-
Name
1-Bromo-2-butanone
- EINECS 212-431-3
- CAS No. 816-40-0
- Article Data38
- CAS DataBase
- Density 1.439 g/cm3
- Solubility
- Melting Point
- Formula C4H7BrO
- Boiling Point 155.9 °C at 760 mmHg
- Molecular Weight 151.003
- Flash Point 68.3 °C
- Transport Information UN 1693
- Appearance colorless to light yellow liquid
- Safety 26-27-36/37/39
- Risk Codes 20/21/22-36/37/38
-
Molecular Structure
-
Hazard Symbols
 Xn
Xn
- Synonyms 1-Bromo-2-butanone;2-Oxobutyl bromide;Bromomethyl ethyl ketone;1-bromobutan-2-one;1-Bromo-2-butanone;
- PSA 17.07000
- LogP 1.36040
Synthetic route
-
-
14091-67-9
1-(trimethylsilyl)-2-butanone

-
-
816-40-0
1-Bromo-2-butanone

| Conditions | Yield |
|---|---|
| With bromine In dichloromethane at -78℃; | 95% |

| Conditions | Yield |
|---|---|
| Stage #1: 1-morpholinopropane-1-one; dimethyltitanocene In toluene at 65℃; Schlenk technique; Inert atmosphere; Stage #2: With bromine In toluene at -78℃; for 0.0333333h; Schlenk technique; Inert atmosphere; Stage #3: With water In toluene at 20℃; for 1h; Schlenk technique; Inert atmosphere; regioselective reaction; | 95% |
-
-
14045-28-4
N-propionylpiperidine

-
-
1271-66-5
dimethyltitanocene

-
A
-
3479-86-5
1,1-dibromo-butan-2-one

-
B
-
816-40-0
1-Bromo-2-butanone

| Conditions | Yield |
|---|---|
| Stage #1: N-propionylpiperidine; dimethyltitanocene In toluene at 65℃; Schlenk technique; Inert atmosphere; Stage #2: With bromine In toluene at -78℃; for 0.0333333h; Schlenk technique; Inert atmosphere; Stage #3: With water In toluene at 20℃; for 1h; Schlenk technique; Inert atmosphere; regioselective reaction; | A n/a B 94% |

-
-
4553-05-3
1-pyrrolidin-1-yl-propan-1-one

-
-
1271-66-5
dimethyltitanocene

-
A
-
3479-86-5
1,1-dibromo-butan-2-one

-
B
-
816-40-0
1-Bromo-2-butanone

| Conditions | Yield |
|---|---|
| Stage #1: 1-pyrrolidin-1-yl-propan-1-one; dimethyltitanocene In toluene at 65℃; Schlenk technique; Inert atmosphere; Stage #2: With bromine In toluene at -78℃; for 0.0333333h; Schlenk technique; Inert atmosphere; Stage #3: With water In toluene at 20℃; for 1h; Schlenk technique; Inert atmosphere; regioselective reaction; | A n/a B 93% |


| Conditions | Yield |
|---|---|
| Stage #1: dimethyltitanocene; N,N-dipropylpropionamide In toluene at 65℃; Schlenk technique; Inert atmosphere; Stage #2: With bromine In toluene at -78℃; for 0.0333333h; Schlenk technique; Inert atmosphere; Stage #3: With water In toluene at 20℃; for 1h; Schlenk technique; Inert atmosphere; regioselective reaction; | 91% |

| Conditions | Yield |
|---|---|
| Stage #1: dimethyltitanocene; N,N-diethylpropanamide In toluene at 65℃; Schlenk technique; Inert atmosphere; Stage #2: With bromine In toluene at -78℃; for 0.0333333h; Schlenk technique; Inert atmosphere; Stage #3: With water In toluene at 20℃; for 1h; Schlenk technique; Inert atmosphere; regioselective reaction; | 90% |

| Conditions | Yield |
|---|---|
| Stage #1: dimethyltitanocene; N,N-dimethyl-propanamide In toluene at 65℃; Schlenk technique; Inert atmosphere; Stage #2: With bromine In toluene at -78℃; for 0.0333333h; Schlenk technique; Inert atmosphere; Stage #3: With water In toluene at 20℃; for 1h; Solvent; Temperature; Reagent/catalyst; Schlenk technique; Inert atmosphere; regioselective reaction; | 90% |
-
-
78-93-3
butanone

-
A
-
2648-69-3
3,3-dibromobutan-2-one

-
B
-
3479-86-5
1,1-dibromo-butan-2-one

-
C
-
816-40-0
1-Bromo-2-butanone

-
D
-
814-75-5
3-bromo-butanone

| Conditions | Yield |
|---|---|
| With N-Bromosuccinimide; silica gel In methanol for 0.166667h; Reflux; | A n/a B n/a C 14% D 83% |


| Conditions | Yield |
|---|---|
| With Oxone; ammonium bromide In methanol at 20℃; for 8h; regioselective reaction; | A 80% B 9% |
| With 3-bromo-6-chloroimidazo<1,2-b>pyridazine hydrobromide-bromine for 0.5h; Ambient temperature; | A 19% B 58% |
| With potassium chlorate; bromine at 50℃; |

| Conditions | Yield |
|---|---|
| With N-bromo-N-sodiopolystyrenesulphonamide; sulfuric acid In chloroform for 8h; Heating; | 50% |
| With bromine | |
| With potassium chlorate; bromine |
-
-
1271-66-5
dimethyltitanocene

-
-
758-96-3
N,N-dimethyl-propanamide

-
A
-
815-51-0
1,3-dibromobutan-2-one

-
B
-
816-40-0
1-Bromo-2-butanone

| Conditions | Yield |
|---|---|
| Stage #1: dimethyltitanocene; N,N-dimethyl-propanamide With bromine at 70℃; for 2h; Stage #2: With water | A n/a B 35% |


| Conditions | Yield |
|---|---|
| With diethyl ether beim folgenden Einleiten von HBr bei 0grad; |

| Conditions | Yield |
|---|---|
| With hypobromous acid |

| Conditions | Yield |
|---|---|
| With chromic acid | |
| With potassium dichromate; sulfuric acid |
-
-
64-19-7
acetic acid

-
-
78-93-3
butanone

-
A
-
4906-24-5
3-acetoxy-2-butanone

-
B
-
1575-57-1
2-oxobutyl acetate

-
C
-
816-40-0
1-Bromo-2-butanone

-
D
-
814-75-5
3-bromo-butanone

| Conditions | Yield |
|---|---|
| With bromine; sodium acetate In water Further byproducts given; |

-
-
78-93-3
butanone

-
A
-
4906-24-5
3-acetoxy-2-butanone

-
B
-
1575-57-1
2-oxobutyl acetate

-
C
-
816-40-0
1-Bromo-2-butanone

-
D
-
814-75-5
3-bromo-butanone

| Conditions | Yield |
|---|---|
| With bromine; sodium acetate In water; acetic acid Further byproducts given; |


| Conditions | Yield |
|---|---|
| With hydrogen bromide 1.) diethyl ether, 2.) diethyl ether; Multistep reaction; |

| Conditions | Yield |
|---|---|
| With sodium bromide In N-methyl-acetamide |
-
-
78-92-2, 15892-23-6
iso-butanol

-
A
-
78-93-3
butanone

-
B
-
816-40-0
1-Bromo-2-butanone

-
C
-
814-75-5
3-bromo-butanone

| Conditions | Yield |
|---|---|
| With sodium bromate; sulfuric acid; sodium bromide In water at 60℃; for 0.166667h; Microwave irradiation; |

-
-
6863-73-6
3-chloropyrazin-2-amine

-
-
816-40-0
1-Bromo-2-butanone

-
-
391954-17-9
8-chloro-2-ethylimidazo[1,2-a]pyrazine

| Conditions | Yield |
|---|---|
| at 90℃; for 18h; | 100% |
| With sodium carbonate In 1,4-dioxane; dimethylsulfoxide-d6; diethyl ether; water | 74% |
| In tert-butyl alcohol Heating; | 44% |

| Conditions | Yield |
|---|---|
| In N,N-dimethyl-formamide at 20℃; for 3.75h; | 100% |
-
-
35302-72-8
pyrrol-2-yl trichloromethyl ketone

-
-
816-40-0
1-Bromo-2-butanone

-
-
1613023-09-8
3-ethyl-1H-pyrrolo[2,1-c][1,4]oxazin-1-one

| Conditions | Yield |
|---|---|
| With potassium carbonate In acetone at 20℃; for 19h; | 100% |

| Conditions | Yield |
|---|---|
| With bromocyane; triethylamine In ethanol at 0 - 20℃; for 0.00138889h; Sealed tube; | 100% |

| Conditions | Yield |
|---|---|
| With bromocyane; triethylamine In ethanol at 0 - 20℃; for 0.00138889h; Sealed tube; | 100% |
-
-
21052-18-6
2-thio-1-(2',3',5'-tri-O-benzoyl-β-D-ribofuranosyl)-(3H)pyrimidine-2,4-dione

-
-
816-40-0
1-Bromo-2-butanone

-
-
155006-17-0
2-(2-oxobutyl)thio-1-(2,3,5-tri-O-benzoyl-β-D-ribofuranosyl)-4(1H)-pyrimidinone

| Conditions | Yield |
|---|---|
| With triethylamine In dichloromethane for 1h; Ambient temperature; | 99% |

-
-
816-40-0
1-Bromo-2-butanone

| Conditions | Yield |
|---|---|
| With caesium carbonate In N,N-dimethyl-formamide at 20℃; for 12h; | 99% |
-
-
158980-46-2
N-(Diphenylmethyl)glycine 1,1-dimethylethyl ester

-
-
816-40-0
1-Bromo-2-butanone

-
-
647857-04-3
[benzhydryl-(2-oxobutyl)amino]acetic tert-butyl ester

| Conditions | Yield |
|---|---|
| With N-ethyl-N,N-diisopropylamine In acetone at -60℃; Heating / reflux; | 98% |
-
-
28443-50-7
2-amino-5-chlorophenol

-
-
816-40-0
1-Bromo-2-butanone

| Conditions | Yield |
|---|---|
| With potassium carbonate In acetone at 20℃; for 3h; Cooling with ice; | 98% |
-
-
951771-70-3
5-chloro-6-(4-chlorophenylamino)nicotinamidine

-
-
816-40-0
1-Bromo-2-butanone

| Conditions | Yield |
|---|---|
| With potassium hydrogencarbonate In tetrahydrofuran at 60 - 80℃; for 2h; | 97% |

| Conditions | Yield |
|---|---|
| Stage #1: 2-nitro-5-trifluoromethylphenol With sodium hydride In N,N-dimethyl-formamide; mineral oil at 20℃; for 0.333333h; Inert atmosphere; Stage #2: 1-Bromo-2-butanone In N,N-dimethyl-formamide; mineral oil at 20℃; for 21.5h; Inert atmosphere; | 97% |

-
-
816-40-0
1-Bromo-2-butanone

| Conditions | Yield |
|---|---|
| Stage #1: tert-butyl (2-bromo-4-methyl-6-nitrophenyl)carbamate With caesium carbonate In N,N-dimethyl-formamide at 20℃; for 0.0833333h; Stage #2: 1-Bromo-2-butanone In N,N-dimethyl-formamide; acetonitrile at 20℃; for 0.25h; | 97% |
-
-
603-35-0
triphenylphosphine

-
-
816-40-0
1-Bromo-2-butanone

-
-
94953-37-4
2-oxobutyltriphenylphosphonium bromide

| Conditions | Yield |
|---|---|
| In tetrahydrofuran for 4h; Reflux; | 96% |
| In tetrahydrofuran for 3h; Cooling with ice; Reflux; | 95% |

-
-
816-40-0
1-Bromo-2-butanone

| Conditions | Yield |
|---|---|
| With tetra-(n-butyl)ammonium iodide; potassium carbonate In N,N-dimethyl-formamide at 20℃; for 2h; Inert atmosphere; | 96% |

-
-
816-40-0
1-Bromo-2-butanone

| Conditions | Yield |
|---|---|
| In acetonitrile for 2h; Reflux; | 96% |
-
-
816-40-0
1-Bromo-2-butanone

-
-
197089-39-7
4-acetylamino-3-amino-benzoic acid t-butyl ester

-
-
233671-43-7
4-acetylamino-3-(2'-oxobutyl)-aminobenzoic acid t-butyl ester

| Conditions | Yield |
|---|---|
| With sodium hydrogencarbonate In N,N-dimethyl-formamide Ambient temperature; | 95% |
-
-
689251-37-4
3-(4-nitrophenyl)propanethioamide

-
-
816-40-0
1-Bromo-2-butanone

-
-
689251-39-6
4-ethyl-2-[2-(4-nitrophenyl)ethyl]-1,3-thiazole

| Conditions | Yield |
|---|---|
| In tert-butyl alcohol for 0.5h; Heating / reflux; | 95% |
-
-
2365-48-2
Methyl thioglycolate

-
-
816-40-0
1-Bromo-2-butanone

-
-
61363-63-1
methyl 2-<(3-methylacetonyl)thio>acetate

| Conditions | Yield |
|---|---|
| With sodium methylate In methanol a) room temperature, 25 min, b) 57 deg C, 25 min; | 94% |
-
-
996-82-7, 34727-00-9, 73177-21-6
sodium diethylmalonate

-
-
816-40-0
1-Bromo-2-butanone

-
-
1907-97-7
<2-Oxo-butyl>-malonsaeure-diethylester

| Conditions | Yield |
|---|---|
| In tetrahydrofuran Ambient temperature; | 94% |

-
-
816-40-0
1-Bromo-2-butanone

| Conditions | Yield |
|---|---|
| With potassium carbonate In acetone at 20℃; for 16h; | 94% |
-
-
26395-26-6
3-methoxy-2-methylpyridine

-
-
816-40-0
1-Bromo-2-butanone

-
-
177559-02-3
3-Methoxy-2-methyl-1-(2-oxobutyl)-pyridinium Bromide

| Conditions | Yield |
|---|---|
| at 70℃; for 0.166667h; | 93.8% |
-
-
23051-16-3
4-thioureidobenzoic acid ethyl ester

-
-
816-40-0
1-Bromo-2-butanone

-
-
960324-91-8
4-(4-ethylthiazol-2-ylamino)-benzoic acid ethyl ester

| Conditions | Yield |
|---|---|
| In 1,4-dioxane at 110℃; for 0.25h; | 93% |
| In 1,4-dioxane at 110℃; for 0.25h; Microwave irradiation; | 93% |
-
-
5369-16-4
meta-iso-propyl aniline

-
-
816-40-0
1-Bromo-2-butanone

-
-
41904-39-6
3-chloro-benzeneacetyl chloride

| Conditions | Yield |
|---|---|
| With pyridine; N-ethyl-N,N-diisopropylamine In toluene | 93% |

-
-
816-40-0
1-Bromo-2-butanone

-
-
177560-06-4
ethyl 3-benzyloxy-2-pyridineacetate

-
-
177558-64-4
Ethyl (8-Benzyloxy-2-ethylindolizin-1-yl)carboxylate

| Conditions | Yield |
|---|---|
| With sodium hydrogencarbonate In butanone for 24h; Heating; | 92% |
| With sodium hydrogencarbonate In butanone | 92% |
| With sodium hydrogencarbonate In butyraldehyde for 24h; Heating / reflux; | 92% |
| With sodium hydrogencarbonate In butyraldehyde for 24h; Heating / reflux; | 92% |
-
-
816-40-0
1-Bromo-2-butanone

-
-
463975-31-7
N-methoxy-N-methyl-2-(5-thioxo-1-trimethylsilanylmethyl-pyrrolidin-2-yl)-acetamide

-
-
463975-33-9
N-methoxy-N-methyl-2-[5-(2-oxo-butylidene)-1-trimethylsilanylmethyl-pyrrolidin-2-yl]-acetamide

| Conditions | Yield |
|---|---|
| Stage #1: 1-Bromo-2-butanone; N-methoxy-N-methyl-2-(5-thioxo-1-trimethylsilanylmethyl-pyrrolidin-2-yl)-acetamide In acetonitrile at 23℃; Stage #2: With triethylamine; triphenylphosphine In acetonitrile at 23℃; | 92% |
-
-
896722-44-4
2-(5-methoxy-pyridin-2-yl)-1-(3,4,5-trimethoxyphenyl)ethanone

-
-
816-40-0
1-Bromo-2-butanone

| Conditions | Yield |
|---|---|
| With sodium hydrogencarbonate In acetone for 20h; | 92% |
1-Bromo-2-butanone Specification
The 1-Bromo-2-butanone, with the CAS registry number 816-40-0 and EINECS registry number 212-431-3, has the systematic name and IUPAC name of 1-bromobutan-2-one. It is a kind of colorless to light yellow liquid, and belongs to the product category of Halogen compounds. And the molecular formula of the chemical is C4H7BrO.
The characteristics of 1-Bromo-2-butanone are as followings: (1)ACD/LogP: 1.07; (2)# of Rule of 5 Violations: 0; (3)ACD/LogD (pH 5.5): 1.07; (4)ACD/LogD (pH 7.4): 1.07; (5)ACD/BCF (pH 5.5): 3.84; (6)ACD/BCF (pH 7.4): 3.84; (7)ACD/KOC (pH 5.5): 91.15; (8)ACD/KOC (pH 7.4): 91.15; (9)#H bond acceptors: 1; (10)#H bond donors: 0; (11)#Freely Rotating Bonds: 2; (12)Polar Surface Area: 17.07 Å2; (13)Index of Refraction: 1.452; (14)Molar Refractivity: 28.34 cm3; (15)Molar Volume: 104.8 cm3; (16)Polarizability: 11.23×10-24cm3; (17)Surface Tension: 31.5 dyne/cm; (18)Density: 1.439 g/cm3; (19)Flash Point: 68.3 °C; (20)Enthalpy of Vaporization: 39.26 kJ/mol; (21)Boiling Point: 155.9 °C at 760 mmHg; (22)Vapour Pressure: 2.96 mmHg at 25°C.
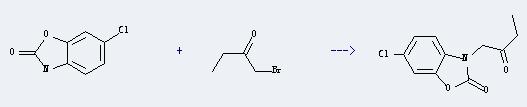
Uses of 1-Bromo-2-butanone: It can react with 6-chloro-3H-benzooxazol-2-one to 6-chloro-3-(2-oxo-butyl)-3H-benzooxazol-2-one. This reaction will need reagent sodium ethoxide, and the menstruum ethanol. The reaction time is 3 hours, and the yield is about 35%.
You should be cautious while dealing with this chemical. It irritates to eyes, respiratory system and skin, and it is also harmful by inhalation, in contact with skin and if swallowed. Therefore, you had better take the following instructions: Wear suitable protective clothing, gloves and eye/face protection, and if in case of contacting with eyes, rinse immediately with plenty of water and seek medical advice; Take off immediately all contaminated clothing.
Addtionally, the following datas could be converted into the molecular structure:
(1)SMILES: BrCC(=O)CC
(2)InChI: InChI=1/C4H7BrO/c1-2-4(6)3-5/h2-3H2,1H3
(3)InChIKey: CCXQVBSQUQCEEO-UHFFFAOYAC
The toxicity data is as follows:
| Organism | Test Type | Route | Reported Dose (Normalized Dose) | Effect | Source |
|---|---|---|---|---|---|
| mouse | LCLo | inhalation | 500mg/m3/10M (500mg/m3) | National Defense Research Committee, Office of Scientific Research and Development, Progress Report.Vol. No.9-4-1-9, Pg. 1943, |
Related Products
- 1-Bromo-2-butanone
- 81-64-1
- 816418-32-3
- 816429-58-0
- 816-43-3
- 816444-90-3
- 816454-25-8
- 816456-44-7
- 816458-31-8
- 81646-13-1
- 81647-80-5
Hot Products
About|Contact|Cas|Product Name|Molecular|Country|Encyclopedia
Message|New Cas|MSDS|Service|Advertisement|CAS DataBase|Article Data|Manufacturers | Chemical Catalog
©2008 LookChem.com,License: ICP
NO.:Zhejiang16009103
complaints:service@lookchem.com Desktop View