-
Name
Squalene
- EINECS 203-826-1
- CAS No. 111-02-4
- Article Data69
- CAS DataBase
- Density 0.858
- Solubility <0.1 g/100 mL at 19 ºC
- Melting Point -75 ºC
- Formula C30H50
- Boiling Point 285 ºC (25 mmHg)
- Molecular Weight 410.727
- Flash Point 254.1°C
- Transport Information
- Appearance Clear, slightly yellow liquid with a faint odor.
- Safety Moderately toxic by intravenous route. Slightly toxic by ingestion. When heated to decomposition it emits acrid smoke and irritating vapors.
- Risk Codes
-
Molecular Structure
- Hazard Symbols
- Synonyms 2,6,10,14,18,22-Tetracosahexaene,2,6,10,15,19,23-hexamethyl-, (all-E)- (8CI); (E,E,E,E)-Squalene;2,6,10,15,19,23-Hexamethyl-2,6,10,14,18,22-tetracosahexaene; Nikko Squalane EX;Spinacen; Spinacene; Squalen; Squalene; Super Squalene; all-trans-Squalene;trans-Squalene
- PSA 0.00000
- LogP 10.60500
Synthetic route

-
-
111-02-4
2,6,10,15,19,23-hexamethyltetracosa-2,6,10,14,18,22-hexaene

| Conditions | Yield |
|---|---|
| With bis(diphenylphosphino)propanepalladium(II) dichloride; lithium triethylborohydride In tetrahydrofuran at -78 - 20℃; for 50h; | 88.7% |
-
-
106-28-5
Farnesol

-
A
-
111-02-4
2,6,10,15,19,23-hexamethyltetracosa-2,6,10,14,18,22-hexaene

-
B
-
3899-18-1
(6E,10E)-2,6,10-trimethyldodeca-2,6,10-triene

-
C
-
66633-32-7
(E)-3,7,11-trimethyldodeca-1,6,10-triene

| Conditions | Yield |
|---|---|
| With bis(cyclopentadienyl)titanium dichloride; manganese In tetrahydrofuran for 2h; Solvent; Inert atmosphere; Reflux; regioselective reaction; | A 9% B 8% C 78% |

-
-
1299297-72-5
[(6E,10E,14E,18E)-13-(1-ethoxyethoxy)-2,6,10,15,19,23-hexamethyltetracosa-2,6,10,14,18,22-hexaen-12-yl]sulfonylbenzene

-
-
111-02-4
2,6,10,15,19,23-hexamethyltetracosa-2,6,10,14,18,22-hexaene

| Conditions | Yield |
|---|---|
| With (1,2-bis(diphenylphosphino)ethane)palladium(II) chloride; lithium triethylborohydride In tetrahydrofuran; 1,4-dioxane at 20 - 70℃; for 10h; Inert atmosphere; | 77% |

-
-
111-02-4
2,6,10,15,19,23-hexamethyltetracosa-2,6,10,14,18,22-hexaene

| Conditions | Yield |
|---|---|
| With 2,6-dimethylpyridine; trimethylsilyl trifluoromethanesulfonate at -78℃; | 70% |
| With 2,6-dimethylpyridine; trimethylsilyl trifluoromethanesulfonate In toluene at -78℃; for 12h; | 70% |

| Conditions | Yield |
|---|---|
| Stage #1: Farnesol With tert.-butyl lithium In tetrahydrofuran; n-heptane at -78 - 20℃; for 0.333333h; Inert atmosphere; Stage #2: With bis(cyclopentadienyl)titanium dichloride; manganese In tetrahydrofuran for 10h; Inert atmosphere; Reflux; | 67% |
| Multi-step reaction with 2 steps 1: PBr3 / tetrahydrofuran / 0.5 h / 0 °C 2: CuI; pyrrolidine; n-BuLi / diethyl ether / 1 h / -38 °C View Scheme | |
| Multi-step reaction with 2 steps 1: PBr3 / tetrahydrofuran / 0.5 h / 0 °C 2: CuI; pyrrolidine; n-BuLi / diethyl ether / 1 h / -38 °C View Scheme | |
| Multi-step reaction with 3 steps 1: triethylamine / CH2Cl2 / 0.5 h / -40 °C 2: lithium bromide / CH2Cl2; tetrahydrofuran / 1 h / 0 °C 3: 1) lithium biphenylide, barium iodide / 1) THF, -78 deg C, 30 min, 2) THF, a) -78 deg C, 3 h, b) 23 deg C, 16 h View Scheme | |
| Multi-step reaction with 3 steps 1: triethylamine / CH2Cl2 / 0.5 h / -40 °C 2: lithium chloride / CH2Cl2; tetrahydrofuran / 1 h / 0 °C 3: 1) lithium biphenylide, barium iodide / 1) THF, -78 deg C, 30 min, 2) THF, a) -78 deg C, 3 h, b) 23 deg C, 16 h View Scheme |

-
-
111-02-4
2,6,10,15,19,23-hexamethyltetracosa-2,6,10,14,18,22-hexaene

| Conditions | Yield |
|---|---|
| With 2,4,6-trimethyl-pyridine; bis(cyclopentadienyl)titanium dichloride; manganese; chloro-trimethyl-silane; triphenylphosphine; palladium dichloride In tetrahydrofuran at 20℃; for 16h; Wurtz type coupling reaction; Inert atmosphere; stereoselective reaction; | 60% |
-
-
24163-93-7, 24163-94-8, 56881-57-3, 6874-67-5, 28290-41-7
farnesyl bromide

-
A
-
111-02-4
2,6,10,15,19,23-hexamethyltetracosa-2,6,10,14,18,22-hexaene

-
B
-
86342-81-6
(6E,14E)-2,6,10,11,15,19-Hexamethyl-10,11-divinyl-icosa-2,6,14,18-tetraene

-
C
-
86342-79-2
(6E,10E,16E)-2,6,10,13,17,21-hexamethyl-13-vinyl-docosa-2,6,10,16,20-pentaene

| Conditions | Yield |
|---|---|
| With chlorotris(triphenylphosphine)cobalt(I) In benzene for 0.5h; Ambient temperature; | A 55% B 12% C 22% |
| With chlorotris(triphenylphosphine)cobalt(I) In benzene for 3h; Ambient temperature; | A 55% B 12% C 22% |

-
-
106-28-5
Farnesol

-
A
-
111-02-4
2,6,10,15,19,23-hexamethyltetracosa-2,6,10,14,18,22-hexaene

-
B
-
66633-32-7
(E)-3,7,11-trimethyldodeca-1,6,10-triene

| Conditions | Yield |
|---|---|
| With bis(cyclopentadienyl)titanium dichloride; manganese In tetrahydrofuran for 6h; Inert atmosphere; Reflux; | A 23% B 42% |
| With bis(cyclopentadienyl)titanium dichloride; manganese In tetrahydrofuran for 4h; Time; Inert atmosphere; Reflux; | A 36% B 24% |
-
-
24163-93-7, 24163-94-8, 56881-57-3, 6874-67-5, 28290-41-7
farnesyl bromide

-
A
-
111-02-4
2,6,10,15,19,23-hexamethyltetracosa-2,6,10,14,18,22-hexaene


| Conditions | Yield |
|---|---|
| With copper(l) iodide; lithium pyrrolidide | A 15% B 33% C 10% |

-
-
130673-53-9
4-{Phenyl-[(3E,7E)-4,8,12-trimethyl-1-((1E,5E)-2,6,10-trimethyl-undeca-1,5,9-trienyl)-trideca-3,7,11-trienyl]-phosphinoyl}-morpholine

-
-
111-02-4
2,6,10,15,19,23-hexamethyltetracosa-2,6,10,14,18,22-hexaene

| Conditions | Yield |
|---|---|
| With lithium; ethylamine; tert-butyl alcohol In tetrahydrofuran at 0℃; for 2.5h; | 31% |
-
-
24163-93-7, 24163-94-8, 28290-41-7, 56881-57-3, 6874-67-5
farnesyl bromide

-
-
111-02-4
2,6,10,15,19,23-hexamethyltetracosa-2,6,10,14,18,22-hexaene

| Conditions | Yield |
|---|---|
| With diethyl ether; lithium |
-
-
4128-17-0
(2E,6E)-farnesyl acetate

-
A
-
111-02-4
2,6,10,15,19,23-hexamethyltetracosa-2,6,10,14,18,22-hexaene

-
B
-
56782-22-0
(6E,10Z,14Z,18E)-squalene

-
C
-
86342-80-5
(6E,10Z,16E)-2,6,10,13,17,21-Hexamethyl-13-vinyl-docosa-2,6,10,16,20-pentaene

-
D
-
86342-79-2
(6E,10E,16E)-2,6,10,13,17,21-hexamethyl-13-vinyl-docosa-2,6,10,16,20-pentaene

| Conditions | Yield |
|---|---|
| With [2,2]bipyridinyl; copper; zinc; molybdenum hexacarbonyl In 1,4-dioxane for 115h; Heating; Yield given. Further byproducts given. Yields of byproduct given; |

-
-
4128-17-0
(2E,6E)-farnesyl acetate

-
A
-
111-02-4
2,6,10,15,19,23-hexamethyltetracosa-2,6,10,14,18,22-hexaene

-
B
-
86342-80-5
(6E,10Z,16E)-2,6,10,13,17,21-Hexamethyl-13-vinyl-docosa-2,6,10,16,20-pentaene

-
C
-
86342-79-2
(6E,10E,16E)-2,6,10,13,17,21-hexamethyl-13-vinyl-docosa-2,6,10,16,20-pentaene

| Conditions | Yield |
|---|---|
| With zinc; tetrakis(triphenylphosphine) palladium(0) In tetrahydrofuran; methanol for 2.5h; Ambient temperature; Yield given. Further byproducts given. Yields of byproduct given; |

-
-
24163-93-7, 24163-94-8, 56881-57-3, 6874-67-5, 28290-41-7
farnesyl bromide

-
-
6784-45-8, 67023-85-2, 72132-86-6, 67023-84-1
(2E,6E)-farnesyl chloride

-
-
111-02-4
2,6,10,15,19,23-hexamethyltetracosa-2,6,10,14,18,22-hexaene

| Conditions | Yield |
|---|---|
| With lithium biphenylide; barium(II) iodide 1) THF, -78 deg C, 30 min, 2) THF, a) -78 deg C, 3 h, b) 23 deg C, 16 h; Yield given. Multistep reaction; |
-
-
85611-33-2
(E)-3,7,11-trimethyldodeca-1,6,10-trien-3-yl acetate

-
A
-
111-02-4
2,6,10,15,19,23-hexamethyltetracosa-2,6,10,14,18,22-hexaene

-
B
-
24566-13-0
squalene

-
C
-
56782-22-0
(6E,10Z,14Z,18E)-squalene

-
D
-
86342-79-2
(6E,10E,16E)-2,6,10,13,17,21-hexamethyl-13-vinyl-docosa-2,6,10,16,20-pentaene

| Conditions | Yield |
|---|---|
| With [2,2]bipyridinyl; zinc; molybdenum hexacarbonyl In 1,4-dioxane for 72h; Heating; Yield given. Further byproducts given. Yields of byproduct given; | |
| With [2,2]bipyridinyl; zinc; molybdenum hexacarbonyl In 1,4-dioxane for 72h; Heating; Yield given. Further byproducts given. Yields of byproduct given; |

-
-
6784-45-8, 67023-85-2, 72132-86-6, 67023-84-1
(2E,6E)-farnesyl chloride

-
A
-
111-02-4
2,6,10,15,19,23-hexamethyltetracosa-2,6,10,14,18,22-hexaene

-
B
-
86342-79-2
(6E,10E,16E)-2,6,10,13,17,21-hexamethyl-13-vinyl-docosa-2,6,10,16,20-pentaene

| Conditions | Yield |
|---|---|
| With lithium biphenylide; barium(II) iodide In tetrahydrofuran at -78℃; for 1h; Yield given. Yields of byproduct given; | |
| With lithium biphenylide; barium(II) iodide In tetrahydrofuran at -78℃; for 1h; Yield given. Yields of byproduct given. Title compound not separated from byproducts; |

-
-
111-02-4
2,6,10,15,19,23-hexamethyltetracosa-2,6,10,14,18,22-hexaene

| Conditions | Yield |
|---|---|
| With PP1; Tween-80 Kinetics; Mechanism; buffer (KF, MgCl2, DTT, NADHP); |

-
-
111-02-4
2,6,10,15,19,23-hexamethyltetracosa-2,6,10,14,18,22-hexaene

| Conditions | Yield |
|---|---|
| With (ammonium analogue); inhib. phosphonophosphate 8 Kinetics; Mechanism; |

| Conditions | Yield |
|---|---|
| With lithium triethylborohydride; bis(diphenylphosphino)propanepalladium(II) dichloride In tetrahydrofuran at 0℃; Yield given. Yields of byproduct given; |

| Conditions | Yield |
|---|---|
| With iron sulfide; hydrogen sulfide In water at 100℃; under 1500.1 Torr; for 144h; Yield given; |

-
-
99548-50-2
3,6-dichloro-octane-2,7-dione

-
-
111-02-4
2,6,10,15,19,23-hexamethyltetracosa-2,6,10,14,18,22-hexaene

| Conditions | Yield |
|---|---|
| With diethyl ether at -70℃; Behandeln des Reaktionsprodukts mit methanol.Kalilauge, dann mit Natriumjodid und Natriumacetat in Essigsaeure und Propionsaeure bei -20grad bis +20grad; dann mit Zinn(II)-chlorid in Aether und Pyridin und mit Phosphoroxychlorid; |

-
-
111-02-4
2,6,10,15,19,23-hexamethyltetracosa-2,6,10,14,18,22-hexaene

| Conditions | Yield |
|---|---|
| With tetrahydrofuran; n-butyllithium; diethyl ether Erwaermen des Reaktionsprodukts mit 6,10-Dimethyl-undeca-5t,9-dien-2-on; |

-
A
-
111-02-4
2,6,10,15,19,23-hexamethyltetracosa-2,6,10,14,18,22-hexaene

-
B
-
42719-34-6
(3S,7S,10S,13R,16S,20S)-2,3,7,10,13,16,20,21-octamethyl-6,17-bis-methylene-10-vinyl-docosa-1,11t,21-triene

-
C
-
88361-07-3, 127483-82-3, 127483-86-7
(6E,11E,16E)-2,6,10,13,17,21-hexamethyl-10-vinyldocosa-2,6,11,16,20-pentaene

| Conditions | Yield |
|---|---|
| With sodium <14C>bicarbonate Further byproducts given. Title compound not separated from byproducts; |


-
A
-
111-02-4
2,6,10,15,19,23-hexamethyltetracosa-2,6,10,14,18,22-hexaene

-
B
-
42719-34-6
(3S,7S,10S,13R,16S,20S)-2,3,7,10,13,16,20,21-octamethyl-6,17-bis-methylene-10-vinyl-docosa-1,11t,21-triene

-
C
-
88361-07-3, 127483-82-3, 127483-86-7
(6E,11E,16E)-2,6,10,13,17,21-hexamethyl-10-vinyldocosa-2,6,11,16,20-pentaene

| Conditions | Yield |
|---|---|
| With sodium <14C>bicarbonate Further byproducts given. Title compound not separated from byproducts; |


-
A
-
111-02-4
2,6,10,15,19,23-hexamethyltetracosa-2,6,10,14,18,22-hexaene

-
B
-
88361-07-3, 127483-82-3, 127483-86-7
(6E,11E,16E)-2,6,10,13,17,21-hexamethyl-10-vinyldocosa-2,6,11,16,20-pentaene

| Conditions | Yield |
|---|---|
| With sodium <14C>bicarbonate Further byproducts given. Title compound not separated from byproducts; |

-
-
24163-93-7, 24163-94-8, 56881-57-3, 6874-67-5, 28290-41-7
farnesyl bromide

-
B
-
111-02-4
2,6,10,15,19,23-hexamethyltetracosa-2,6,10,14,18,22-hexaene

| Conditions | Yield |
|---|---|
| With manganese; bis(cyclopentadienyl)titanium dichloride In tetrahydrofuran at 20℃; |

-
-
6138-90-5
trans-geranyl bromide

-
-
24163-93-7, 24163-94-8, 56881-57-3, 6874-67-5, 28290-41-7
farnesyl bromide

-
A
-
111-02-4
2,6,10,15,19,23-hexamethyltetracosa-2,6,10,14,18,22-hexaene

-
B
-
35162-77-7
digeranyl

-
C
-
38235-83-5
2,6,10,15,19-pentamethyleicosa-(6E,10E,14E)-2,6,10,14,18-pentaene

| Conditions | Yield |
|---|---|
| With pyrrolidine; copper(l) iodide; n-butyllithium In diethyl ether at -38℃; for 1h; | A n/a B 20 mg C 28 mg |

-
-
24163-93-7, 24163-94-8, 56881-57-3, 6874-67-5, 28290-41-7
farnesyl bromide

-
-
870-63-3
prenyl bromide

-
B
-
111-02-4
2,6,10,15,19,23-hexamethyltetracosa-2,6,10,14,18,22-hexaene

| Conditions | Yield |
|---|---|
| With pyrrolidine; copper(l) iodide; n-butyllithium In diethyl ether at -38℃; for 1h; |


| Conditions | Yield |
|---|---|
| Multi-step reaction with 2 steps 1: PBr3 / tetrahydrofuran / 0.5 h / 0 °C 2: CuI; pyrrolidine; n-BuLi / diethyl ether / 1 h / -38 °C View Scheme |
-
-
111-02-4
2,6,10,15,19,23-hexamethyltetracosa-2,6,10,14,18,22-hexaene

-
-
111-01-3
2,6,10,15,19,23-hexamethyltetracosane

| Conditions | Yield |
|---|---|
| With hydrogen In ethanol at 50℃; under 760.051 Torr; for 6h; Reagent/catalyst; Temperature; Solvent; chemoselective reaction; | 99.6% |
| With hydrogen; nickel | |
| With palladium 10% on activated carbon; hydrogen In ethanol at 100℃; under 3750.38 Torr; for 1h; Autoclave; |
-
-
111-02-4
2,6,10,15,19,23-hexamethyltetracosa-2,6,10,14,18,22-hexaene

-
-
654052-53-6
2,3,22,23-Dioxidosqualene

| Conditions | Yield |
|---|---|
| Stage #1: 2,6,10,15,19,23-hexamethyltetracosa-2,6,10,14,18,22-hexaene With N-Bromosuccinimide; water In tetrahydrofuran for 3h; Stage #2: With potassium carbonate In methanol for 2h; Further stages.; | 90% |
| With potassium carbonate; 3-chloro-benzenecarboperoxoic acid or NBS; Multistep reaction; | |
| Multi-step reaction with 2 steps 1: 22 percent / dimethyldioxirane 2: 60 percent / methyl(trifluoromethyl)dioxirane View Scheme |
-
-
111-02-4
2,6,10,15,19,23-hexamethyltetracosa-2,6,10,14,18,22-hexaene

-
-
10008-64-7
(rac)-hexaepoxysqualene

| Conditions | Yield |
|---|---|
| With polyoxotungstate PW4O24[methyltri((C8-C10)alkyl)ammonium]3 In water at 20℃; for 12h; pH=7.0; Green chemistry; | 89% |
-
-
111-02-4
2,6,10,15,19,23-hexamethyltetracosa-2,6,10,14,18,22-hexaene

-
-
77-76-9
2,2-dimethoxy-propane

-
-
642093-28-5
(3R)-2,3-isopropylidenedioxy-2,3-dihydrosqualene

| Conditions | Yield |
|---|---|
| Stage #1: 2,6,10,15,19,23-hexamethyltetracosa-2,6,10,14,18,22-hexaene With potassium osmate(VI); methanesulfonamide; potassium hexacyanoferrate(III); chiral monocinchona derivative C70H63N5O3; potassium carbonate In water; tert-butyl alcohol at 0℃; for 20h; Stage #2: 2,2-dimethoxy-propane With toluene-4-sulfonic acid at 23℃; for 1h; Further stages.; | 88% |
-
-
111-02-4
2,6,10,15,19,23-hexamethyltetracosa-2,6,10,14,18,22-hexaene

-
-
56882-09-8
(4E,8E,12E)-4,9,13,17-Tetramethyloctadeca-4,8,12,16-tetraen-1-al

| Conditions | Yield |
|---|---|
| With periodic acid In tetrahydrofuran; water at 0 - 20℃; for 10h; | 86% |
| Stage #1: 2,6,10,15,19,23-hexamethyltetracosa-2,6,10,14,18,22-hexaene With 3-chloro-benzenecarboperoxoic acid In dichloromethane Epoxidation; Stage #2: With periodic acid Oxidation; | |
| Stage #1: 2,6,10,15,19,23-hexamethyltetracosa-2,6,10,14,18,22-hexaene With 3-chloro-benzenecarboperoxoic acid In dichloromethane at 0℃; for 2h; Stage #2: With periodic acid In diethyl ether | 3.1 g |
-
-
111-02-4
2,6,10,15,19,23-hexamethyltetracosa-2,6,10,14,18,22-hexaene

-
-
57434-13-6
2,6,10,15,19,23-hexamethyltetracosane-3,7,11,14,18,22-hexaol

| Conditions | Yield |
|---|---|
| Stage #1: 2,6,10,15,19,23-hexamethyltetracosa-2,6,10,14,18,22-hexaene With sodium tetrahydroborate; boron trifluoride diethyl etherate In tetrahydrofuran for 1.33333h; Stage #2: With dihydrogen peroxide In tetrahydrofuran for 0.5h; Further stages.; | 67% |
| Stage #1: 2,6,10,15,19,23-hexamethyltetracosa-2,6,10,14,18,22-hexaene With borane-THF; 2-methyl-but-2-ene In tetrahydrofuran at 0 - 4℃; for 77h; Stage #2: With dihydrogen peroxide; sodium hydroxide In tetrahydrofuran; water at 20℃; for 12h; | 51% |
-
-
111-02-4
2,6,10,15,19,23-hexamethyltetracosa-2,6,10,14,18,22-hexaene

| Conditions | Yield |
|---|---|
| Stage #1: 2,6,10,15,19,23-hexamethyltetracosa-2,6,10,14,18,22-hexaene With N-Bromosuccinimide; water In tetrahydrofuran for 0.75h; Stage #2: With methanol; potassium carbonate for 0.333333h; | 62% |
| With 3,3-dimethyldioxirane | 22% |
| With sodium hydrogencarbonate; 3-chloro-benzenecarboperoxoic acid In dichloromethane at 0℃; for 1h; | 14% |
-
-
111-02-4
2,6,10,15,19,23-hexamethyltetracosa-2,6,10,14,18,22-hexaene

-
-
63976-65-8
(3R)-(6E,10E,14E,18E)-2,3-dihydroxy-2,3-dihydrosqualene

| Conditions | Yield |
|---|---|
| With potassium osmate(VI); methanesulfonamide; potassium carbonate; potassium hexacyanoferrate(III) In water; butan-1-ol at 0℃; for 19h; ligand: 1,4-bisnaphthopyridazine Monomethiodide Salt; | 62% |
| With osmium(VIII) oxide; methanesulfonamide; potassium carbonate; 1,4-bis(9-O-dihydroquinidine)phthalazine; potassium hexacyanoferrate(III) In tert-butyl alcohol at 0℃; for 48h; optical yield given as %ee; enantioselective reaction; | 12% |
-
-
111-02-4
2,6,10,15,19,23-hexamethyltetracosa-2,6,10,14,18,22-hexaene

| Conditions | Yield |
|---|---|
| With N-chloro-succinimide; selenenyl bromide In dichloromethane at 20℃; for 8h; | A 18% B 55% |
-
-
111-02-4
2,6,10,15,19,23-hexamethyltetracosa-2,6,10,14,18,22-hexaene

-
-
65746-05-6
squalene monobromohydrin: 3-bromo-2,6,10,15,19,23-hexamethyl-6,10,14,18,22-tetracosapentaen-2-ol (all E)

| Conditions | Yield |
|---|---|
| With N-Bromosuccinimide; water In tetrahydrofuran at 20℃; for 1h; | 51% |
| With N-Bromosuccinimide; water In tetrahydrofuran for 1h; Ambient temperature; | 35% |
| With N-Bromosuccinimide In tetrahydrofuran; water 1.) 0 deg C, 30 min; 2.) RT, 1 h; | 35% |
-
-
111-02-4
2,6,10,15,19,23-hexamethyltetracosa-2,6,10,14,18,22-hexaene

| Conditions | Yield |
|---|---|
| With sodium periodate; ruthenium(IV) oxide In water; ethyl acetate; acetonitrile for 0.5h; | 50% |
-
-
111-02-4
2,6,10,15,19,23-hexamethyltetracosa-2,6,10,14,18,22-hexaene

| Conditions | Yield |
|---|---|
| With sodium periodate; ruthenium(IV) oxide In ethanol; water; acetonitrile at 0℃; for 0.5h; | 50% |
| With sodium periodate; ruthenium tetroxide | |
| With sodium periodate; ruthenium tetroxide |
-
-
111-02-4
2,6,10,15,19,23-hexamethyltetracosa-2,6,10,14,18,22-hexaene

| Conditions | Yield |
|---|---|
| With Hoveyda-Grubbs catalyst second generation In dichloromethane at 40℃; for 1h; Cross Metathesis; regioselective reaction; | A 45% B 6.3% C 20.3% D 8.5% |

-
-
111-02-4
2,6,10,15,19,23-hexamethyltetracosa-2,6,10,14,18,22-hexaene

| Conditions | Yield |
|---|---|
| With potassium osmate(VI); methanesulfonamide; potassium hexacyanoferrate(III); chiral monocinchona derivative C70H63N5O3; potassium carbonate In methanol; water; tert-butyl alcohol at 0℃; for 3h; | 40% |
-
-
111-02-4
2,6,10,15,19,23-hexamethyltetracosa-2,6,10,14,18,22-hexaene

-
A
-
65746-05-6
squalene monobromohydrin: 3-bromo-2,6,10,15,19,23-hexamethyl-6,10,14,18,22-tetracosapentaen-2-ol (all E)

-
B
-
110390-75-5
squalene dibromohydrin: 3,22-dibromo-2,6,10,15,19,23-hexamethyl-6,10,14,18-tetracosatetraen-2,23-diol (all E)

| Conditions | Yield |
|---|---|
| With N-Bromosuccinimide In tetrahydrofuran; water 1.) 30 min, 0 deg C; 2.) 1 h, RT; | A n/a B 30% |
| With N-Bromosuccinimide; water In tetrahydrofuran for 1h; Ambient temperature; | A n/a B 30% |
| With N-Bromosuccinimide; water In tetrahydrofuran at 0℃; for 1.5h; |

| Conditions | Yield |
|---|---|
| With sodium periodate; ruthenium(IV) oxide In ethanol; water; acetonitrile at 0℃; for 0.0666667h; | A 5% B 4.5% C 4.3% D 28% |

-
-
111-02-4
2,6,10,15,19,23-hexamethyltetracosa-2,6,10,14,18,22-hexaene

-
-
110390-75-5
squalene dibromohydrin: 3,22-dibromo-2,6,10,15,19,23-hexamethyl-6,10,14,18-tetracosatetraen-2,23-diol (all E)

| Conditions | Yield |
|---|---|
| With N-Bromosuccinimide In tetrahydrofuran at 0℃; | 27% |
| With 2,4,4,6-Tetrabromo-2,5-cyclohexadien-1-one In 1,2-dimethoxyethane; dichloromethane; water Ambient temperature; | |
| With N-Bromosuccinimide; water In tetrahydrofuran |
-
-
111-02-4
2,6,10,15,19,23-hexamethyltetracosa-2,6,10,14,18,22-hexaene

-
-
67858-78-0
(4E, 8E)-5,9,13-trimethyl-4,8,12-tetradecatrienal

| Conditions | Yield |
|---|---|
| With MMPP In tetrahydrofuran; water at 25℃; for 48h; | 26% |
| Multi-step reaction with 2 steps 1: MCPBA / CH2Cl2 / 0 °C 2: HIO4 / diethyl ether / 25 °C View Scheme |
-
-
111-02-4
2,6,10,15,19,23-hexamethyltetracosa-2,6,10,14,18,22-hexaene

-
-
62399-03-5
(2E,6E,10E,14E,18E,22E)-2,6,10,15,19,23-Hexamethyl-tetracosa-2,6,10,14,18,22-hexaenedioic acid

| Conditions | Yield |
|---|---|
| soil bacterium; | 26% |
-
-
111-02-4
2,6,10,15,19,23-hexamethyltetracosa-2,6,10,14,18,22-hexaene

-
B
-
97232-74-1
(6E,10E,14E,18E)-2,6,10,15,19,23-hexamethyltetracosadeca-1,6,10,14,18,22-hexaen-3-ol

-
C
-
79370-71-1
(2E,6E,10E,14E,18E,22E)-2,6,10,15,19,23-hexamethyltetracosa-2,6,10,14,18,22-hexaene-1-ol

-
D
-
79370-70-0
(2E,6E,10E,14E,18E,22E)-2,6,10,15,19,23-hexamethyltetracosadeca-2,6,10,14,18,22-hexaene-1,24-diol

| Conditions | Yield |
|---|---|
| Multistep reaction; | A 9% B 26% C 20% D 6% |

(E,E,E,E)-Squalene Chemical Properties
IUPAC Name: (6Z,10E,14Z,18E)-2,6,10,15,19,23-Hexamethyltetracosa-2,6,10,14,18,22-hexaene (111-02-4)
Synonyms : Trans-squalene ; (all-E)-2,6,10,15,19,23-Hexamethyl-2,6,10,14,18,22-tetracosahexaene
Molecular Structure: 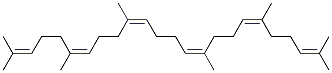
Molecular Formula: C30H50
Molecular Weight : 410.72
CAS Registry Number : 111-02-4
EINECS : 203-826-1
Product Categories: Biochemistry;Terpenes;Terpenes (Others)
Mol File: 111-02-4.mol
Surface Tension: 29.2 dyne/cm
Density: 0.848 g/cm3
Flash Point: 254.1 °C
Melting point -75 ºC
Enthalpy of Vaporization: 65.81 kJ/mol
Boiling Point: 429.3 °C at 760 mmHg
Vapour Pressure: 3.56E-07 mmHg at 25°C
Refractive index: 1.4945-1.4965
Water solubility: <0.1 g/100 mL at 19 ºC
refractive index : n20/D 1.494(lit.)
storage temp: 2-8°C
Water Solubility: <0.1 g/100 mL at 19 ºC
Appearance: Trans-squalene (111-02-4) is clear colourless to faint yellow oil .
(E,E,E,E)-Squalene Uses
Trans-squalene (111-02-4) is used for nutrition medicine,mainly for oral treatment of high-, low blood pressure, anemia, diabetes, liver cirrhosis, cancer, constipation, cavities; topical treatment of tonsillitis, wheezing, bronchitis, influenza, tuberculosis, rhinitis, gastric ulcer, duodenal ulcer, gall bladder stone , rheumatism and neuralgia.
(E,E,E,E)-Squalene Toxicity Data With Reference
| Organism | Test Type | Route | Reported Dose (Normalized Dose) | Effect | Source |
|---|---|---|---|---|---|
| mouse | LD50 | intravenous | 1800mg/kg (1800mg/kg) | Japanese Journal of Cancer Research. Vol. 76, Pg. 1021, 1985. | |
| mouse | LD50 | oral | 5gm/kg (5000mg/kg) | Japanese Journal of Cancer Research. Vol. 76, Pg. 1021, 1985. |
(E,E,E,E)-Squalene Consensus Reports
Reported in EPA TSCA Inventory.
(E,E,E,E)-Squalene Safety Profile
Safety Statements 24/25
24/25: Trans-squalene (111-02-4) used, avoid contact with skin and eyes
WGK Germany 2
RTECS XB6010000
F 8-10-23
HS Code 29012980
(E,E,E,E)-Squalene Specification
Chemical Properties: clear colourless to faint yellow oil
General Description Clear, slightly yellow liquid with a faint odor.
Air & Water Reactions : May become discolored on exposure to air. Insoluble in water.
Reactivity Profile: (all-E)-2,6,10,15,19,23-Hexamethyl-2,6,10,14,18,22-tetracosahexaene is incompatible with strong oxidizing agents. .
Health Hazard : ACUTE/CHRONIC HAZARDS: When heated to decomposition (all-E)-2,6,10,15,19,23-Hexamethyl-2,6,10,14,18,22-tetracosahexaene emits toxic fumes of carbon monoxide and carbon dioxide.
Fire Hazard : (all-E)-2,6,10,15,19,23-Hexamethyl-2,6,10,14,18,22-tetracosahexaene is probably combustible.
Related Products
- (E)-2-Bromo-4,4,4-trifluoro-2-butenoic acid ethyl ester
- (E)-2-Butenenitrile
- (E)-2-Butenoyl chloride
- (E)-2-Cyano-3-(3,4-dihydroxyphenyl)-2-propenoic acid
- (E)-2-Cyano-3-(4-methylphenyl)prop-2-enoate
- (E)-2-Hexadecenoic acid
- (E)-2-Hexadecenoic acid ethyl ester
- (E)-2-Methyl-2-pentenal
- (E)-2-Octadecenol
- (E)-2-Octenal
- 111024-84-1
- 111025-46-8
- 111-03-5
- 11103-57-4
- 11103-72-3
- 11103-91-6
- 11104-28-2
- 111042-89-8
- 111042-90-1
- 111043-48-2
Hot Products
About|Contact|Cas|Product Name|Molecular|Country|Encyclopedia
Message|New Cas|MSDS|Service|Advertisement|CAS DataBase|Article Data|Manufacturers | Chemical Catalog
©2008 LookChem.com,License: ICP
NO.:Zhejiang16009103
complaints:service@lookchem.com Desktop View