature 2: 85 percent / KHMDS / tetrahydrofuran / 6 h / -78 °C 3: 87 percent / NaI / pyridine / 7.5 h 4: 88 percent / methanol / 2 h / Ambient temperature 5: 70 percent / NaH / toluene / 1.) room temperature, 30 min, 2.) reflux, 5 h 6: 78 percent / Na2CO3*H2O / tetrahydrofuran; H2O / 16 h 7: 96 percent / aq. NaOH / ethanol / 1.) 0 deg C, 1 h, 2.) room temperature, 1.5 h 8: 80 percent / 0.67 h / 105 - 110 °C 9: 94 percent / Na2CO3, aq. H2O2 / H2O / 24 h / Ambient temperature 10: 96 percent / DMAP / CH2Cl2 / 12 h / Ambient temperature 11: 90 percent / TFA / CH2Cl2 / 1 h / Ambient temperature 12: 75 percent / NaBH3CN / acetonitrile / 1 h / Ambient temperature 13: 93 percent / NaNO2 / acetic acid; acetic anhydride / 1.) 0 deg C, 16 h, 2.) room temperature, 8 h View Scheme | |
| Multi-step reaction with 14 steps 1: 96 percent / K2CO3 / acetonitrile / 14 h / Ambient temperature 2: 90 percent / KHMDS / tetrahydrofuran / 1 h / -78 °C 3: 90 percent / NaI / pyridine / 8 h / Heating 4: 96 percent / H2 / Pd/C / methanol / 5 h 5: 88 percent / methanol / 2 h / Ambient temperature 6: 70 percent / NaH / toluene / 1.) room temperature, 30 min, 2.) reflux, 5 h 7: 78 percent / Na2CO3*H2O / tetrahydrofuran; H2O / 16 h 8: 96 percent / aq. NaOH / ethanol / 1.) 0 deg C, 1 h, 2.) room temperature, 1.5 h 9: 80 percent / 0.67 h / 105 - 110 °C 10: 94 percent / Na2CO3, aq. H2O2 / H2O / 24 h / Ambient temperature 11: 96 percent / DMAP / CH2Cl2 / 12 h / Ambient temperature 12: 90 percent / TFA / CH2Cl2 / 1 h / Ambient temperature 13: 75 percent / NaBH3CN / acetonitrile / 1 h / Ambient temperature 14: 93 percent / NaNO2 / acetic acid; acetic anhydride / 1.) 0 deg C, 16 h, 2.) room temperature, 8 h View Scheme |
-
-
208037-72-3
(2R,5S)-5-(2-Carboxy-ethyl)-pyrrolidine-2-carboxylic acid tert-butyl ester

-
-
519-09-5
benzylecgonine

| Conditions | Yield |
|---|---|
| Multi-step reaction with 14 steps 1: 95 percent / AcCl / 14 h / Heating 2: 93 percent / Et3N / methanol / 14 h / Ambient temperature 3: 85 percent / KHMDS / tetrahydrofuran / 6 h / -78 °C 4: 87 percent / NaI / pyridine / 7.5 h 5: 88 percent / methanol / 2 h / Ambient temperature 6: 70 percent / NaH / toluene / 1.) room temperature, 30 min, 2.) reflux, 5 h 7: 78 percent / Na2CO3*H2O / tetrahydrofuran; H2O / 16 h 8: 96 percent / aq. NaOH / ethanol / 1.) 0 deg C, 1 h, 2.) room temperature, 1.5 h 9: 80 percent / 0.67 h / 105 - 110 °C 10: 94 percent / Na2CO3, aq. H2O2 / H2O / 24 h / Ambient temperature 11: 96 percent / DMAP / CH2Cl2 / 12 h / Ambient temperature 12: 90 percent / TFA / CH2Cl2 / 1 h / Ambient temperature 13: 75 percent / NaBH3CN / acetonitrile / 1 h / Ambient temperature 14: 93 percent / NaNO2 / acetic acid; acetic anhydride / 1.) 0 deg C, 16 h, 2.) room temperature, 8 h View Scheme | |
| Multi-step reaction with 15 steps 1: 95 percent / AcCl / 14 h / Heating 2: 96 percent / K2CO3 / acetonitrile / 14 h / Ambient temperature 3: 90 percent / KHMDS / tetrahydrofuran / 1 h / -78 °C 4: 90 percent / NaI / pyridine / 8 h / Heating 5: 96 percent / H2 / Pd/C / methanol / 5 h 6: 88 percent / methanol / 2 h / Ambient temperature 7: 70 percent / NaH / toluene / 1.) room temperature, 30 min, 2.) reflux, 5 h 8: 78 percent / Na2CO3*H2O / tetrahydrofuran; H2O / 16 h 9: 96 percent / aq. NaOH / ethanol / 1.) 0 deg C, 1 h, 2.) room temperature, 1.5 h 10: 80 percent / 0.67 h / 105 - 110 °C 11: 94 percent / Na2CO3, aq. H2O2 / H2O / 24 h / Ambient temperature 12: 96 percent / DMAP / CH2Cl2 / 12 h / Ambient temperature 13: 90 percent / TFA / CH2Cl2 / 1 h / Ambient temperature 14: 75 percent / NaBH3CN / acetonitrile / 1 h / Ambient temperature 15: 93 percent / NaNO2 / acetic acid; acetic anhydride / 1.) 0 deg C, 16 h, 2.) room temperature, 8 h View Scheme |
-
-
208037-85-8
(1R,2R,3S,5S)-2-cyano-3-hydroxy-8-azabicyclo[3.2.1]octane-8-carboxylic acid tert-butyl ester

-
-
519-09-5
benzylecgonine

| Conditions | Yield |
|---|---|
| Multi-step reaction with 5 steps 1: 94 percent / Na2CO3, aq. H2O2 / H2O / 24 h / Ambient temperature 2: 96 percent / DMAP / CH2Cl2 / 12 h / Ambient temperature 3: 90 percent / TFA / CH2Cl2 / 1 h / Ambient temperature 4: 75 percent / NaBH3CN / acetonitrile / 1 h / Ambient temperature 5: 93 percent / NaNO2 / acetic acid; acetic anhydride / 1.) 0 deg C, 16 h, 2.) room temperature, 8 h View Scheme |
-
-
208037-75-6
methyl 8-benzyl-2-hydroxy-8-azabicyclo[3.2.1]oct-2-ene-3-carboxylate

-
-
519-09-5
benzylecgonine

| Conditions | Yield |
|---|---|
| Multi-step reaction with 12 steps 1: 90 percent / NaI / pyridine / 8 h / Heating 2: 96 percent / H2 / Pd/C / methanol / 5 h 3: 88 percent / methanol / 2 h / Ambient temperature 4: 70 percent / NaH / toluene / 1.) room temperature, 30 min, 2.) reflux, 5 h 5: 78 percent / Na2CO3*H2O / tetrahydrofuran; H2O / 16 h 6: 96 percent / aq. NaOH / ethanol / 1.) 0 deg C, 1 h, 2.) room temperature, 1.5 h 7: 80 percent / 0.67 h / 105 - 110 °C 8: 94 percent / Na2CO3, aq. H2O2 / H2O / 24 h / Ambient temperature 9: 96 percent / DMAP / CH2Cl2 / 12 h / Ambient temperature 10: 90 percent / TFA / CH2Cl2 / 1 h / Ambient temperature 11: 75 percent / NaBH3CN / acetonitrile / 1 h / Ambient temperature 12: 93 percent / NaNO2 / acetic acid; acetic anhydride / 1.) 0 deg C, 16 h, 2.) room temperature, 8 h View Scheme |
-
-
208037-88-1
Benzoic acid (1R,2R,3S,5S)-2-carbamoyl-8-aza-bicyclo[3.2.1]oct-3-yl ester

-
-
519-09-5
benzylecgonine

| Conditions | Yield |
|---|---|
| Multi-step reaction with 2 steps 1: 75 percent / NaBH3CN / acetonitrile / 1 h / Ambient temperature 2: 93 percent / NaNO2 / acetic acid; acetic anhydride / 1.) 0 deg C, 16 h, 2.) room temperature, 8 h View Scheme |
-
-
208037-86-9
(1R,2R,3S,5S)-2-Carbamoyl-3-hydroxy-8-aza-bicyclo[3.2.1]octane-8-carboxylic acid tert-butyl ester

-
-
519-09-5
benzylecgonine

| Conditions | Yield |
|---|---|
| Multi-step reaction with 4 steps 1: 96 percent / DMAP / CH2Cl2 / 12 h / Ambient temperature 2: 90 percent / TFA / CH2Cl2 / 1 h / Ambient temperature 3: 75 percent / NaBH3CN / acetonitrile / 1 h / Ambient temperature 4: 93 percent / NaNO2 / acetic acid; acetic anhydride / 1.) 0 deg C, 16 h, 2.) room temperature, 8 h View Scheme |
-
-
208037-82-5
(1R,2S,5S)-2-Phenylselanyl-8-aza-bicyclo[3.2.1]octane-8-carboxylic acid tert-butyl ester

-
-
519-09-5
benzylecgonine

| Conditions | Yield |
|---|---|
| Multi-step reaction with 9 steps 1: 91 percent / NaIO4 / tetrahydrofuran; H2O / 2 h / Ambient temperature 2: 78 percent / Na2CO3*H2O / tetrahydrofuran; H2O / 16 h 3: 96 percent / aq. NaOH / ethanol / 1.) 0 deg C, 1 h, 2.) room temperature, 1.5 h 4: 80 percent / 0.67 h / 105 - 110 °C 5: 94 percent / Na2CO3, aq. H2O2 / H2O / 24 h / Ambient temperature 6: 96 percent / DMAP / CH2Cl2 / 12 h / Ambient temperature 7: 90 percent / TFA / CH2Cl2 / 1 h / Ambient temperature 8: 75 percent / NaBH3CN / acetonitrile / 1 h / Ambient temperature 9: 93 percent / NaNO2 / acetic acid; acetic anhydride / 1.) 0 deg C, 16 h, 2.) room temperature, 8 h View Scheme |
-
-
208038-13-5
(1R,2R,5S)-2-Phenylselanyl-8-aza-bicyclo[3.2.1]octane-8-carboxylic acid tert-butyl ester

-
-
519-09-5
benzylecgonine

| Conditions | Yield |
|---|---|
| Multi-step reaction with 9 steps 1: 91 percent / NaIO4 / tetrahydrofuran; H2O / 2 h / Ambient temperature 2: 78 percent / Na2CO3*H2O / tetrahydrofuran; H2O / 16 h 3: 96 percent / aq. NaOH / ethanol / 1.) 0 deg C, 1 h, 2.) room temperature, 1.5 h 4: 80 percent / 0.67 h / 105 - 110 °C 5: 94 percent / Na2CO3, aq. H2O2 / H2O / 24 h / Ambient temperature 6: 96 percent / DMAP / CH2Cl2 / 12 h / Ambient temperature 7: 90 percent / TFA / CH2Cl2 / 1 h / Ambient temperature 8: 75 percent / NaBH3CN / acetonitrile / 1 h / Ambient temperature 9: 93 percent / NaNO2 / acetic acid; acetic anhydride / 1.) 0 deg C, 16 h, 2.) room temperature, 8 h View Scheme |
-
-
208037-92-7
(1R,2S,5S)-2-Phenylselanyl-8-aza-bicyclo[3.2.1]octane-8-carboxylic acid 1-chloro-ethyl ester

-
-
519-09-5
benzylecgonine

| Conditions | Yield |
|---|---|
| Multi-step reaction with 10 steps 1: 2.) Et3N / 1.) MeOH, reflux, 2 h, 2.) MeOH, room temperature, 15 h 2: 91 percent / NaIO4 / tetrahydrofuran; H2O / 2 h / Ambient temperature 3: 78 percent / Na2CO3*H2O / tetrahydrofuran; H2O / 16 h 4: 96 percent / aq. NaOH / ethanol / 1.) 0 deg C, 1 h, 2.) room temperature, 1.5 h 5: 80 percent / 0.67 h / 105 - 110 °C 6: 94 percent / Na2CO3, aq. H2O2 / H2O / 24 h / Ambient temperature 7: 96 percent / DMAP / CH2Cl2 / 12 h / Ambient temperature 8: 90 percent / TFA / CH2Cl2 / 1 h / Ambient temperature 9: 75 percent / NaBH3CN / acetonitrile / 1 h / Ambient temperature 10: 93 percent / NaNO2 / acetic acid; acetic anhydride / 1.) 0 deg C, 16 h, 2.) room temperature, 8 h View Scheme |

-
-
519-09-5
benzylecgonine

| Conditions | Yield |
|---|---|
| Multi-step reaction with 10 steps 1: 2.) Et3N / 1.) MeOH, reflux, 2 h, 2.) MeOH, room temperature, 15 h 2: 91 percent / NaIO4 / tetrahydrofuran; H2O / 2 h / Ambient temperature 3: 78 percent / Na2CO3*H2O / tetrahydrofuran; H2O / 16 h 4: 96 percent / aq. NaOH / ethanol / 1.) 0 deg C, 1 h, 2.) room temperature, 1.5 h 5: 80 percent / 0.67 h / 105 - 110 °C 6: 94 percent / Na2CO3, aq. H2O2 / H2O / 24 h / Ambient temperature 7: 96 percent / DMAP / CH2Cl2 / 12 h / Ambient temperature 8: 90 percent / TFA / CH2Cl2 / 1 h / Ambient temperature 9: 75 percent / NaBH3CN / acetonitrile / 1 h / Ambient temperature 10: 93 percent / NaNO2 / acetic acid; acetic anhydride / 1.) 0 deg C, 16 h, 2.) room temperature, 8 h View Scheme |
-
-
208037-90-5
(1R,5S)-2-Hydroxy-8-aza-bicyclo[3.2.1]oct-2-ene-3,8-dicarboxylic acid 8-tert-butyl ester 3-methyl ester

-
-
519-09-5
benzylecgonine

| Conditions | Yield |
|---|---|
| Multi-step reaction with 11 steps 1: 87 percent / NaI / pyridine / 7.5 h 2: 88 percent / methanol / 2 h / Ambient temperature 3: 70 percent / NaH / toluene / 1.) room temperature, 30 min, 2.) reflux, 5 h 4: 78 percent / Na2CO3*H2O / tetrahydrofuran; H2O / 16 h 5: 96 percent / aq. NaOH / ethanol / 1.) 0 deg C, 1 h, 2.) room temperature, 1.5 h 6: 80 percent / 0.67 h / 105 - 110 °C 7: 94 percent / Na2CO3, aq. H2O2 / H2O / 24 h / Ambient temperature 8: 96 percent / DMAP / CH2Cl2 / 12 h / Ambient temperature 9: 90 percent / TFA / CH2Cl2 / 1 h / Ambient temperature 10: 75 percent / NaBH3CN / acetonitrile / 1 h / Ambient temperature 11: 93 percent / NaNO2 / acetic acid; acetic anhydride / 1.) 0 deg C, 16 h, 2.) room temperature, 8 h View Scheme |

| Conditions | Yield |
|---|---|
| In dichloromethane | 95% |

| Conditions | Yield |
|---|---|
| Stage #1: benzylecgonine With 1,1'-carbonyldiimidazole In dichloromethane at 20℃; Stage #2: trimethyleneglycol In DMF (N,N-dimethyl-formamide); dichloromethane at 20℃; | 95% |
| Stage #1: benzylecgonine With 1,1'-carbonyldiimidazole In dichloromethane at 20℃; for 24h; Stage #2: trimethyleneglycol In dichloromethane | |
| Stage #1: benzylecgonine With 1,1'-carbonyldiimidazole In dichloromethane for 22h; Stage #2: trimethyleneglycol In dichloromethane at 20℃; for 6h; |

| Conditions | Yield |
|---|---|
| With hydrogenchloride at 0℃; for 48h; | 83% |

| Conditions | Yield |
|---|---|
| With N,N,N,N,-tetramethylethylenediamine In dichloromethane for 72h; Ambient temperature; | 72% |
| With ethanol at 100℃; im Rohr; |
-
-
78277-26-6
benzyl 6-bromohexanoate

-
-
519-09-5
benzylecgonine

-
-
173443-26-0
(1R,2R,3S,5S)-3-Benzoyloxy-8-methyl-8-aza-bicyclo[3.2.1]octane-2-carboxylic acid 5-benzyloxycarbonyl-pentyl ester

| Conditions | Yield |
|---|---|
| With potassium carbonate; potassium iodide In acetonitrile at 80℃; | 72% |

| Conditions | Yield |
|---|---|
| Stage #1: 6-amino-hexanoic acid benzyl ester toluene-4-sulfonic acid; benzylecgonine With dmap In dichloromethane for 1.5h; Inert atmosphere; Cooling with ice; Stage #2: With 1-ethyl-(3-(3-dimethylamino)propyl)-carbodiimide hydrochloride; triethylamine In dichloromethane for 96h; Inert atmosphere; | 71% |
-
-
16367-71-8
t-butyl (RS)-phenylalaninate

-
-
519-09-5
benzylecgonine

| Conditions | Yield |
|---|---|
| With benzotriazol-1-yl-oxy-tris-pyrrolidinophosphonium; triethylamine In dichloromethane for 12h; Ambient temperature; | 65% |

| Conditions | Yield |
|---|---|
| Stage #1: benzylecgonine With oxalyl dichloride at 20℃; for 0.333333h; Stage #2: ethanol at 20℃; for 12h; | 60% |
| With hydrogenchloride | |
| With hydrogenchloride at 0℃; for 48h; |

| Conditions | Yield |
|---|---|
| With t-butyl (RS)-phenylalaninate In dichloromethane Ambient temperature; | A 58% B 15% |

-
-
16367-71-8
t-butyl (RS)-phenylalaninate

-
-
519-09-5
benzylecgonine

-
-
109309-90-2
(S)-N-benzoylphenylalanine tert-butyl ester

| Conditions | Yield |
|---|---|
| With benzotriazol-1-ol; dicyclohexyl-carbodiimide In dichloromethane Ambient temperature; | 34% |
-
-
519-09-5
benzylecgonine

-
-
137718-56-0
3β-(benzoyloxy)-2β-(hydroxymethyl)-8-methyl-8-azabicyclo<3.2.1>octane

| Conditions | Yield |
|---|---|
| With B2H6-THF In tetrahydrofuran at 0 - 20℃; for 3h; | 31% |

| Conditions | Yield |
|---|---|
| With hydrogenchloride |

| Conditions | Yield |
|---|---|
| With 2-methyl-propan-1-ol at 95℃; im Rohr; |

| Conditions | Yield |
|---|---|
| With propan-1-ol at 95℃; im Rohr; |

| Conditions | Yield |
|---|---|
| With ethanol at 95℃; im Rohr; O-benzoyl-l-ecgonine-<β-bromo-ethyl ester>; |

| Conditions | Yield |
|---|---|
| With methanol at 100℃; im Rohr; | |
| With methanol; sodium methylate at 100℃; im Rohr; |

| Conditions | Yield |
|---|---|
| With potassium hydroxide | |
| With potassium hydroxide |

| Conditions | Yield |
|---|---|
| With hydrogenchloride |
-
-
519-09-5
benzylecgonine

| Conditions | Yield |
|---|---|
| With thionyl chloride Behandeln des Reaktionsprodukts mit NaN3 in H2O, Erwaermen mit wss. HCl und anschliessendes Behandeln mit wss. NaOH; |
-
-
519-09-5
benzylecgonine

-
-
41889-45-6
benzoylnorecgonine

| Conditions | Yield |
|---|---|
| With potassium permanganate; sodium carbonate |
-
-
100-27-6
2-(4-nitrophenyl)ethanol

-
-
519-09-5
benzylecgonine

-
-
137819-59-1
3β-(benzoyloxy)-8-methyl-8-azabicyclo<3.2.1>octane-2β-carboxylic acid (p-nitrophenyl)ethyl ester

| Conditions | Yield |
|---|---|
| With 1,1'-carbonyldiimidazole 1.) CH2Cl2, RT, 6 h, 2.) acetone, reflux, 3 h; Yield given. Multistep reaction; |

| Conditions | Yield |
|---|---|
| With 1,1'-carbonyldiimidazole 1.) CH2Cl2, RT, 6 h, 2.) acetone, reflux, 3 h; Yield given. Multistep reaction; |
-
-
74-89-5
methylamine

-
-
519-09-5
benzylecgonine

-
-
137819-57-9
3β-(benzoyloxy)-8-methyl-8-azabicyclo<3.2.1>octane-2β-carboxylic acid N-methylamide

| Conditions | Yield |
|---|---|
| With 1,1'-carbonyldiimidazole 1.) CH2Cl2, RT, 1 h, 2.) CH2Cl2, 3 h; Yield given. Multistep reaction; |

| Conditions | Yield |
|---|---|
| With 1,1'-carbonyldiimidazole 1.) CH2Cl2, RT, 6 h, 2.) acetone, reflux, 3 h; Yield given. Multistep reaction; |
-
-
519-09-5
benzylecgonine

-
-
61194-36-3
benzoylecgonine acid chloride

| Conditions | Yield |
|---|---|
| With thionyl chloride | |
| With thionyl chloride at 0℃; for 4h; | |
| With oxalyl dichloride In dichloromethane for 1h; Ambient temperature; | |
| With oxalyl dichloride for 0.25h; Substitution; |
Related products
Hot Products
About|Contact|Cas|Product Name|Molecular|Country|Encyclopedia
Message|New Cas|MSDS|Service|Advertisement|CAS DataBase|Article Data|Manufacturers | Chemical Catalog
©2008 LookChem.com,License: ICP
NO.:Zhejiang16009103
complaints:service@lookchem.com Desktop View




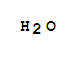






 T;
T; F
F