-
Name
(3S,4E)-3-Hydroxy-7-[(triphenylmethyl)thio]-4-heptenoic acid
- EINECS
- CAS No. 180973-24-4
- Article Data7
- CAS DataBase
- Density 1.201
- Solubility
- Melting Point
- Formula C26H26O3S
- Boiling Point
- Molecular Weight 418.557
- Flash Point
- Transport Information
- Appearance
- Safety
- Risk Codes
-
Molecular Structure
- Hazard Symbols
- Synonyms (E)-(S)-3-hydroxy-7-tritylthio-4-heptenoic acid;(3S,E)-3-hydroxy-7-tritylthio-4-heptenoic acid;(E)-(S)-3-hydroxy-7-tritylsulfanyl-hept-4-enoic acid;4-Heptenoic acid,3-hydroxy-7-[(triphenylmethyl)thio]-,(3S,4E);
- PSA 82.83000
- LogP 5.49360
Synthetic route
-
-
180973-40-4
(E)-(S)-3-Hydroxy-7-tritylsulfanyl-hept-4-enoic acid benzyl ester

-
-
180973-24-4
(E)-(S)-3-hydroxy-7-tritylthio-4-heptenoic acid

| Conditions | Yield |
|---|---|
| With lithium hydroxide In methanol; water | 100% |
-
-
663918-95-4
(3S,4E)-3-hydroxy-1-((4R)-4-isopropyl-2-thioxothiazolidin-3-yl)-7-tritylsulfanyl-hept-4-en-1-one

-
-
180973-24-4
(E)-(S)-3-hydroxy-7-tritylthio-4-heptenoic acid

| Conditions | Yield |
|---|---|
| With lithium hydroxide; water In tetrahydrofuran Cooling with ice; | 96% |
| Stage #1: (3S,4E)-3-hydroxy-1-((4R)-4-isopropyl-2-thioxothiazolidin-3-yl)-7-tritylsulfanyl-hept-4-en-1-one With water; lithium hydroxide In tetrahydrofuran at 0 - 20℃; Stage #2: With hydrogenchloride In tetrahydrofuran; water pH=2; | 86% |
| With lithium hydroxide monohydrate; water In tetrahydrofuran at 0 - 30℃; for 12h; | 13 g |
-
-
878630-47-8
(E)-(S)-3-hydroxy-1-[(R)-4-isopropyl-5,5-diphenyl-2-oxo-oxazolidin-3-yl]-7-tritylthio-4-hepten-1-one

-
-
180973-24-4
(E)-(S)-3-hydroxy-7-tritylthio-4-heptenoic acid

| Conditions | Yield |
|---|---|
| Stage #1: (E)-(S)-3-hydroxy-1-[(R)-4-isopropyl-5,5-diphenyl-2-oxo-oxazolidin-3-yl]-7-tritylthio-4-hepten-1-one With water; sodium hydroxide In tetrahydrofuran; methanol at 0℃; for 1h; Stage #2: With hydrogenchloride In water; ethyl acetate at 0℃; | 92% |
| With sodium hydroxide | 76% |
| With sodium hydroxide In methanol; water at 0 - 20℃; for 1h; | 58% |
-
-
180973-22-2
(2E)-5-[(triphenylmethyl)thio]-2-pentenal

-
-
180973-24-4
(E)-(S)-3-hydroxy-7-tritylthio-4-heptenoic acid

| Conditions | Yield |
|---|---|
| Multi-step reaction with 2 steps 1.1: BuLi / tetrahydrofuran / -78 °C 1.2: Cp2ZrCl2 / tetrahydrofuran / -78 - 20 °C 2.1: 76 percent / NaOH View Scheme | |
| Multi-step reaction with 2 steps 1.1: SmI2 / tetrahydrofuran / -78 °C 1.2: tetrahydrofuran / -78 °C 2.1: 76 percent / NaOH View Scheme | |
| Multi-step reaction with 2 steps 1: 99 percent / Ti(IV) complex with (S)-(-)-binaphthyl amino alcohol 2: 100 percent / LiOH / methanol; H2O View Scheme |
-
-
3695-77-0
triphenylmethanethiol

-
-
180973-24-4
(E)-(S)-3-hydroxy-7-tritylthio-4-heptenoic acid

| Conditions | Yield |
|---|---|
| Multi-step reaction with 5 steps 1: 78 percent / Cs2CO3 2: 91 percent / DIBAL 3: 74 percent / (COCl)2, DMSO, Et3N 4: 99 percent / Ti(IV) complex with (S)-(-)-binaphthyl amino alcohol 5: 100 percent / LiOH / methanol; H2O View Scheme | |
| Multi-step reaction with 4 steps 1.1: triethylamine / dichloromethane / 1 h / 25 °C / Inert atmosphere; Automated synthesizer 2.1: dmap / N,N-dimethyl-formamide / 2 h / 100 °C / Inert atmosphere; Automated synthesizer 2.2: 2 h / -20 - 25 °C / Inert atmosphere; Automated synthesizer 2.3: -78 - 0 °C / Inert atmosphere; Automated synthesizer 3.1: n-butyllithium / tetrahydrofuran; hexane / 1 h / -78 °C / Inert atmosphere; Automated synthesizer 3.2: 1 h / -78 - -30 °C / Inert atmosphere; Automated synthesizer 3.3: 1 h / -50 - 0 °C / Inert atmosphere; Automated synthesizer 4.1: water; sodium hydroxide / tetrahydrofuran; methanol / 1 h / 0 °C 4.2: 0 °C View Scheme | |
| Multi-step reaction with 4 steps 1.1: sodium hydride / tetrahydrofuran / 20 °C / Flow reactor 2.1: diisobutylaluminium hydride / toluene / -97 °C / Flow reactor 3.1: lithium hexamethyldisilazane / tetrahydrofuran / -40 °C / Flow reactor 3.2: 0.02 h / -78 °C / Flow reactor 4.1: sodium hydroxide / methanol; water / 1 h / 0 - 20 °C View Scheme |
-
-
180973-37-9
(E)-5-(tritylthio)-2-penten-1-ol

-
-
180973-24-4
(E)-(S)-3-hydroxy-7-tritylthio-4-heptenoic acid

| Conditions | Yield |
|---|---|
| Multi-step reaction with 3 steps 1: 74 percent / (COCl)2, DMSO, Et3N 2: 99 percent / Ti(IV) complex with (S)-(-)-binaphthyl amino alcohol 3: 100 percent / LiOH / methanol; H2O View Scheme | |
| Multi-step reaction with 3 steps 1.1: manganese(IV) oxide / dichloromethane / 8 h / 25 - 45 °C 2.1: titanium tetrachloride / dichloromethane / 0 - 5 °C 2.2: 2 h / -78 °C 2.3: 0.5 h / -78 °C 3.1: lithium hydroxide monohydrate; water / tetrahydrofuran / 12 h / 0 - 30 °C View Scheme |
-
-
180973-36-8
(E)-5-Tritylsulfanyl-pent-2-enoic acid methyl ester

-
-
180973-24-4
(E)-(S)-3-hydroxy-7-tritylthio-4-heptenoic acid

| Conditions | Yield |
|---|---|
| Multi-step reaction with 4 steps 1: 91 percent / DIBAL 2: 74 percent / (COCl)2, DMSO, Et3N 3: 99 percent / Ti(IV) complex with (S)-(-)-binaphthyl amino alcohol 4: 100 percent / LiOH / methanol; H2O View Scheme |
-
-
2409-87-2
methyl (2E)-penta-2,4-dienoate

-
-
180973-24-4
(E)-(S)-3-hydroxy-7-tritylthio-4-heptenoic acid

| Conditions | Yield |
|---|---|
| Multi-step reaction with 5 steps 1: 78 percent / Cs2CO3 2: 91 percent / DIBAL 3: 74 percent / (COCl)2, DMSO, Et3N 4: 99 percent / Ti(IV) complex with (S)-(-)-binaphthyl amino alcohol 5: 100 percent / LiOH / methanol; H2O View Scheme |
-
-
150350-28-0
S-trityl 3-mercaptopropionaldehyde

-
-
180973-24-4
(E)-(S)-3-hydroxy-7-tritylthio-4-heptenoic acid

| Conditions | Yield |
|---|---|
| Multi-step reaction with 3 steps 1.1: dmap / N,N-dimethyl-formamide / 2 h / 100 °C / Inert atmosphere; Automated synthesizer 1.2: 2 h / -20 - 25 °C / Inert atmosphere; Automated synthesizer 1.3: -78 - 0 °C / Inert atmosphere; Automated synthesizer 2.1: n-butyllithium / tetrahydrofuran; hexane / 1 h / -78 °C / Inert atmosphere; Automated synthesizer 2.2: 1 h / -78 - -30 °C / Inert atmosphere; Automated synthesizer 2.3: 1 h / -50 - 0 °C / Inert atmosphere; Automated synthesizer 3.1: water; sodium hydroxide / tetrahydrofuran; methanol / 1 h / 0 °C 3.2: 0 °C View Scheme | |
| Multi-step reaction with 4 steps 1.1: sodium hydride / tetrahydrofuran; mineral oil / 0.5 h / 0 °C / Inert atmosphere 1.2: 0 - 20 °C / Inert atmosphere 2.1: diisobutylaluminium hydride / toluene / -97 °C / Flow reactor 3.1: lithium hexamethyldisilazane / tetrahydrofuran / -40 °C / Flow reactor 3.2: 0.02 h / -78 °C / Flow reactor 4.1: sodium hydroxide / methanol; water / 1 h / 0 - 20 °C View Scheme | |
| Multi-step reaction with 4 steps 1.1: sodium hydride / tetrahydrofuran; mineral oil / 0.5 h / 0 °C / Inert atmosphere 1.2: 0 - 20 °C / Inert atmosphere 2.1: diisobutylaluminium hydride / toluene / -97 °C / Flow reactor 3.1: lithium hexamethyldisilazane / tetrahydrofuran / -78 °C / Flow reactor 3.2: 0.02 h / Flow reactor 4.1: sodium hydroxide / methanol; water / 1 h / 0 - 20 °C View Scheme | |
| Multi-step reaction with 5 steps 1.1: triphenylphosphine / toluene; water / 6 h / 25 - 105 °C 1.2: 2 h / 0 - 5 °C 2.1: diisobutylaluminium hydride / toluene / 3 h / 0 - 5 °C / Inert atmosphere 3.1: manganese(IV) oxide / dichloromethane / 8 h / 25 - 45 °C 4.1: titanium tetrachloride / dichloromethane / 0 - 5 °C 4.2: 2 h / -78 °C 4.3: 0.5 h / -78 °C 5.1: lithium hydroxide monohydrate; water / tetrahydrofuran / 12 h / 0 - 30 °C View Scheme |
-
-
1309766-86-6
ethyl (E)-5-(triphenylmethylthio)-2-pentenoate

-
-
180973-24-4
(E)-(S)-3-hydroxy-7-tritylthio-4-heptenoic acid

| Conditions | Yield |
|---|---|
| Multi-step reaction with 3 steps 1.1: diisobutylaluminium hydride / toluene / -97 °C / Flow reactor 2.1: lithium hexamethyldisilazane / tetrahydrofuran / -40 °C / Flow reactor 2.2: 0.02 h / -78 °C / Flow reactor 3.1: sodium hydroxide / methanol; water / 1 h / 0 - 20 °C View Scheme | |
| Multi-step reaction with 3 steps 1.1: diisobutylaluminium hydride / toluene / -97 °C / Flow reactor 2.1: lithium hexamethyldisilazane / tetrahydrofuran / -78 °C / Flow reactor 2.2: 0.02 h / Flow reactor 3.1: sodium hydroxide / methanol; water / 1 h / 0 - 20 °C View Scheme | |
| Multi-step reaction with 4 steps 1.1: diisobutylaluminium hydride / toluene / 3 h / 0 - 5 °C / Inert atmosphere 2.1: manganese(IV) oxide / dichloromethane / 8 h / 25 - 45 °C 3.1: titanium tetrachloride / dichloromethane / 0 - 5 °C 3.2: 2 h / -78 °C 3.3: 0.5 h / -78 °C 4.1: lithium hydroxide monohydrate; water / tetrahydrofuran / 12 h / 0 - 30 °C View Scheme |
-
-
76-83-5
trityl chloride

-
-
180973-24-4
(E)-(S)-3-hydroxy-7-tritylthio-4-heptenoic acid

| Conditions | Yield |
|---|---|
| Multi-step reaction with 8 steps 1.1: toluene / 6 h / 25 - 30 °C 2.1: sodium tetrahydroborate / tetrahydrofuran / 0.17 h / 0 - 5 °C 2.2: 3 h / 0 - 30 °C 3.1: oxalyl dichloride; dimethyl sulfoxide / dichloromethane / 2 h / -75 - -70 °C 3.2: 2 h / -75 - 5 °C 4.1: triphenylphosphine / toluene; water / 6 h / 25 - 105 °C 4.2: 2 h / 0 - 5 °C 5.1: diisobutylaluminium hydride / toluene / 3 h / 0 - 5 °C / Inert atmosphere 6.1: manganese(IV) oxide / dichloromethane / 8 h / 25 - 45 °C 7.1: titanium tetrachloride / dichloromethane / 0 - 5 °C 7.2: 2 h / -78 °C 7.3: 0.5 h / -78 °C 8.1: lithium hydroxide monohydrate; water / tetrahydrofuran / 12 h / 0 - 30 °C View Scheme |
-
-
27144-18-9
3-(tritylthio) propanoic acid

-
-
180973-24-4
(E)-(S)-3-hydroxy-7-tritylthio-4-heptenoic acid

| Conditions | Yield |
|---|---|
| Multi-step reaction with 7 steps 1.1: sodium tetrahydroborate / tetrahydrofuran / 0.17 h / 0 - 5 °C 1.2: 3 h / 0 - 30 °C 2.1: oxalyl dichloride; dimethyl sulfoxide / dichloromethane / 2 h / -75 - -70 °C 2.2: 2 h / -75 - 5 °C 3.1: triphenylphosphine / toluene; water / 6 h / 25 - 105 °C 3.2: 2 h / 0 - 5 °C 4.1: diisobutylaluminium hydride / toluene / 3 h / 0 - 5 °C / Inert atmosphere 5.1: manganese(IV) oxide / dichloromethane / 8 h / 25 - 45 °C 6.1: titanium tetrachloride / dichloromethane / 0 - 5 °C 6.2: 2 h / -78 °C 6.3: 0.5 h / -78 °C 7.1: lithium hydroxide monohydrate; water / tetrahydrofuran / 12 h / 0 - 30 °C View Scheme |
-
-
100360-56-3
3-(tritylthio)propan-1-ol

-
-
180973-24-4
(E)-(S)-3-hydroxy-7-tritylthio-4-heptenoic acid

| Conditions | Yield |
|---|---|
| Multi-step reaction with 6 steps 1.1: oxalyl dichloride; dimethyl sulfoxide / dichloromethane / 2 h / -75 - -70 °C 1.2: 2 h / -75 - 5 °C 2.1: triphenylphosphine / toluene; water / 6 h / 25 - 105 °C 2.2: 2 h / 0 - 5 °C 3.1: diisobutylaluminium hydride / toluene / 3 h / 0 - 5 °C / Inert atmosphere 4.1: manganese(IV) oxide / dichloromethane / 8 h / 25 - 45 °C 5.1: titanium tetrachloride / dichloromethane / 0 - 5 °C 5.2: 2 h / -78 °C 5.3: 0.5 h / -78 °C 6.1: lithium hydroxide monohydrate; water / tetrahydrofuran / 12 h / 0 - 30 °C View Scheme |
-
-
180973-32-4
(S)-2-{(Z)-2-[(S)-2-((R)-2-Amino-3-methyl-butyrylamino)-3-tritylsulfanyl-propionylamino]-but-2-enoylamino}-3-methyl-butyric acid methyl ester

-
-
180973-24-4
(E)-(S)-3-hydroxy-7-tritylthio-4-heptenoic acid

-
-
180973-33-5
(5E,7S,11R,14S,17E,20S)-methyl 17-ethylidene-7-hydroxy-11,20-diisopropyl-9,12,15,18-tetraoxo-1,1,1-triphenyl-14-(tritylthiomethyl)-2-thia-10,13,16,19-tetraazahenicos-5-en-21-oate

| Conditions | Yield |
|---|---|
| With (benzotriazo-1-yloxy)tris(dimethylamino)phosphonium hexafluorophosphate; N-ethyl-N,N-diisopropylamine | 95% |
-
-
1196992-63-8
C52H49N5O7S

-
-
180973-24-4
(E)-(S)-3-hydroxy-7-tritylthio-4-heptenoic acid

-
-
1196992-65-0
C63H63N5O7S2

| Conditions | Yield |
|---|---|
| Stage #1: C52H49N5O7S With diethylamine In acetonitrile at 20℃; for 2h; Inert atmosphere; Stage #2: (E)-(S)-3-hydroxy-7-tritylthio-4-heptenoic acid With benzotriazol-1-yloxyl-tris-(pyrrolidino)-phosphonium hexafluorophosphate; N-ethyl-N,N-diisopropylamine In dichloromethane; acetonitrile at 0 - 20℃; Inert atmosphere; | 95% |
-
-
1196992-16-1
(R)-4-(9H-fluoren-9-ylmethoxycarbonylamino)-4-{(S)-1-[1-(methoxycarbonylmethyl-carbamoyl)-cyclopropylcarbamoyl]-2-tritylsulfanyl-ethylcarbamoyl}-butyric acid tert-butyl ester

-
-
180973-24-4
(E)-(S)-3-hydroxy-7-tritylthio-4-heptenoic acid

-
-
1196992-18-3
(R)-4-((E)-(S)-3-hydroxy-7-tritylsulfanyl-hept-4-enoylamino)-4-{(S)-1-[1-(methoxycarbonylmethyl-carbamoyl)-cyclopropylcarbamoyl]-2-tritylsulfanyl-ethylcarbamoyl}-butyric acid tert-butyl ester

| Conditions | Yield |
|---|---|
| Stage #1: (R)-4-(9H-fluoren-9-ylmethoxycarbonylamino)-4-{(S)-1-[1-(methoxycarbonylmethyl-carbamoyl)-cyclopropylcarbamoyl]-2-tritylsulfanyl-ethylcarbamoyl}-butyric acid tert-butyl ester With diethylamine In acetonitrile at 20℃; for 1h; Stage #2: (E)-(S)-3-hydroxy-7-tritylthio-4-heptenoic acid With benzotriazol-1-yloxyl-tris-(pyrrolidino)-phosphonium hexafluorophosphate; N-ethyl-N,N-diisopropylamine In dichloromethane; acetonitrile at 0 - 20℃; | 91% |
-
-
1196992-76-3
[(1-{(S)-2-[(R)-2-(9H-Fluoren-9-ylmethoxycarbonylamino)-3-phenyl-propionylamino]-3-tritylsulfanyl-propionylamino}-cyclopropanecarbonyl)-amino]-acetic acid methyl ester

-
-
180973-24-4
(E)-(S)-3-hydroxy-7-tritylthio-4-heptenoic acid

-
-
1196992-77-4
[(1-{(S)-2-[(R)-2-((E)-(S)-3-hydroxy-7-tritylsulfanyl-hept-4-enoylamino)-3-phenyl-propionylamino]-3-tritylsulfanyl-propionylamino}-cyclopropanecarbonyl)-amino]-acetic acid methyl ester

| Conditions | Yield |
|---|---|
| Stage #1: [(1-{(S)-2-[(R)-2-(9H-Fluoren-9-ylmethoxycarbonylamino)-3-phenyl-propionylamino]-3-tritylsulfanyl-propionylamino}-cyclopropanecarbonyl)-amino]-acetic acid methyl ester With diethylamine In acetonitrile at 20℃; for 2h; Inert atmosphere; Stage #2: (E)-(S)-3-hydroxy-7-tritylthio-4-heptenoic acid With benzotriazol-1-yloxyl-tris-(pyrrolidino)-phosphonium hexafluorophosphate; N-ethyl-N,N-diisopropylamine In dichloromethane; acetonitrile at 0 - 20℃; Inert atmosphere; | 87% |
-
-
1196992-05-8
(R)-4-(9H-fluoren-9-ylmethoxycarbonylamino)-4-{(S)-1-[1-(methoxycarbonylmethyl-carbamoyl)-1-methyl-ethylcarbamoyl]-2-tritylsulfanyl-ethylcarbamoyl}-butyric acid tert-butyl ester

-
-
180973-24-4
(E)-(S)-3-hydroxy-7-tritylthio-4-heptenoic acid

-
-
1196992-07-0
(R)-4-((E)-(S)-3-hydroxy-7-tritylsulfanyl-hept-4-enoylamino)-4-{(S)-1-[1-(methoxycarbonylmethyl-carbamoyl)-1-methyl-ethylcarbamoyl]-2-tritylsulfanyl-ethylcarbamoyl}-butyric acid tert-butyl ester

| Conditions | Yield |
|---|---|
| Stage #1: (R)-4-(9H-fluoren-9-ylmethoxycarbonylamino)-4-{(S)-1-[1-(methoxycarbonylmethyl-carbamoyl)-1-methyl-ethylcarbamoyl]-2-tritylsulfanyl-ethylcarbamoyl}-butyric acid tert-butyl ester With diethylamine In acetonitrile at 20℃; Stage #2: (E)-(S)-3-hydroxy-7-tritylthio-4-heptenoic acid With benzotriazol-1-yloxyl-tris-(pyrrolidino)-phosphonium hexafluorophosphate; N-ethyl-N,N-diisopropylamine In dichloromethane; acetonitrile at 0 - 20℃; | 85% |
-
-
2916-68-9
2-(Trimethylsilyl)ethanol

-
-
180973-24-4
(E)-(S)-3-hydroxy-7-tritylthio-4-heptenoic acid

-
-
1043577-31-6
(S,E)-2-(trimethylsilyl)ethyl 3-(((S)-2-amino-3-methylbutanoyl)oxy)-7-(tritylthio)hept-4-enoate

| Conditions | Yield |
|---|---|
| With dmap; dicyclohexyl-carbodiimide In dichloromethane Cooling with ice; | 80% |
| With dmap; dicyclohexyl-carbodiimide In dichloromethane at 0 - 5℃; for 0.916667h; | 2.5 g |
-
-
1196992-69-4
[(1-{(S)-2-[(R)-3-(3-chloro-phenyl)-2-(9H-fluoren-9-ylmethoxycarbonylamino)-propionylamino]-3-tritylsulfanyl-propionylamino}-cyclopropanecarbonyl)-amino]-acetic acid methyl ester

-
-
180973-24-4
(E)-(S)-3-hydroxy-7-tritylthio-4-heptenoic acid

-
-
1196992-72-9
[(1-{(S)-2-[(R)-3-(3-chloro-phenyl)-2-((E)-(S)-3-hydroxy-7-tritylsulfanyl-hept-4-enoylamino)-propionylamino]-3-tritylsulfanyl-propionylamino}-cyclopropanecarbonyl)-amino]-acetic acid methyl ester

| Conditions | Yield |
|---|---|
| Stage #1: [(1-{(S)-2-[(R)-3-(3-chloro-phenyl)-2-(9H-fluoren-9-ylmethoxycarbonylamino)-propionylamino]-3-tritylsulfanyl-propionylamino}-cyclopropanecarbonyl)-amino]-acetic acid methyl ester With diethylamine In dichloromethane; acetonitrile at 20℃; for 1.5h; Inert atmosphere; Stage #2: (E)-(S)-3-hydroxy-7-tritylthio-4-heptenoic acid With benzotriazol-1-yloxyl-tris-(pyrrolidino)-phosphonium hexafluorophosphate; N-ethyl-N,N-diisopropylamine In dichloromethane; acetonitrile at 20℃; for 16h; Inert atmosphere; | 80% |
-
-
1196992-35-4
(R)-4-(9H-fluoren-9-ylmethoxycarbonylamino)-4-{(S)-1-[1-(methoxycarbonylmethyl-carbamoyl)-cyclobutylcarbamoyl]-2-tritylsulfanyl-ethylcarbamoyl}-butyric acid tert-butyl ester

-
-
180973-24-4
(E)-(S)-3-hydroxy-7-tritylthio-4-heptenoic acid

-
-
1196992-37-6
(R)-4-((E)-(S)-3-hydroxy-7-tritylsulfanyl-hept-4-enoylamino)-4-{(S)-1-[1-(methoxycarbonylmethyl-carbamoyl)-cyclobutylcarbamoyl]-2-tritylsulfanyl-ethylcarbamoyl}-butyric acid tert-butyl ester

| Conditions | Yield |
|---|---|
| Stage #1: (R)-4-(9H-fluoren-9-ylmethoxycarbonylamino)-4-{(S)-1-[1-(methoxycarbonylmethyl-carbamoyl)-cyclobutylcarbamoyl]-2-tritylsulfanyl-ethylcarbamoyl}-butyric acid tert-butyl ester With diethylamine In acetonitrile at 20℃; Inert atmosphere; Stage #2: (E)-(S)-3-hydroxy-7-tritylthio-4-heptenoic acid With benzotriazol-1-yloxyl-tris-(pyrrolidino)-phosphonium hexafluorophosphate; N-ethyl-N,N-diisopropylamine In dichloromethane; acetonitrile at 0 - 20℃; Inert atmosphere; | 75% |
-
-
1196991-91-9
(R)-4-(9H-fluoren-9-ylmethoxycarbonylamino)-4-{(S)-1-[1-(1-hydroxy-2-methoxycarbonyl-ethyl)-cyclopropylcarbamoyl]-2-tritylsulfanyl-ethylcarbamoyl}-butyric acid tert-butyl ester

-
-
180973-24-4
(E)-(S)-3-hydroxy-7-tritylthio-4-heptenoic acid

-
-
1196991-93-1
(R)-4-{(S)-1-[1-(1-hydroxy-2-methoxycarbonyl-ethyl)-cyclopropylcarbamoyl]-2-tritylsulfanyl-ethylcarbamoyl}-4-((E)-(S)-3-hydroxy-7-tritylsulfanyl-hept-4-enoylamino)-butyric acid tert-butyl ester

| Conditions | Yield |
|---|---|
| Stage #1: (R)-4-(9H-fluoren-9-ylmethoxycarbonylamino)-4-{(S)-1-[1-(1-hydroxy-2-methoxycarbonyl-ethyl)-cyclopropylcarbamoyl]-2-tritylsulfanyl-ethylcarbamoyl}-butyric acid tert-butyl ester With diethylamine In dichloromethane; acetonitrile for 1.5h; Stage #2: (E)-(S)-3-hydroxy-7-tritylthio-4-heptenoic acid With benzotriazol-1-yloxyl-tris-(pyrrolidino)-phosphonium hexafluorophosphate; N-ethyl-N,N-diisopropylamine In acetonitrile at 20℃; for 16h; Inert atmosphere; | 72% |

-
-
180973-24-4
(E)-(S)-3-hydroxy-7-tritylthio-4-heptenoic acid

-
-
881845-29-0
(3S,4R)-3-Hydroxy-4-{(S)-2-[(R)-2-((E)-(S)-3-hydroxy-7-tritylsulfanyl-hept-4-enoylamino)-propionylamino]-3-tritylsulfanyl-propionylamino}-5-methyl-hexanoic acid allyl ester

| Conditions | Yield |
|---|---|
| With benzotriazol-1-yloxyl-tris-(pyrrolidino)-phosphonium hexafluorophosphate; N-ethyl-N,N-diisopropylamine | 65% |
-
-
1196992-82-1
[(1-{(S)-2-[(R)-2-(9H-Fluoren-9-ylmethoxycarbonylamino)-3-pyridin-4-yl-propionylamino]-3-tritylsulfanyl-propionylamino}-cyclopropanecarbonyl)-amino]-acetic acid methyl ester

-
-
180973-24-4
(E)-(S)-3-hydroxy-7-tritylthio-4-heptenoic acid

-
-
1196992-84-3
[(1-{(S)-2-[(R)-2-((E)-(S)-3-hydroxy-7-tritylsulfanyl-hept-4-enoylamino)-3-pyridin-4-yl-propionylamino]-3-tritylsulfanyl-propionylamino}-cyclopropanecarbonyl)-amino]-acetic acid methyl ester

| Conditions | Yield |
|---|---|
| Stage #1: [(1-{(S)-2-[(R)-2-(9H-Fluoren-9-ylmethoxycarbonylamino)-3-pyridin-4-yl-propionylamino]-3-tritylsulfanyl-propionylamino}-cyclopropanecarbonyl)-amino]-acetic acid methyl ester With diethylamine In acetonitrile at 20℃; Inert atmosphere; Stage #2: (E)-(S)-3-hydroxy-7-tritylthio-4-heptenoic acid With benzotriazol-1-yloxyl-tris-(pyrrolidino)-phosphonium hexafluorophosphate; N-ethyl-N,N-diisopropylamine In dichloromethane; acetonitrile at 0 - 20℃; Inert atmosphere; | 61% |
-
-
1196992-56-9
C46H44N4O7S

-
-
180973-24-4
(E)-(S)-3-hydroxy-7-tritylthio-4-heptenoic acid

-
-
1196992-58-1
[(1-{(S)-2-[2-((E)-(S)-3-hydroxy-7-tritylsulfanyl-hept-4-enoylamino)-acetylamino]-3-tritylsulfanyl-propionylamino}-cyclopropanecarbonyl)-amino]-acetic acid methyl ester

| Conditions | Yield |
|---|---|
| Stage #1: C46H44N4O7S With diethylamine In acetonitrile at 20℃; for 2h; Inert atmosphere; Stage #2: (E)-(S)-3-hydroxy-7-tritylthio-4-heptenoic acid With benzotriazol-1-yloxyl-tris-(pyrrolidino)-phosphonium hexafluorophosphate; N-ethyl-N,N-diisopropylamine In dichloromethane; acetonitrile at 20℃; for 16h; Inert atmosphere; | 56% |
-
-
1196992-44-5
((R)-2-{(S)-2-[2-(9H-Fluoren-9-ylmethoxycarbonylamino)-2-methyl-propionylamino]-3-tritylsulfanyl-propionylamino}-3-methyl-butyrylamino)-acetic acid methyl ester

-
-
180973-24-4
(E)-(S)-3-hydroxy-7-tritylthio-4-heptenoic acid

-
-
1196992-46-7
((R)-2-{(S)-2-[2-((E)-(S)-3-hydroxy-7-tritylsulfanyl-hept-4-enoylamino)-2-methyl-propionylamino]-3-tritylsulfanyl-propionylamino}-3-methyl-butyrylamino)-acetic acid methyl ester

| Conditions | Yield |
|---|---|
| Stage #1: ((R)-2-{(S)-2-[2-(9H-Fluoren-9-ylmethoxycarbonylamino)-2-methyl-propionylamino]-3-tritylsulfanyl-propionylamino}-3-methyl-butyrylamino)-acetic acid methyl ester With diethylamine In acetonitrile at 20℃; for 1.25h; Inert atmosphere; Stage #2: (E)-(S)-3-hydroxy-7-tritylthio-4-heptenoic acid With benzotriazol-1-yloxyl-tris-(pyrrolidino)-phosphonium hexafluorophosphate; N-ethyl-N,N-diisopropylamine In dichloromethane; acetonitrile at 0 - 20℃; Inert atmosphere; | 51% |

-
-
180973-24-4
(E)-(S)-3-hydroxy-7-tritylthio-4-heptenoic acid

| Conditions | Yield |
|---|---|
| Stage #1: [2-((S)-2-{[1-(9H-fluoren-9-ylmethoxycarbonylamino)-cyclopropanecarbonyl]-amino}-3-tritylsulfanyl-propionylamino)-3-methyl-butyrylamino]-acetic acid methyl ester With diethylamine In acetonitrile at 20℃; Inert atmosphere; Stage #2: (E)-(S)-3-hydroxy-7-tritylthio-4-heptenoic acid With benzotriazol-1-yloxyl-tris-(pyrrolidino)-phosphonium hexafluorophosphate; N-ethyl-N,N-diisopropylamine In dichloromethane; acetonitrile at 0 - 20℃; Inert atmosphere; | 41% |
-
-
180973-24-4
(E)-(S)-3-hydroxy-7-tritylthio-4-heptenoic acid

| Conditions | Yield |
|---|---|
| Multi-step reaction with 4 steps 1: 65 percent / N,N-diisopropylethylamine; benzotriazole-1-yl-oxy-tris-pyrrolidino-phosphonium PF6(1-) 2: 87 percent / morpholine / Pd(PPh3)4 / methanol 3: 67 percent / MNBA; DMAP / CH2Cl2 4: 100 percent / I2 / methanol; CH2Cl2 View Scheme |
-
-
180973-24-4
(E)-(S)-3-hydroxy-7-tritylthio-4-heptenoic acid

| Conditions | Yield |
|---|---|
| Multi-step reaction with 3 steps 1: 65 percent / N,N-diisopropylethylamine; benzotriazole-1-yl-oxy-tris-pyrrolidino-phosphonium PF6(1-) 2: 87 percent / morpholine / Pd(PPh3)4 / methanol 3: 100 percent / I2 / methanol; CH2Cl2 View Scheme |
-
-
180973-24-4
(E)-(S)-3-hydroxy-7-tritylthio-4-heptenoic acid

-
-
881845-68-7
(2S,6R,9S,12R,13S)-13-Hydroxy-12-isopropyl-6-methyl-2-((E)-4-tritylsulfanyl-but-1-enyl)-9-tritylsulfanylmethyl-1-oxa-5,8,11-triaza-cyclopentadecane-4,7,10,15-tetraone

| Conditions | Yield |
|---|---|
| Multi-step reaction with 3 steps 1: 65 percent / N,N-diisopropylethylamine; benzotriazole-1-yl-oxy-tris-pyrrolidino-phosphonium PF6(1-) 2: 87 percent / morpholine / Pd(PPh3)4 / methanol 3: 67 percent / MNBA; DMAP / CH2Cl2 View Scheme |
-
-
180973-24-4
(E)-(S)-3-hydroxy-7-tritylthio-4-heptenoic acid

-
-
878630-45-6
(3S,4R)-3-Hydroxy-4-{(S)-2-[(R)-2-((E)-(S)-3-hydroxy-7-tritylsulfanyl-hept-4-enoylamino)-propionylamino]-3-tritylsulfanyl-propionylamino}-5-methyl-hexanoic acid

| Conditions | Yield |
|---|---|
| Multi-step reaction with 2 steps 1: 65 percent / N,N-diisopropylethylamine; benzotriazole-1-yl-oxy-tris-pyrrolidino-phosphonium PF6(1-) 2: 87 percent / morpholine / Pd(PPh3)4 / methanol View Scheme |
-
-
180973-24-4
(E)-(S)-3-hydroxy-7-tritylthio-4-heptenoic acid

-
-
128517-07-7
romidepsin

| Conditions | Yield |
|---|---|
| Multi-step reaction with 4 steps 1: 95 percent / (benzotriazol-1-yloxy)tris(dimethylamino)phosphonium hexafluorophosphate, DIEA 2: 98 percent / LiOH 3: EDCl, DMAP, TsOH 4: 84 percent / I2 / methanol; H2O View Scheme |
-
-
180973-24-4
(E)-(S)-3-hydroxy-7-tritylthio-4-heptenoic acid

-
-
180973-35-7
(3S,9S,12R,16S)-6-eth-(Z)-ylidene-3,12-diisopropyl-16-((E)-4-tritylsulfanyl-but-1-enyl)-9-tritylsulfanylmethyl-1-oxa-4,7,10,13-tetraazacyclohexadecane-2,5,8,11,14-pentaone

| Conditions | Yield |
|---|---|
| Multi-step reaction with 3 steps 1: 95 percent / (benzotriazol-1-yloxy)tris(dimethylamino)phosphonium hexafluorophosphate, DIEA 2: 98 percent / LiOH 3: EDCl, DMAP, TsOH View Scheme |
-
-
180973-24-4
(E)-(S)-3-hydroxy-7-tritylthio-4-heptenoic acid

-
-
180973-34-6
(5E,7S,11R,14S,17E,20S)-17-ethylidene-7-hydroxy-11,20-diisopropyl-9,12,15,18-tetraoxo-1,1,1-triphenyl-14-(tritylthiomethyl)-2-thia-10,13,16,19-tetraazahenicos-5-en-21-oic acid

| Conditions | Yield |
|---|---|
| Multi-step reaction with 2 steps 1: 95 percent / (benzotriazol-1-yloxy)tris(dimethylamino)phosphonium hexafluorophosphate, DIEA 2: 98 percent / LiOH View Scheme |

| Conditions | Yield |
|---|---|
| With benzotriazol-1-yloxyl-tris-(pyrrolidino)-phosphonium hexafluorophosphate; N-ethyl-N,N-diisopropylamine In dichloromethane for 24h; |
(3S,4E)-3-Hydroxy-7-[(triphenylmethyl)thio]-4-heptenoic acid Chemical Properties
Molecular Structure of (3S,4E)-3-Hydroxy-7-[(triphenylmethyl)thio]-4-heptenoic acid (CAS No.180973-24-4):
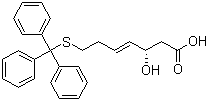
Molecular Formula: C26H26O3S
Molecular Weight: 418.5478
CAS No: 180973-24-4
H bond acceptors: 3
H bond donors: 2
Freely Rotating Bonds: 11
Polar Surface Area: 82.83 Å2
Index of Refraction: 1.624
Molar Refractivity: 123.08 cm3
Molar Volume: 348.423 cm3
Surface Tension: 52.238 dyne/cm
Density: 1.201 g/cm3
Flash Point: 308.363 °C
Enthalpy of Vaporization: 92.097 kJ/mol
Boiling Point: 586.266 °C at 760 mmHg
Vapour Pressure: 0 mmHg at 25°C
Systematic Name: (3S,4E)-3-Hydroxy-7-(tritylsulfanyl)hept-4-enoic acid
InChI: InChI=1/C26H26O3S/c27-24(20-25(28)29)18-10-11-19-30-26(21-12-4-1-5-13-21,22-14-6-2-7-15-22)23-16-8-3-9-17-23/h1-10,12-18,24,27H,11,19-20H2,(H,28,29)/b18-10+/t24-/m1/s1
InChIKey: BGUJCVRJDCZLQS-XMZCUOJSBV
Std. InChI: InChI=1S/C26H26O3S/c27-24(20-25(28)29)18-10-11-19-30-26(21-12-4-1-5-13-21,22-14-6-2-7-15-22)23-16-8-3-9-17-23/h1-10,12-18,24,27H,11,19-20H2,(H,28,29)/b18-10+/t24-/m1/s1
Std. InChIKey: BGUJCVRJDCZLQS-XMZCUOJSSA-N
Related Products
- (3S-(3alpha,6alpha(R*)))-Tetrahydro-2,2,6-trimethyl-6-(4-methyl-3-
- (3s)-( )-3-(1-methyl-1h-indol-3-yl)
- (3S)-(-)-3-(Ethylamino)pyrrolidine
- (3S)-(-)-3-Acetamidopyrrolidine
- (3S)-(+)-1-Benzyl-3-(methylamino)pyrrolidine
- (3S)-(+)-3-Aminopyrrolidine dihydrochloride
- (3S)-(+)-3-Fluoropyrrolidine hydrochloride
- (3S)-1-(2-Aminoethyl)-3-pyrrolidinol
- (3S)-1-(Phenylmethyl)-3-pyrrolidinemethanamine
- (3S)-1,2,3,4-Tetrahydro-3-isoquinolinemethanamine
- 180975-51-3
- 180982-90-5
- 180994-87-0
- 180995-02-2
- 180995-12-4
- 18101-58-1
- 181021-20-5
- 18102-22-2
- 18102-30-2
- 1810-23-7
Hot Products
About|Contact|Cas|Product Name|Molecular|Country|Encyclopedia
Message|New Cas|MSDS|Service|Advertisement|CAS DataBase|Article Data|Manufacturers | Chemical Catalog
©2008 LookChem.com,License: ICP
NO.:Zhejiang16009103
complaints:service@lookchem.com Desktop View